Back to Journals » Cancer Management and Research » Volume 15
Outcomes of Antineoplastic Immunotherapy at a Large Healthcare Organization: Impact of Provider, Race and Socioeconomic Status
Authors Mirsky MM , Mitchell C, Hong A, Cao S, Fu P, Margevicius S, Wu S, Dowlati A, Nelson A, Selfridge JE, Ramaiya N, Hoimes C , Alahmadi A, Bruno DS
Received 17 January 2023
Accepted for publication 20 April 2023
Published 1 September 2023 Volume 2023:15 Pages 913—927
DOI https://doi.org/10.2147/CMAR.S403569
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Ahmet Emre Eşkazan
Matthew M Mirsky,1 Carley Mitchell,1 Augustine Hong,1 Shufen Cao,2 Pingfu Fu,2 Seunghee Margevicius,2 Sulin Wu,1 Afshin Dowlati,1 Ariel Nelson,1 J Eva Selfridge,1 Nikhil Ramaiya,1 Christopher Hoimes,1 Asrar Alahmadi,1,* Debora S Bruno1,*
1University Hospitals Cleveland Medical Center, Case Western Reserve University School of Medicine, Cleveland, OH, USA; 2Department of Population and Quantitative Health Sciences, Case Western Reserve University School of Medicine, Cleveland, OH, USA
*These authors contributed equally to this work
Correspondence: Debora S Bruno, University Hospitals Cleveland Medical Center, Case Western Reserve University School of Medicine, 11100 Euclid Avenue, Cleveland, OH, 44106, USA, Tel +1 216 844-3951, Email [email protected]
Purpose: Disparities in cancer care delivery remain a pressing health-care crisis within the United States (US). The use of immune checkpoint inhibitors (ICIs) and their management may be a disparity generator that impacts survival. This retrospective study assessed disparities in a cohort of patients with a variety of solid tumors treated with ICIs within a single health-care organization focusing on the impact of race, socioeconomic status (SES) and site of care delivery on survival and the development of severe immune-related adverse events (irAEs).
Patients and Methods: Manual chart review was performed on all patients with solid tumors treated with ICIs within a health-care organization from 2012 to 2018. Care delivery was dichotomized as DOP (disease-oriented provider at academic center) and COP (community oncology provider). Primary and secondary outcomes were overall survival (OS) and rates of grade 3– 4 irAEs, respectively. Relationships with covariates of interest, including race, socioeconomic status and type of care delivery, were assessed among both outcomes.
Results: A total of 1070 eligible patients were identified. Of those, 11.4% were of Black race, 59.7% had either non-small cell lung cancer (NSCLC) or melanoma and 82.8% had stage IV disease. Patients of Black race and lower SES were more likely to be treated by DOPs (p< 0.0001). A superior OS was associated with care delivered by DOPs when compared to COPs (HR 0.68; 95% CI 0.56– 0.84; p=0.0002), which was durable after accounting for race, SES, histopathologic diagnosis and disease stage. Melanoma patients experienced higher rates of severe irAEs (HR 2.37; 95% CI 1.42– 3.97; p=0.001). Race, SES and site of care delivery were not related to rates of severe irAEs.
Conclusion: In a large health-care organization, patients treated with checkpoint inhibitors by DOPs benefited from a significant OS advantage that was durable after controlling for racial and socioeconomic factors, providing evidence that disease-oriented care has the potential to mitigate racial and socioeconomic disparities.
Keywords: disparities, healthcare delivery, immunotherapy outcomes
Introduction
Since the United States (US) Food and Drug Administration’s (FDA) approval of ipilimumab in 2011, the use of immune checkpoint inhibitors (ICI) has revolutionized the treatment paradigm of most advanced solid tumors.1,2 With 21 indications and 10 available agents on the market, medical oncologists are able to extend the survival of patients with solid tumors beyond any historical perspective.3,4 Immune-related adverse events (irAEs) occur in 15–90% of patients treated with ICIs, which may affect any organ system with variable timing from ICI initiation.5 Medical oncologists must become experts at prompt identification, severity grading and treatment of irAEs.6 They should also be comfortable initiating, managing and treating related side effects of immunosuppressive therapies. All things considered, ICIs have made a remarkable impact on cancer treatment, and further insight into how to best leverage their benefits will remain of value. As such, initiatives addressing cancer-related health disparities and optimizing cancer-care delivery models are imperative.7,8
While innovation and novel treatment approaches drive the field of oncology forward, they also create the potential to exacerbate already existing gaps in health outcomes within the US. It is well established that socioeconomic and racial disparities persist throughout various aspects of cancer-care.9–13 Continued system-level interventions and research efforts are warranted to help address these gaps. Additionally, the effect of increasing cancer-care complexity on varying care models, and their relationship to patient outcomes, is another important area of consideration. While most oncology patients are treated within community settings, previous data reported superior patient outcomes when surgical oncology care was received via high-volume provider practices in academic settings compared to their community affiliates.14,15 Similarly, radiotherapy delivered in high-volume facilities may also lead to superior survival in multiple cancers.16,17 However, these associations are less frequently examined at the medical oncology level. Moreover, the impact that high-volume, disease-oriented providers (DOPs) have on mitigating cancer health disparities is a novel and unanswered question.
We performed a retrospective cohort study of patients diagnosed with a broad range of solid tumor malignancies treated with ICIs at a large health-care organization over the span of 7 years. We focused on the impact of medical care provided by DOPs versus community-oriented providers (COPs) on survival outcomes. Additionally, these outcomes were explored on the basis of socioeconomic status and race.
Materials and Methods
Study Design
This study took place at a 3108-bed health system consisting of 11 community-based hospitals, plus the 1032 bed main academic campus location. All affiliated sites are integrated under a single electronic medical record (EMR). Care providers were designated as a DOP if they specialized in a specific disease type (ie thoracic oncology, urologic oncology, cutaneous malignancies, etc.) and treated patients at the main campus location. Whereas a COP practiced general oncology, seeing multiple different cancer types, and treated patients at any of the 19 community clinic locations.
Following Institutional Review Board approval, all patients ages ≥18 years, with solid tumors and recipients of antineoplastic immunotherapy between the years 2012–2018 were identified by medical record number (MRN) for purposes of this study. Manual chart review was performed on all 1070 eligible MRNs with data entry into institutional REDCap v10.9.1 for purposes of deidentification and further data analysis.18
Demographic data was gathered including age, gender, marital status, employment status, and self-reported race, if listed. Residential zip codes were also collected and categorized into one of three groups based on the percentage of individuals living below the poverty line (≤5%, 6% to 10%, and ≥11%). This attribution was performed utilizing the Northeast Ohio Community and Neighborhood Data for Organizing (NEO CANDO) website developed and validated by Case Western Reserve University.19 Payer information was also abstracted from EMR.
Clinical data was gathered including histopathologic diagnosis, disease stage, ICI agent, date of first treatment administration and irAE development. When available, Eastern Cooperative Oncology Group (ECOG) performance status at the time of CIT initiation was included and graded from 0 to 5, with 0 indicating a fully active patient, able to carry out all pre-disease activities without restrictions and 5 indicating death. Charlson Comorbidity Index (CCI) was calculated based on EMR review. Patients were deemed to have severe, grade 3 or 4 irAEs if they required hospitalization for management of their condition.
The primary outcome was overall survival (OS) from date of immunotherapy initiation to the date of death or date of last follow-up. This was analyzed in relation to covariates of interest. Secondary outcomes included cumulative incidence rates of grade 3 or 4 irAEs in relation to covariates of interest.
Statistical Methodology
Overall survival (OS) was measured from the date of immunotherapy initiation to the date of death and was censored at the date of last follow-up for survivors. Survivor distribution was estimated using Kaplan–Meier methods, and the difference in OS between and among groups was examined by Log rank test.20 Primary independent variables included provider type, race, histopathologic diagnosis, disease stage and development of grade 3 or 4 irAEs. The effects of variables of interest on OS were evaluated using a multivariable Cox model after adjusting for confounding factors.21 The cumulative incidence of irAE development was estimated with death as the competing risk. The Fine and Gray method was used for comparisons of cumulative incidence between groups.22 The effect of important factors on time to irAE was further evaluated using multivariable Fine and Gray method. All tests are two-sided and p-values ≤0.05 were considered statistically significant.
Results
Baseline Demographic and Clinical Data
Between January 1, 2012, and December 31, 2018, a total of 1070 patients with solid tumors were treated with ICIs throughout the health system. The mean patient age was 64.34 years with a greater proportion of male patients compared to female patients (57.1% v 42.9%). The racial distribution of interest included 783 (73.2%) White individuals and 122 (11.4%) Black individuals. Most identified Medicare as their primary health insurance, followed by private insurance plans and Medicaid (49.95% v 40.09% v 9.95%, respectively). Similarly, most patients were retired followed by actively employed and unemployed (47.71% v 36.9% v 15.38%, respectively). Married patients accounted for 62.97% of the cohort, the rest identifying as single, divorced or widowed. Finally, neighborhood poverty level was assessed, and a majority of the cohort (44.74%) resided in areas where ≤5% of the population fell below the federal poverty level, whereas 22.72% of patients resided in areas were ≥11% of the population fell below the federal poverty level (Table 1A and B).
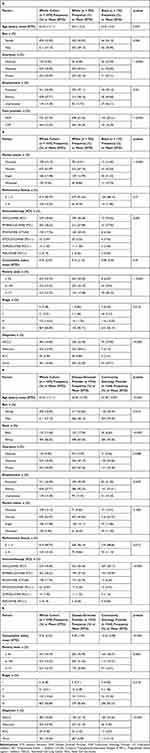 |
Table 1 Patient Characteristics by Race (A) and Care Provider (B) |
Non-small cell lung cancer (NSCLC) was the most common individual diagnosis followed by melanoma (35.83% v 23.95%, respectively), and 82.89% of patients had stage IV disease at the time of immunotherapy initiation. Patients were treated most frequently with nivolumab followed by pembrolizumab and ipilimumab (49.44% v 28.22% v 17.76%, respectively). DOPs cared for 67.76% of patients while COPs cared for 32.24% of patients. Overall, 89.75% of patients had a favorable ECOG performance status of 0–2 with a mean CCI of 9.76 for the entire cohort (Table 1A and B).
When comparing the two racial groups (Table 1A) of interest, Black patients were more likely than White patients to be female (54.1% vs 40.87%, p = 0.006), single (41.32% v 13.99%, p < 0.0001), unemployed (26.21% v 12.73%, p = 0.001), utilize Medicaid as their primary health insurance (24.59% v 6.99%, p < 0.0001) and live in neighborhoods where ≥11% of the population fell below the federal poverty level (58.33% v 17.08%, p < 0.0001). Additionally, Black patients in his cohort were more frequently diagnosed with NSCLC (57.85% v 32.95%, p < 0.0001), whereas White patients were more frequently diagnosed with melanoma (28.61% v 4.13%, p < 0.0001). Treatment differed slightly with Black patients more likely to receive nivolumab (59.02% v 48.28%, p = 0.006) and White patients more likely to receive ipilimumab (20.43% v 6.56%, p = 0.006). Finally, Black patients received primary oncology care from DOPs significantly more often than their White counterparts (83.61% v 63.76%, p < 0.0001).
When comparing care models utilizing DOPs versus COPs (Table 1B), DOPs were significantly more likely to care for patients of younger mean age (62.95 v 67.28, p < 0.0001) and Black race (17% v 6.6%, p < 0.0001). Additionally, they tended to see a population that was more frequently employed (39.4% v 31.06%, p = 0.0472), more likely to have either private insurance (42.42% v 35.38%, p = 0.0388) or Medicaid (39.4% v 31.06%, p = 0.0388) and more often resided in areas where the poverty level was ≥11% of the population (25.8% v 16.91%, p = 0.0067) compared to their COP counterparts. From a clinical standpoint, DOPs treated significantly more melanoma patients (31.44% v 7.56%, p < 0.0001) and utilized ipilimumab to a greater extent (23.79% v 4.36%, p < 0.0001), whereas COPs treated significantly more NSCLC patients (54.36% vs 27.15%, p < 0.0001) and utilized nivolumab to a greater extent (60.17% vs 44.54%, p < 0.0001). While both subgroups had similar ECOG performance status scores, the CCI was significantly higher among patients treated by COPs (10.32 v 9.49, p < 0.0001).
Overall Survival Analysis
The median OS from time of immunotherapy initiation for the entire cohort was 13.1 months (95% Confidence Interval (CI) 10.9–14.8) with 24- and 48-month survival probabilities of 35.33% and 27.51%, respectively. A survival advantage was significantly associated with care delivered by DOPs compared to COPs in both univariate analysis (Hazard Ratio (HR) of death 0.65; 95% CI, 0.55–0.76; p < 0.0001) and multivariate regression, after controlling for the effects of age, performance status, histopathologic diagnosis and disease stage (HR 0.68; 95% CI, 0.56–0.84; p = 0.0002). The median OS among the DOP cohort was 15.8 months (CI 13.7–19.2) compared to 8.7 months (CI 6.4–10.3) for the non-DOP cohort, p < 0.001 (Figure 1). Other factors significantly associated with improved OS on both univariate analysis and multivariate regression included a melanoma diagnosis (multivariate HR 0.44; 95% CI, 0.33–0.58; p < 0.0001), favorable performance status (ECOG 0–2) (multivariate HR 0.46; 95% CI, 0.35–0.59; p < 0.0001) and non-metastatic disease (Stages I and II vs stage IV) (multivariate HR 0.48; 95% CI 0.25–0.94; p = 0.0329) and stage III vs stage IV (multivariate HR 0.57; 95% CI, 0.43–0.76; p = 0.0001) (Table 2).
 |
Table 2 Cox Regression on Overall Survival |
Immune Related Adverse Events
The cumulative incidence of experiencing a severe, grade 3 or 4 irAE at 48- and 96-weeks following immunotherapy initiation was 8.0% and 9.3%, respectively. A melanoma diagnosis was significantly associated with severe irAEs in both univariate analysis and multivariate regression, after controlling for the effects of age, gender, CCI, care provider, disease stage and histopathologic diagnosis (multivariate HR 2.37; 95% CI 1.42–3.97; p = 0.001). While DOPs were associated with a significant increase in severe irAEs on univariate analysis (HR 1.88; 95% CI, 1.12–3.15; p = 0.017), this was not sustained on multivariable regression (HR 1.53; 95% CI, 0.88–2.65; p = 0.13) (Table 3).
 |
Table 3 The Results from Cox Regression with Death as Competing Risk (Fine and Gray Method) on Time to Adverse Event (TTAE) |
Discussion
This retrospective study, conducted within a large single health-care organization, provides important insight into outcomes when utilizing CIT in a real-world setting. Most notably, results indicate a significant differential survival advantage of 7.1 months when patients receive care from DOPs compared to COPs (HR of death 0.65; 95% CI, 0.55–0.76; p < 0.0001). This effect was sustained after a multivariable regression controlling for covariates of interest including age, performance status, comorbidity index, histopathologic diagnosis and disease stage (HR of death 0.68; 95% CI, 0.56–0.84; p < 0.0001). Despite a paucity of related research in the solid tumor medical oncology setting, these results support previous outcomes identifying superior OS among patients receiving care from high-volume, disease specific, medical oncology providers in the setting of pancreatic and esophago-gastric malignancies.23,24 To our knowledge, this is the first description of superior outcomes of patients treated with checkpoint inhibitors by DOPs. Continued research will be necessary to further corroborate these findings.
Like most institutions, DOPs within this study practice at a large, academic referral center, as opposed to smaller community-based locations. Furthermore, DOPs are identified as experts treating their respective disease niches and care for these patients in a high-volume setting. Whereas non-DOPs frequently practice at community-based sites, and while equipped to adequately treat a range of cancer diagnoses, they inevitably see a lower volume of each individual disease entity. This volume–outcome relationship, and its associated factors, likely account for these results. Contributing factors may be recognized at both the provider and institutional level, even when integrated into one large health system. At the provider level, it is speculated that variations may include overall experience and confidence when treating a specific diagnosis or utilizing specific treatment regimens. At the institutional level, there may be discrepancies in early access to diagnostic technology or therapeutic approaches along with differing levels of multidisciplinary engagement when comparing academic and community-based sites. In the age of genomic sequencing, targeted therapy/precision medicine and immunotherapy, treatment of each individual cancer diagnosis is becoming highly nuanced.25 As an example, there are currently 13 subpopulations of NSCLC that are treated differently based on genomic and programmed death-ligand 1 (PD-L1) testing results.26 This rapid evolution makes it uniquely challenging for COPs to stay abreast of all aspects of an ever-changing field. Additionally, DOPs at academic institutions frequently have access to and experience treating patients within clinical trials studying novel therapeutic approaches, like CIT, prior to their FDA approval. Over time, this fosters greater confidence and clinical aptitude when managing individual patient variations and complications, such as irAEs, in response to treatment. As such, there is often a slower uptake of newly approved treatment regimens within the community setting. A 2011–2015 study assessing CIT utilization in the metastatic melanoma population found that greater than 80% of health systems treating the largest fractions of patients with immunotherapy were academic centers.27 Additionally, the academic environment provides an infrastructure with greater access to diagnostic technology, multidisciplinary support and collaborative subspecialist care when needed, preferentially benefiting DOPs.
While evidence that differential patient outcomes exist between care providers lessens an important knowledge gap in the medical oncology literature, how we counteract this discrepancy is of greater value (Figure 2). Most recently, oncology clinical pathways have been strategically adopted by a variety of cancer centers in order to promote the delivery of evidence-based care. The American Society of Clinical Oncology (ASCO) defines clinical pathways as “evidenced-based treatment protocols for delivering cancer care to patients with specific disease types and stages.”28 From 2014 to 2016, there was a 42% increase in the number of oncology practices compliant with oncology clinical pathways.29 And in 2016, the ASCO Task Force on Clinical Pathways defined many criteria for high-quality oncology pathway program development. Among others, they should be expert driven, transparent, evidence-based, patient focused and promote participation in clinical trials.30 Hybrid academic-community practice institutions have reported on the benefits of integrating such multidisciplinary oncology pathways into EMR, with community oncologists specifically reporting greater practice efficiency while decreasing necessity to obtain treatment opinions from their disease-oriented, academic colleagues.31,32 This application also has the potential for reducing drug spending.33 However, restrictions that inherently permeate clinical pathways may also negatively impact patient care and uptake by providers. Payers may utilize such resources in order to limit the reimbursement of different therapies, creating a myriad of stipulations and barriers that prevent access to certain standard of care options that may not be included in a certain pathway.32
Vertical integration of oncology practices has increased remarkably over the past two decades. In 2017, 55–60% of the medical oncology practices were acquired by larger hospital-based health-care organizations.34,35 The model of full-spectrum health-care organizations offering primary through quaternary care often involves an academic medical center with their faculty and community practice physicians.36 The resulting interface between providers with different scopes of practice has the potential to further promote the delivery of evidence-based, high-quality oncology care to patients closer to home. For instance, combined community and academic virtual tumor boards have the potential to offer COPs the opportunity to obtain a multidisciplinary review of their challenging cases within a convenient platform. Real-world effects of such integration have resulted in treatment regimen modifications and facilitation of appropriate clinical trial participation.37 Additionally, creating an infrastructure that allows COPs to actively participate and enroll their patients in clinical trials without the need for provider referral would be of great value. This would afford superior patient access to treatment that may otherwise be prohibitive based on treatment location restraints. Further, providers would gain familiarity and necessary experience utilizing new and upcoming therapies. Successful integration of this measure would rely on strong educational initiatives provided by principal investigators to oncologists at affiliated sites along with institutional buy-in for resource allocation to develop extended clinical trial support staff.
Other areas of benefit include development of standardized, disease-specific programs promoting compliance with high-quality oncology care – such as precision medicine – throughout the whole health-care organization. A recent real-world analysis determined rates of comprehensive genomic testing for patients with advanced/metastatic NSCLC treated mainly at community sites remains very low, with less than 50% of patients tested in compliance with national guidelines.10 To address such gaps, our institution has established a precision medicine program within the thoracic oncology domain. In this model, reflex, centralized testing with next-generation sequencing (NGS) and PD-L1 analysis are obtained for all newly diagnosed stage IV NSCLC patients throughout both academic and community sites.38 Resulting information is used at a comprehensive molecular tumor board, providing community oncologists with treatment recommendations based on actionable genomic alterations.38 Following implementation of this program, there was a statistically significant increase in utilization of targeted therapies, as well as a significant decrease in the turn-around time for both NGS and PD-L1 test results.38 Finally, providing COPs within the community direct, one-on-one access to specific DOPs would allow for greater patient care collaboration. This could materialize in multiple ways such as integration of DOPs into community sites where they would not only see patients but also provide guidance to other providers on an as needed basis. Another feasible option would be identification of specific DOPs who could act as academic liaisons, or a point of contact, for a subset of COPs. This would allow DOPs the opportunity to guide COPs when addressing more nuanced areas of patient management as the need arose. Finally, periodic disease-specific educational sessions and meetings could be held to discuss topics such as new treatment recommendations, clinical trial availability and any areas of provider concern regarding disease workup and management. Though prior examples certainly do not embody the full scope of integrative opportunities, they do provide a foundation upon which to act.
While opportunity for improved patient outcomes exists with the implementation of the above initiatives, the practicality of such engagement for COPs remains a difficult obstacle to overcome. With such a wide scope of practice, it may be challenging to actively engage in multiple tumor boards or have the bandwidth to become familiar with various clinical trials among a range of diagnoses. However, as oncologic innovation continues to accelerate, treatment of individual malignancies will likely become more precise with greater individual variation placing increasing demands on COPs. Recognizing this, some aspects of sub-specialization at the community level may be of value. Whether this entails providers preferentially caring for one disease entity or a larger yet limited number, many benefits may be realized including, improved ability to stay up to date within focused disease areas, enhanced confidence in patient management, ability to attend necessary tumor board or educational meetings, and the opportunity for improved collaboration within clinical groups as those with a disease focus can provide expertise that other colleagues may lack.39 Of course, implementation of such care would hinge on the size and scope of an individual practice and would not be practical for all. Nonetheless, efforts to promote varying models of sub-specialization within the community may also help improve patient outcomes.
Importantly, results from this study also show that the DOP cohort included a significantly higher prevalence of Black patients compared to the COP cohort (17% v 6.60%), and those of Black race were more likely to be unemployed (26.21% v 12.73%), live in a neighborhood with a poverty rate ≥11% (58.33% v 17.08%) and have Medicaid as their primary health insurance (24.59% v 6.99%). Based on a multitude of previous research, low socioeconomic status (SES) has the potential to promote poor cancer outcomes due to increased risk of environmental exposures, unhealthy behaviors such as excess tobacco and alcohol use, and lack of health-care resources, most notably insurance.9 Additionally, while cancer-associated mortality has declined for all racial groups since the early 1990s, the Black population continues to experience the highest mortality rate for most cancer subtypes, and their 5-year survival rate is typically lower in comparison to their White counterparts, at every stage of diagnosis.40 Despite these existing data, racial disparities were not identified in this study and differential survival outcomes were not realized among other social determinants of health including poverty level, employment status and health insurance type, all major contributors to SES and resulting cancer health disparities. These results are remarkably important, suggesting that access to expert level, disease-oriented care has the potential to mitigate pervasive racial and socioeconomic cancer health disparities.
The academic institution where this study took place is located within Ward 6, one of Cleveland’s most impoverished communities. Here, the poverty rate reaches 41.62%, with Black individuals accounting for 75.58% of the population.19 This local demographic datum likely accounts for a portion of the differences in racial distribution seen between DOPs and COPs. Historically, racial minorities are less likely to travel to receive medical care and are more likely to seek care at safety-net or public hospitals that may lack the supportive infrastructure of academic centers.4 However, Cleveland’s main safety net hospital requires an approximate one-hour commute via public transportation for individuals residing in this general location. This makes the academic center of this large health-care organization uniquely situated to provide expert-level care to a characteristically vulnerable population that may not have the opportunity to access such resources otherwise. Hence, we hypothesize that DOPs may afford all patients superior centralized care, optimized treatment schedules, improved identification of disease progression and easy access to subspecialty consultants that deliver expert management of clinical intercurrences. Access to clinical trials may also be superior for DOP-treated patients. Accordingly, racial and socioeconomic disparities were not identified in this study, potentially due to preferential benefits experienced by vulnerable and disadvantaged populations.
Analyzing the rate of severe, grade 3 or 4 irAEs identified significant differences among disease diagnoses. At 48 and 96 weeks following the initiation of immunotherapy, incidence of severe irAEs for melanoma patients reached 13.4% and 15.2%, respectively. Incidence among NSCLC and renal cell carcinoma (RCC) patients over the same time frames only reached 4.7% and 5.0%, and 6.3% and 6.3%, respectively. While there was a significantly higher rate of severe irAEs identified among the DOP cohort compared to the COP cohort, this difference was not upheld within a multivariable regression after controlling for factors such as age, sex, comorbidity index and histopathologic diagnosis. These results are likely secondary to the frequency and combination use of certain ICIs among differing cancer diagnoses. Any grade irAEs occur in 60% of patients treated with ipilimumab, the only cytotoxic T-lymphocyte associated protein 4 (CTLA-4) antibody, with 10–30% categorized as severe.41 In comparison, the incidence of irAEs in relation to anti-programmed cell death protein 1 (PD-1) and PD-L1 antibodies is significantly less frequent with only 10% of patients developing severe irAEs.32 Furthermore, the combination of anti-CTLA-4 and anti-PD-1 antibodies, ipilimumab and nivolumab, leads to both increased incidence and severity of irAEs.41 Compared to other diagnoses, melanoma is most frequently treated with this combination regimen. Furthermore, results did not identify any racial or socioeconomic differences in the setting of immune-related toxicities.
It is recognized that there are many limitations to the present study. First, this was a retrospective design that does not hold the same weight as a prospective cohort study addressing the same question of interest. Variables were assessed based on chart review of patients cared for by a heterogeneous group of providers over a seven-year period. Subjective data, including performance status, were dependent on interpretation of the provider. Additionally, development of severe irAEs was determined based on patient hospitalization. This relies heavily on individual physician judgment as irAEs may be misdiagnosed or erroneously graded. Furthermore, some patient data could not be identified based on chart review. Variable rates of missing data were apparent for all patient characteristic categories except age, sex, immunotherapy type and comorbidity index. Missing data were most prominent among employment status with 16% unavailable, poverty level with 7% unavailable and performance status with 4% unavailable. While this does not account for a significant proportion of available data, its potential effect on the above results is unknown. Information regarding clinical trial participation was not obtained and, hence, not utilized as a covariate of interest that could have affected outcomes.
Finally, this retrospective study offers insight into real-world patient outcomes utilizing CIT for multiple malignancy types in a large health-care organization. The results demonstrate a superior survival benefit among patients receiving care from DOPs in an academic setting, adding to a limited fund of existing data suggesting similar provider-level variations through a solid tumor and medical oncology lens. Furthermore, no survival disparities were identified based on race or SES. In fact, it is important to acknowledge that DOPs cared for a significantly larger proportion of patients living in highly impoverished communities and of Black race. This suggests that access to expert-level care at an institution with a strong infrastructure can potentially counterbalance some longstanding cancer health disparities. These provocative results suggest a potential benefit for greater collaboration between disease-oriented and community oncologists along with improved system-wide care coordination through multidisciplinary approaches, especially in large health-care organizations such as the one described in this study. Furthermore, multiple potential areas for improvement have been highlighted above. In light of rising cancer-care complexity, implementation of these changes must be prioritized to help close the apparent gaps generated by differential access to expert-level care.
Conclusion
This is a retrospective large cohort of patients with solid tumors who received immune checkpoint inhibitors at a large health-care organization over a seven-year period. Patients treated by academic clinicians experienced superior overall survival when compared to patients treated by community-based oncologists. Patients of Black race and lower socioeconomic status were more likely to be treated by academic oncologists. As consensus disparities data show worse health outcomes for Black and patients of lower socioeconomic status, this study demonstrated no significant differences in overall survival based on race, payer or socioeconomic status. This study highlights the opportunities associated with vertical integration of oncology practices for delivery of high-value oncology care.
Research Ethics and Consent
This study received IRB approval (reference number STUDY20221434) through Case Western Reserve University and University Hospitals meeting international guidelines for research on humans. The dataset is owned by authors and all data acquired has been anonymized and deidentified. Informed consent for participation was specifically waived by local Institutional Review Board office. This research is in compliance with Declaration of Helsinki.
Disclosure
Christopher Hoimes reports grants, personal fees from Merck, BMS, Genentech, Seagen, Astellas, and Eisai, during the conduct of the study. Debora S Bruno reports personal fees from Bristol Myers Squibb, Novartis, Eli Lilly, Amgen, Mirati Therapeutics, AstraZeneca, TEMPUS, Daiichi-Sankyo, and grants from AstraZeneca, outside the submitted work. Afshin Dowlati reports being on the advisory board for Ipsen, BMS, Merck, Astra Zeneca, and Seattle Genetics, during the conduct of the study. The authors report no other conflicts of interest in this work.
References
1. Haslam A, Prasad V. Estimation of the percentage of US patients with cancer who are eligible for and respond to checkpoint inhibitor immunotherapy drugs. JAMA Netw Open. 2019;2(5):e192535. doi:10.1001/jamanetworkopen.2019.2535
2. Lipson EJ, Drake CG. Ipilimumab: an anti-CTLA-4 antibody for metastatic melanoma. Clin Cancer Res. 2011;17(22):6958–6962. doi:10.1158/1078-0432.CCR-11-1595
3. Michot JM, Bigenwald C, Champiat S, et al. Immune-related adverse events with immune checkpoint blockade: a comprehensive review. Eur J Cancer. 2016;54:139–148. doi:10.1016/j.ejca.2015.11.016
4. Vaddepally RK, Kharel P, Pandey R, Garje R, Chandra AB. Review of indications of FDA-approved immune checkpoint inhibitors per NCCN guidelines with the level of evidence. Cancers. 2020;12(3):738. doi:10.3390/cancers12030738
5. Thompson JA, Schneider BJ, Brahmer J, et al. NCCN guidelines insights: management of immunotherapy-related toxicities, version 1.2020. J Natl Compr Canc Netw. 2020;18(3):230–241. doi:10.6004/jnccn.2020.0012
6. Schneider BJ, Naidoo J, Santomasso BD, et al. Management of immune-related adverse events in patients treated with immune checkpoint inhibitor therapy: ASCO guideline update. J Clin Oncol. 2021;39(36):4073–4126. doi:10.1200/JCO.21.01440
7. Verhoeven DC, Chollette V, Lazzara EH, Shuffler ML, Osarogiagbon RU, Weaver SJ. The anatomy and physiology of teaming in cancer care delivery: a conceptual framework. J Natl Cancer Inst. 2021;113(4):360–370. doi:10.1093/jnci/djaa166
8. Noyes K, Monson JR, Rizvi I, Savastano A, Green JS, Sevdalis N. Regional multiteam systems in cancer care delivery. J Oncol Pract. 2016;12(11):1059–1066. doi:10.1200/JOP.2016.013896
9. American Association for Cancer Research. Cancer disparities progress report 2022; 2022.
10. Bruno DS, Hess LM, Li X, Su EW, Patel M. Disparities in biomarker testing and clinical trial enrollment among patients with lung, breast, or colorectal cancers in the United States. JCO Precis Oncol. 2022;6:e2100427. doi:10.1200/PO.21.00427
11. Turner M, Adam MA, Sun Z, et al. Insurance status, not race, is associated with use of minimally invasive surgical approach for rectal cancer. Ann Surg. 2017;265(4):774–781. doi:10.1097/SLA.0000000000001781
12. Corso CD, Park HS, Kim AW, Yu JB, Husain Z, Decker RH. Racial disparities in the use of SBRT for treating early-stage lung cancer. Lung Cancer. 2015;89(2):133–138. doi:10.1016/j.lungcan.2015.05.002
13. Kann BH, Park HS, Johnson SB, Chiang VL, Yu JB. Radiosurgery for brain metastases: changing practice patterns and disparities in the United States. J Natl Compr Canc Netw. 2017;15(12):1494–1502. doi:10.6004/jnccn.2017.7003
14. Killeen SD, O’Sullivan MJ, Coffey JC, Kirwan WO, Redmond HP. Provider volume and outcomes for oncological procedures. Br J Surg. 2005;92(4):389–402. doi:10.1002/bjs.4954
15. Boffa DJ, Mallin K, Herrin J, et al. Survival after cancer treatment at top-ranked US cancer hospitals vs affiliates of top-ranked cancer hospitals. JAMA Netw Open. 2020;3(5):e203942. doi:10.1001/jamanetworkopen.2020.3942
16. Naghavi AO, Echevarria MI, Strom TJ, et al. Patient choice for high-volume center radiation impacts head and neck cancer outcome. Cancer Med. 2018;7(10):4964–4979. doi:10.1002/cam4.1756
17. Koshy M, Malik R, Spiotto M, Mahmood U, Weichselbaum R, Sher D. Disparities in treatment of patients with inoperable stage I non-small cell lung cancer: a population-based analysis. J Thorac Oncol. 2015;10(2):264–271. doi:10.1097/JTO.0000000000000418
18. Harris PA, R Taylor R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap) – A metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42(2):377–381.
19. NEOCANDO at the Center on Urban Poverty and Community Development. Neighborhood data warehouse; 2020. Available from: https://neocando.case.edu/neighborhood-data.html.
20. Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. J Am Stat Assoc. 1958;53(282):457–481. doi:10.1080/01621459.1958.10501452
21. Cox DR. Regression models and life-tables. J R Stat Soc Series B. 1972;34(2):187–220.
22. Fine JP, Gray RJ. A proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc. 1999;94(446):496–509. doi:10.1080/01621459.1999.10474144
23. Hallet J, Davis L, Mahar A, et al. Benefits of high-volume medical oncology care for noncurable pancreatic adenocarcinoma: a population-based analysis. J Natl Compr Canc Netw. 2020;18(3):297–303. doi:10.6004/jnccn.2019.7361
24. Hallet J, Davis LE, Mahar AL, et al. Variation in receipt of therapy and survival with provider volume for medical oncology in non-curative esophago-gastric cancer: a population-based analysis. Gastric Cancer. 2020;23(2):300–309. doi:10.1007/s10120-019-01012-z
25. City of Hope. Why cancer care is different; 2021. Available from: https://www.cityofhope.org/breakthroughs/why-cancer-care-is-different.
26. National Comprehensive Cancer Network. Non-small cell lung cancer (version 6.2022); 2022. Available from: https://www.nccn.org/professionals/physician_gls/pdf/nscl.pdf.
27. Krimphove MJ, Tully KH, Friedlander DF, et al. Adoption of immunotherapy in the community for patients diagnosed with metastatic melanoma. J Immunother Cancer. 2019;7(1):289. doi:10.1186/s40425-019-0782-y
28. American Society of Clinical Oncology. Clinical Pathways. Available from: https://old-prod.asco.org/news-initiatives/current-initiatives/cancer-care-initiatives/clinical-pathways#:~:text=High%2Dquality%20oncology%20clinical%20pathways,care%20quality%20and%20reducing%20costs.
29. Daly B, Zon RT, Page RD, et al. Oncology clinical pathways: charting the landscape of pathway providers. J Oncol Pract. 2018;14(3):e194–e200. doi:10.1200/JOP.17.00033
30. Zon RT, Edge SB, Page RD, et al. American Society of clinical oncology criteria for high-quality clinical pathways in oncology. J Oncol Pract. 2017;13(3):207–210. doi:10.1200/JOP.2016.019836
31. Bosserman LD, Cianfrocca M, Yuh B, et al. Integrating academic and community cancer care and research through multidisciplinary oncology pathways for value-based care: a review and the city of hope experience. J Clin Med. 2021;10(2):188. doi:10.3390/jcm10020188
32. Caffrey M. From making oncology clinical pathways multidisciplinary, to adding the patient voice. Evid Based Oncol. 2020;26(9):SP306.
33. Hertler A, Chau S, Khetarpal R, et al. Utilization of clinical pathways can reduce drug spend within the oncology care model. JCO Oncol Pract. 2020;16(5):e456–e63. doi:10.1200/JOP.19.00753
34. Hu X, Lipscomb J, Graetz I. The impact of vertical integration of oncologists on cancer outcomes and healthcare costs among metastatic prostate cancer patients. J Clin Oncol. 2022;40(16_suppl):e18786–e. doi:10.1200/JCO.2022.40.16_suppl.e18786
35. Alpert A, Hsi H, Jacobson M. Evaluating the role of payment policy in driving vertical integration in the oncology market. Health Aff. 2017;36(4):680–688. doi:10.1377/hlthaff.2016.0830
36. Stamy CD, Schwartz CC, Han LP, Schwinn DA. Community and academic physicians working together in integrated health care systems. Mayo Clin Proc Innov Qual Outcomes. 2021;5(5):951–960. doi:10.1016/j.mayocpiqo.2021.06.008
37. Karimi M, Wang C, Bahadini B, Hajjar G, Fakih M. Integrating academic and community practices in the management of colorectal cancer: the city of hope model. J Clin Med. 2020;9(6):1687. doi:10.3390/jcm9061687
38. Bruno D, Donner A, Kopp S, et al. Implementation of a precision medicine thoracic (PREDICT) service using reflex testing in a large academic-community practice network. J Clin Oncol. 2022;40:6572. doi:10.1200/JCO.2022.40.16_suppl.6572
39. Gesme DH, Wiseman M. Subspecialization in community oncology: option or necessity? J Oncol Pract. 2011;7(3):199–201. doi:10.1200/JOP.2011.000292
40. Giaquinto AN, Miller KD, Tossas KY, Winn RA, Jemal A, Siegel RL. Cancer statistics for African American/black people 2022. CA Cancer J Clin. 2022;72(3):202–229. doi:10.3322/caac.21718
41. Martins F, Sofiya L, Sykiotis GP, et al. Adverse effects of immune-checkpoint inhibitors: epidemiology, management and surveillance. Nat Rev Clin Oncol. 2019;16(9):563–580. doi:10.1038/s41571-019-0218-0
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.