Back to Journals » Journal of Blood Medicine » Volume 14
Outcomes of Hydroxyurea Accessed via Various Means and Barriers Affecting Its Usage Among Children with Sickle Cell Anaemia in North-Western Tanzania
Authors Ambrose EE , Kidenya BR, Charles M, Ndunguru J, Jonathan A, Makani J , Minja IK , Ruggajo P, Balandya E
Received 16 July 2022
Accepted for publication 5 December 2022
Published 21 January 2023 Volume 2023:14 Pages 37—47
DOI https://doi.org/10.2147/JBM.S380901
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Martin H Bluth
Emmanuela E Ambrose,1 Benson R Kidenya,2 Mwesige Charles,3 Joyce Ndunguru,4 Agnes Jonathan,4 Julie Makani,4 Irene K Minja,4,5 Paschal Ruggajo,4,6 Emmanuel Balandya4,7
1Department of Paediatrics and Child Health, Catholic University of Health and Allied Sciences, Mwanza, Tanzania; 2Department of Biochemistry and Molecular Biology, Catholic University of Health and Allied Sciences, Mwanza, Tanzania; 3Department of Laboratory Services, Bugando Medical Centre, Mwanza, Tanzania; 4Department of Haematology and Blood Transfusion, Muhimbili University of Health and Allied Sciences, Dar es Salaam, Tanzania; 5Department of Restorative Dentistry, Muhimbili University of Health and Allied Sciences, Dar es Salaam, Tanzania; 6Department of Internal Medicine, Muhimbili University of Health and Allied Sciences, Dar es Salaam, Tanzania; 7Department of Physiology, Muhimbili University of Health and Allied Sciences, Dar es Salaam, Tanzania
Correspondence: Emmanuela E Ambrose, Department of Paediatrics and Child Health, Catholic University of Health and Allied Sciences, P.O. Box 1464, Mwanza, Tanzania, Tel +255789733833, Fax +255282500799, Email [email protected]
Purpose: To assess clinical and haematological outcomes of Hydroxyurea accessed via various access means and uncover the barriers to its utilization in children with Sickle cell anaemia (SCA), North-western Tanzania.
Patients and Methods: A retrospective study was conducted between October 2020 and April 2021 at Bugando Medical Centre (BMC) through review of medical files to compare the clinical and haematological outcomes among children with SCA at baseline and followed up retrospectively for at least one year of hydroxyurea utilization, accessed via cash, insurance and projects. Subsequently, a cross-sectional survey was conducted among parents and caregivers to ascertain the barriers to access of hydroxyurea via the various means. The p-values < 0.05 were considered statistically significant.
Results: We identified 87 children with SCA who were on hydroxyurea for at least one year. The median age at baseline (before hydroxyurea) was 99 [78– 151] months, and 52/87 (59.8%) were male. Compared to baseline, there was a significant reduction in proportion of patients reporting vaso-occlusive crisis, admissions and blood transfusions, a significant increase in Haemoglobin and mean corpuscular volume, conversely a significant reduction in absolute neutrophil and reticulocytes to both insurance and project participants. There was no significant change in most of these parameters among patients who accessed hydroxyurea via cash. Further, a total of 24/87 (27.6%) participants reported different barriers to access of hydroxyurea, where 10/24 (41.7%) reported hydroxyurea to be very expensive, 10/24 (41.7%) reported insurance challenges, and 4/21 (16.6%) reported unavailability of the drug.
Conclusion: The paediatric patients utilizing hydroxyurea accessed via insurance and projects, but not cash, experienced significant improvement in the clinical and haematological outcomes. Several barriers for access to hydroxyurea were observed which appeared to impact these outcomes. These findings call for concerted efforts to improve the sustainable access to hydroxyurea among all patients with SCA.
Keywords: sickle cell anaemia, hydroxyurea, access, outcomes, North-western Tanzania
Introduction
Sickle cell anaemia (SCA) is the most common genetic disorder of the red blood cells (RBCs) characterized by a single base mutation on the 6th codon of the β globin gene, resulting in substitution of amino acid valine for glutamic acid.1 This change causes the RBCs to change to a sickle shape under hypoxic conditions due to polymerization of the haemoglobin molecules,1,2 which is the cause for the multitude of complications in SCA such as cerebro-vascular accidents, vaso-occlusive crisis (VOC), acute chest syndrome, spleen sequestration and end organ damage.2
Worldwide, Tanzania ranks fourth among countries with the highest annual births of individuals with SCA.3 The high disease burden is mostly seen in regions along the Lake Victoria zone of the country.3–6 Subsequently, an overwhelming number of patients with a myriad of SCA-related complications attend at the Bugando Medical Centre (BMC), the zonal tertiary referral hospital.7
Hydroxyurea is the first disease modifying agent to be approved for use in SCA.8 It does so by inducing foetal haemoglobin (HbF) production, decreasing intracellular sickle haemoglobin (HbS) polymerization within RBCs, decreasing RBCs adhesion to endothelium,9 and increasing release of the potent vasodilator nitric oxide.9 The use of hydroxyurea has been shown to reduce the frequency of pain crisis, acute chest syndrome and blood transfusions in patients with SCA.9 The overall safety and benefits of hydroxyurea have been demonstrated in diverse SCA populations, including children with SCA in sub-Saharan Africa (SSA).10–12 These benefits however depend of sustained exposure to the medication.13 The use of hydroxyurea in SCA is recommended from as early as 9 months of age and should generally be continued throughout life.14
While the use of hydroxyurea is common in the developed countries, most patients with SCA in SSA are not able to access the medication. In this setting, several challenges have been reported spanning from the introduction of hydroxyurea to utilisation by the patients. These challenges include bottlenecks in registration of hydroxyurea as a drug for patients with SCA, shortage of healthcare workers, high costs of the medication and misconceptions regarding use of hydroxyurea for SCA among patients, caregivers and prescribers.15–17 In order to improve the utilisation of hydroxyurea among patients with SCA in SSA, it is imperative to ensure that the drug is available and affordable to all in need and that healthcare workers caring for patients with SCA have proper training on prescription of hydroxyurea.15,16
Along the shores of Lake Victoria, hydroxyurea is obtained either through self-purchase out-of-pocket, health insurance services, different donations by private/non-governmental organizations or through research projects. This applies to most other parts of the country as well since there is currently no universal health insurance policy. All these means of access pose different challenges with regard to eligibility and therefore number of patients that can be accommodated, age at initiation of access to hydroxyurea, and continuity of access to the drug. Since the goal is to ensure as many patients as possible are initiated on hydroxyurea early on and continue to take the medication for life with minimal interruptions, it is imperative to evaluate the clinical effects and challenges associated with the use of hydroxyurea accessed via the different means.
The present study therefore evaluated the clinical and haematological outcomes and barriers for access to hydroxyurea via cash, insurance and projects among paediatric SCA patients attended at the BMC in Mwanza, Tanzania. Insights from this study will inform policy on the best way or combination of ways towards achieving universal and optimal access to hydroxyurea among patients with SCA as the standard of care in Tanzania and other countries in the developing world.
Materials and Methods
Study Area
This study was conducted at the department of paediatrics and child health of the BMC in Tanzania. BMC is a 900-bed consultant and teaching hospital located along the shores of Lake Victoria in Mwanza region, and serves as the tertiary referral hospital for the Lake Victoria and Western zones of the United Republic of Tanzania. It has a catchment area of eight regions including Mwanza, Geita, Kigoma, Kagera, Mara, Shinyanga, Simiyu and Tabora, serving an estimated population of 16 million people. The department of peadiatrics and child health has a capacity of 128 beds. The department is subdivided into inpatient and outpatient sections. From January 2020 to June 2020, a total of 300 children were admitted with SCA-related complications. Paediatric SCA clinic at BMC serves 80 patients per week aged 6 months to 12 years. Majority of children with SCA seen at the clinic are diagnosed with sickling test or sickle SCAN point-of-care test (Biomedomics Inc., USA) and confirmed by haemoglobin electrophoresis (HbE), Isoelectric focusing (IEF) or high performance liquid chromatography (HPLC). Routine care for patients with SCA include folic acid and Pen V (available to all patients) and also hydroxyurea if available through projects or if the parents can afford to subscribe to health insurance or purchase the drug out-of-pocket. During the study period, approximately 80% of all patients with SCA seen at BMC were children, and only about 26% of them were on hydroxyurea.
Study Design
The study was conducted between October 2020 and April 2021. The first part of the study involved retrospective evaluation of the medical files of paediatric patients with SCA who attended at the hospital between June 2019 and October 2020 to ascertain who were on hydroxyurea, duration on the drug, and outcomes/parameters extracted from the patients’ medical files prior initiation of hydroxyurea. After written informed consent was obtained from the parents or guardians, and assent from children where appropriate, patients who were on hydroxyurea for a least one year were enrolled into a cross-sectional survey to ascertain the clinical outcomes after hydroxyurea initiation, means of access (16 patients-cash, 43 patients-insurance or 28 patients- projects) and barriers to access of hydroxyurea. Demographic and clinical information as well as information on hydroxyurea access was collected using a standardized questionnaire. A venous blood sample was then collected for assessment of the complete blood count (CBC), reticulocyte count and HbF at the time of enrolment.
Ethical Issues
Ethical clearance for the research proposal was sought from the joint Bugando Medical Centre and Catholic University of Health and Allied Sciences (BMC/CUHAS) ethics and review committee with a certificate number CREC/437/2020. Furthermore, written informed consent was obtained from the parents of children with SCA who participated in the study after they clearly understood the objectives of the study which was presented using the information sheet and consent form. Assent was sought for children above the age of 10 years. The informed consent forms were then stored safely together with the data collection forms in a safe location. All activities performed in this study comply with the Declaration of Helsinki.
Laboratory Methods
Two millilitres (mls) of peripheral venous blood were collected in ethylene-diamine-tetra-acetic acid (EDTA) tubes from all children on hydroxyurea for at least one year at the time of enrolment into the cross-sectional survey. A complete blood count with reticulocyte count was performed using the Cell-Dyn 3700 Haematology Analyzer (Abbott Diagnostics, Lake Bluff, IL, USA). The identification of HbA, HbS, HbF and other haemoglobin variants was done using HbE (Biotec Fisher Filipo) or IEF (GE Healthcare Bio Sciences AB, Bjorktan 30,751 84 Uppsala Sweden). The identification and quantification of HbA, HbS, HbF and other variants was done using high-performance liquid chromatography (HPLC) using the Variant I Haemoglobin Testing System (Bio-Rad, Laboratories, Inc., Hercules, CA, USA). Patients with HbS and no detectable HbA were considered to have SCA. Patients with HbS and low levels of detectable HbA on HPLC or light HbA band on HbE/IEF were considered to have sickle beta plus thalassemia (HbSβ+ thal) and were excluded from the study. All tests were performed under the experimental conditions specified by the manufacturers.
Data Analysis
Data was entered into Microsoft excel database and analyzed by using STATA version 16 (Stata Corp, College Station, Texas, USA). Descriptive statistics were summarized in frequencies and proportions (percent) (for categorical variables), means with standard deviations or medians and interquartile ranges (for continuous variables, depending on distribution), and were presented in tables and figures. The prevalence of clinical outcomes (VOC, admissions, blood transfusion) were compared before and after hydroxyurea initiation using two-sample test of proportions. Haematological outcomes (Hb, MCV and ANC) before and after hydroxyurea initiation were compared using Wilcoxon matched-pairs signed-ranks test for continuous variables. P-values of <0.05 were considered statistically significant.
Results
Enrolment
From October 2020 to April 2021, a total of 132 children with SCA were screened for eligibility. Of these, 87 children were on hydroxyurea for more than one year. Past medical history and medical files were reviewed. The median age of patients prior to hydroxyurea initiation was 99 [78–151] months and the median duration on hydroxyurea was 22.6 [16.6–37.2] months at study enrolment. From the 87 participants, 16/87 (18.4%) were accessing hydroxyurea through cash, 43/87 (49.4%) through health insurance coverage, and 28/87 (32.2%) through projects (Figure 1).
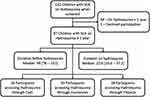 |
Figure 1 Enrolment flowchart of sickle cell anaemia participant on Hydroxyurea from October 2020 to April 2021. |
Baseline History and Clinical Characteristics of 87 Children with SCA on Hydroxyurea for at Least One Year
The median age at enrolment in the cross-sectional survey for the 87 children with SCA patients prior to hydroxyurea initiation was 99 [78–151] months and the median duration on hydroxyurea was 22.6 [16.6–37.2] months at study enrolment, 52/87 (59.8%) were male. Majority of the children were from Mwanza region 66/87 (75.6%). Almost all children reported different complications encountered before initiation on hydroxyurea. Majority of participants had reported to have had malaria 76/87 (87.4%) and 55/87 (63.2%) had acute severe pulmonary event (acute chest syndrome or pneumonia). Stroke was reported by 22/87 (25.3%) participants (Table 1).
 |
Table 1 Baseline Characteristics of Sickle Cell Anaemia (SCA) Participants |
Clinical and Haematological Outcomes of Hydroxyurea Usage by Means of Access
With regard to hydroxyurea access means (insurance, cash or projects), the median duration on hydroxyurea was 26.7 [18.9–38.7] months, 22.9 [16.5–42.2] months and 19.7 [14.6–25.4] months for insurance coverage, cash and projects, respectively.
Following hydroxyurea utilization, there was improvement of children’s clinical outcomes with significant reduction in the proportions of patients reporting vaso occlusive crisis, P=<0.0001 in insurance group, hospital admissions (P = 0.002, <0.0001 and 0.01, in cash, insurance and Project) and blood transfusions (P = 0.002, <0.0001 and <0.0001, in cash, insurance and Project) (Figure 2 and Table 2). Furthermore, the use of hydroxyurea was associated with significant improvement of haemoglobin (Hb) and mean corpuscular volume (MCV), as well as reduction of absolute neutrophil count (ANC) and reticulocytes among patients in the insurance and projects groups. The median Hb and MCV increased by 1.1g/dl and 7.3 um3, respectively, among patients in the insurance group, and by 1.2g/dl and 7.4 um3, respectively, among patients in the projects group, which was statistically significant (Table 2). Conversely, the mean ANC and percentage reticulocytes decreased by 0.8 × 109/L and 5.7%, respectively, among patients in the insurance group, and by 1.1 × 109/L and 6.3%, respectively, among patients in the projects group (Table 2). The changes in these parameters however did not reach statistical significance for patients who accessed hydroxyurea through cash (Table 2). The trends in Hb, MCV, ANC and reticulocytes by means of hydroxyurea access are depicted in Figure 3.
 |
Table 2 Haematological Outcomes of 87 Children with Sickle Cell Anaemia on Hydroxyurea |
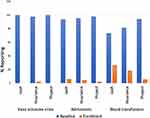 |
Figure 2 Reported clinical presentations at baseline and one year of Hydroxyurea. |
 |
Figure 3 Showing the trend of haematological parameters ((A) Haemoglobin, (B) MCV, (C) ANC and (D) reticulocytes) to participants at baseline (before Hydroxyurea) to the time of Enrolment. |
Barriers to Hydroxyurea Access
Participants faced different barriers in accessing Hydroxyurea. These barriers were exclusively reported by patients in the cash group (14% of patients) and insurance group (10% of patients; Figure 4). The reported barriers included drug unavailability in pharmacies, long distance to travel to get hydroxyurea due to unavailability in districts hospitals and also high cost of hydroxyurea that made it hard for patients in the cash group to adhere to everyday dose. For patients in the insurance group, inadequate coverage of hydroxyurea in the insurance plans and unavailability of hydroxyurea in some hospitals were the main challenges reported (Figure 4).
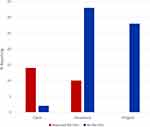 |
Figure 4 Barriers to Hydroxyurea access to 87 sickle cell anaemia participants on Hydroxyurea from October 2020 to April 2021. |
Discussion
Understanding the barriers for access to hydroxyurea once initiated is critical in ensuring sustainability of its clinical benefits. Here, we show that patients who accessed hydroxyurea via cash experienced the most challenges in getting the drug and subsequently the least haematological benefits from the drug. To the best of our knowledge, our study is one of the first to investigate the clinical and haematological benefits of hydroxyurea across various access means (cash, insurance, projects), and contributes to emerging literature on this subject. The major strength of our study is the use of two-way review (pre-hydroxyurea and post-hydroxyurea) following more than six months of use of hydroxyurea to determine its clinical and haematologic benefits in our participants.
Clinical Outcome of Hydroxyurea Usage
Our study reported significant improvement of clinical outcomes in paediatric patients with SCA on hydroxyurea, including reduction of admissions and reduction on vaso-occlusive events, which is similar to what has been reported in other studies.9–12 Importantly, these benefits have also been observed among children with SCA in sub-Saharan Africa. The REACH trial in sub-Saharan Africa showed clearly that the use of hydroxyurea in children had significantly reduced SCA-associated complications, blood transfusion needs and hospital admissions.11 Similarly, the NORHAM study in Uganda showed decrement of vaso-occlusive crisis in children with SCA.12 Given the mounting evidence of the clinical benefits of hydroxyurea in sub-Saharan Africa, it is high time to invest in scaling up its availability in the region with the aim to reach most if not all patients with SCA. Such roll-out will have the added advantage of facilitating cohort studies to investigate the long-term effects of hydroxyurea use in the sub-Saharan Africa settings.
Haematological Outcome of Hydroxyurea Usage by Means of Access
Dramatic improvement of laboratory values was observed in this study with increment of haemoglobin and MCV, as well as decrement of ANC and reticulocytes. Similar improvements in laboratory parameters have also been reported among children with SCA in Africa,18 including recently by the REACH study.11 Nahavandi et al and Cokic et al showed that the beneficial effects of hydroxyurea in patient with SCA were mediated via induction of NO, cGMP, and HbF.19,20 Besides the increase in Hb and HbF, the changes in haematological parameters induced by hydroxyurea have various additional benefits to the patients. The reduction in reticulocyte count is thought to improve overall erythrocytes deformability and hence significantly reduce blood viscosity.21 Further, the reduction in chronic inflammation medicated by hydroxyurea also presents a major therapeutic benefit.22
Between the three groups, we observed that those who used cash to access hydroxyurea did not experience significant changes in all the haematological parameters studied. The same group also reported inconsistent use of the drug due to financial constraints. The sub-optimal dosing of hydroxyurea that ensued likely explains the lack of haematological benefits in this group and brings to attention issues related to long-term sustainability of access to hydroxyurea, especially among the majority of patients with SCD who live in resource limited settings in sub-Saharan Africa.
Barriers to Hydroxyurea Access
Our study pointed some barriers of hydroxyurea access and the most commonly pointed out were high drug cost, insurance challenges and unavailability in most parts in the Lake Zone regions of the country where SCA is mostly prevalent. We found that majority of the participants had to travel long distances to obtain hydroxyurea at a high cost. These challenges have also been reported by other groups.23–25
In order to reduce the cost of hydroxyurea and thus increase its affordability and accessibility to patients with SCA, local production in countries that are heavily burdened with SCA may be the solution. In Africa, Nigeria has succeeded in this by having a pharmaceutical company that is producing hydroxyurea locally which has significantly reduced its costs to patients.26,27 In Tanzania, a proof-of-concept project at the Muhimbili University of Health and Allied Sciences (MUHAS) showed that local compounding of hydroxyurea was feasible and would reduce the cost of the drug significantly.28 Such initiatives should receive full support from the industry for scale up.
Limitation
As our study was conducted during the peak of COVID-19 outbreak, we were only able to recruit a relatively small number of patients that limited our ability to examine some important factors with high resolution, particularly the barriers to hydroxyurea access across the various access means.
Conclusion
Hydroxyurea significantly increased the haemoglobin and MCV, and reduced ANC and reticulocytes in paediatric patients with SCA in Tanzania. These benefits were observed in SCA patients accessing hydroxyurea via insurance and projects, but not cash. Further, patients accessing hydroxyurea via cash reported the most challenges in accessing the drug, likely contributing to inconsistent dosing and impaired haematological benefits. While these findings support the roll-out of hydroxyurea to increased number of patients with SCA in sub-Saharan Africa, they also highlight the need to ensure its long-term affordability among patients. Future longitudinal studies should investigate the clinical and haematological outcomes as well as barriers to access of hydroxyurea across the various access means among large sample sizes of patients in North-western and Eastern parts of the country where the magnitude of SCA is highest.
Acknowledgments
We thank the patients and staff of Bugando Medical Centre. We also acknowledge support from the National Heart, Lung, and Blood Institute of the National Institutes of Health under Award Number U24 HL135881 (Sickle Pan-African Research Consortium—SPARCO) and U01 HL156853 (SPARCO-Tanzania) that made this study possible. The content of this paper is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Disclosure
The authors declare no competing interests in this work.
References
1. Ilesanmi OO. Pathological basis of symptoms and crises in sickle cell disorder: implications for counseling and psychotherapy. Hematol Rev. 2010;2:10–22.
2. Prithu Sundd MT, Gladwin EMN. Pathophysiology of Sickle Cell Disease. Physiol Behav. 2018;176:139–148.
3. Therrell BLLloyd-Puryear MA, Ohene-Frempong K, et al. Empowering newborn screening programs in African countries through establishment of an international collaborative effort. J Community Genet. 2019;1:18.
4. Ambrose EE, Makani J, Chami N, et al. High birth prevalence of sickle cell disease in Northwestern Tanzania. Pediatr Blood Cancer. 2018;65:e26735. doi:10.1002/pbc.26735
5. Ambrose EE, Smart LR, Charles M, et al. Geospatial mapping of sickle cell disease in northwest Tanzania: the Tanzania sickle surveillance study (TS3). Blood. 2018;132:3662. doi:10.1182/blood-2018-99-113939
6. Rwezaula S, Magesa PM, Mgaya J, et al. Newborn screening for hemoglobinopathies at Muhimbili National Hospital, Dar es Salaam–Tanzania. East Afr J Public Health. 2015;12:948–955.
7. Saidi H, Smart LR, Kamugisha E, et al. Complications of sickle cell anaemia in children in Northwestern Tanzania. Hematology. 2016;21(4):248–256. doi:10.1080/10245332.2015.1101976
8. Wong TE, Brandow AM, Lim W, Lottenberg R. Update on the use of hydroxyurea therapy in sickle cell disease. Blood. 2014;124(26):3850–3857. doi:10.1182/blood-2014-08-435768
9. Charache S, Terrin ML, Moore RD, et al. Effect of hydroxyurea on the frequency of painful crises in sickle cell anemia. N Engl J Med. 1995;332(20):1317–1322. doi:10.1056/NEJM199505183322001
10. Cunningham-Myrie C, Abdulkadri A, Waugh A, et al. Hydroxyurea use in prevention of stroke recurrence in children with sickle cell disease in a developing country: a cost effectiveness analysis. Pediatr Blood Cancer. 2015;62(10):1862–1864. doi:10.1002/pbc.25563
11. Tshilolo L, Tomlinson G, Williams TN, et al. Hydroxyurea for children with sickle cell anemia in Sub-Saharan Africa. N Engl J Med. 2019;380(2):121–131. doi:10.1056/NEJMoa1813598
12. Opoka RO, Ndugwa CM, Latham TS, et al. Novel use Of Hydroxyurea in an African Region with Malaria (NOHARM): a trial for children with sickle cell anemia. Blood. 2017. doi:10.1182/blood-2017-06-788935
13. Candrilli SD, O’Brien SH, Ware RE, et al. Hydroxyurea adherence and associated outcomes among Medicaid enrollees with sickle cell disease. Am J Hematol. 2011;86(3):273–277. doi:10.1002/ajh.21968
14. Qureshi A, Kaya B, Pancham S, et al. Guidelines for the use of hydroxycarbamide in children and adults with sickle cell disease: a British Society for Haematology Guideline. Br J Haematol. 2018;181:460–475. doi:10.1111/bjh.15235
15. Ofakunrin AOD, S Okpe E, O Afolaranmi T, et al. Level of utilization and provider-related barriers to the use of hydroxyurea in the treatment of sickle cell disease patients in jos, north-central Nigeria. Afr Health Sci. 2021;21:765–774. doi:10.4314/ahs.v21i2.36
16. Power-Hays A, Ware RE. Effective use of hydroxyurea for sickle cell anemia in low-resource countries. Curr Opin Hematol. 2020;27(3):172–180. doi:10.1097/moh.0000000000000582
17. Kilonzi M, Mlyuka HJ, Felician FF, et al. Barriers and Facilitators of Use of Hydroxyurea among Children with Sickle Cell Disease: experiences of Stakeholders in Tanzania. Hemato. 2021;2(4):713–726. doi:10.3390/hemato2040048
18. Ware RE. Optimizing hydroxyurea therapy for sickle cell anemia. Hematol Am Soc Hematol Educ Progr. 2015;2015:436–443. doi:10.1182/asheducation.V2015.1.436.3917688
19. Nahavandi M, Tavakkoli F, Wyche MQ, et al. Nitric oxide and cyclic GMP levels in sickle cell patients receiving hydroxyurea. Br J Haematol. 2002;119(3):855–857. doi:10.1046/j.1365-2141.2002.03919.x
20. Cokic VP, Andric SA, Stojilkovic SS, Noguchi CT, Schechter AN. Hydroxyurea nitrosylates and activates soluble guanylyl cyclase in human erythroid cells. Blood. 2008;111:1117. doi:10.1182/blood-2007-05-088732
21. Davies SC, Gilmore A. The role of hydroxyurea in the management of sickle cell disease. Blood Rev. 2003;17:99–109. doi:10.1016/S0268-960X(02)00074-7
22. Conran N, Belcher JD. Inflammation in sickle cell disease. Clin Hemorheol Microcirculat. 2018. doi:10.3233/CH-189012
23. Badawy SM, Thompson AA, Penedo FJ, et al. Barriers to hydroxyurea adherence and health-related quality of life in adolescents and young adults with sickle cell disease. Eur J Haematol. 2017;98:608–614. doi:10.1111/ejh.12878
24. Adeyemo TA, Diaku-Akinwunmi IN, Ojewunmi OO, Bolarinwa AB, Adekile AD. Barriers to the use of hydroxyurea in the management of sickle cell disease in Nigeria. Hemoglobin. 2019;43:188–192. doi:10.1080/03630269.2019.1649278
25. Okocha EC, Gyamfi J, Ryan N, et al. Barriers to therapeutic use of hydroxyurea for sickle cell disease in Nigeria: a cross-sectional survey. Front Genet. 2022;12:2834. doi:10.3389/fgene.2021.765958
26. Abdullahi SU, Jibir BW, Bello-Manga H, et al. Hydroxyurea for primary stroke prevention in children with sickle cell anaemia in Nigeria (SPRING): a double-blind, multicentre, randomised, Phase 3 trial. Lancet Haematol. 2022;9:e26–e37. doi:10.1016/S2352-3026(21)00368-9
27. Galadanci NA, Abdullahi SU, Ali Abubakar S, et al. Moderate fixed-dose hydroxyurea for primary prevention of strokes in Nigerian children with sickle cell disease: final results of the SPIN trial. Am J Hematol. 2020;95:E247. doi:10.1002/ajh.25900
28. Costa E, Tibalinda P, Sterzi E, et al. Making hydroxyurea affordable for sickle cell disease in Tanzania is essential (HASTE): how to meet major health needs at a reasonable cost. Am J Hematol. 2021;96:E2–E5. doi:10.1002/ajh.26007
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
