Back to Journals » Infection and Drug Resistance » Volume 16
Outcomes and Risk Factor Analysis of Plastic Bronchitis Among 321 Children with Influenza Pneumonia After Bronchoscopy Examination
Authors Hu Q , Wu J, Wang C , Liang W, Wang Y, Zheng Y , Wen F, Wang W, Yu U
Received 19 January 2023
Accepted for publication 15 June 2023
Published 21 June 2023 Volume 2023:16 Pages 4001—4011
DOI https://doi.org/10.2147/IDR.S405444
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Prof. Dr. Héctor Mora-Montes
Qian Hu,1 Jianle Wu,1 Chengqian Wang,1 Wen Liang,1 Yulei Wang,1 Yuejie Zheng,1 Feiqiu Wen,2 Wenjian Wang,1,* Uet Yu2,*
1Department of Respiratory Diseases, Shenzhen Children’s Hospital, Shenzhen, People’s Republic of China; 2Department of Hematology and Oncology, Shenzhen Children’s Hospital, Shenzhen, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Uet Yu, Department of Hematology and Oncology, Shenzhen Children’s Hospital, 7019, Yitian, Road, Shenzhen, 518038, People’s Republic of China, Email [email protected] Wenjian Wang, Department of Respiratory Diseases, Shenzhen Children’s Hospital, 7019, Yitian, Road, Shenzhen, 518038, People’s Republic of China, Email [email protected]
Background: Plastic bronchitis (PB) is a rare and severe lung disease. It can be triggered by influenza virus infection, which is a common respiratory infection in children. Bronchoscopy can aid in the early detection and treatment of PB. However, the outcomes and risk for PB development in pediatric patients with influenza virus infection are not fully understood.
Methods: Data from 321 children diagnosed with influenza virus pneumonia who underwent bronchoscopy examinations between 1st January, 2009 and 31st December, 2020 were retrospectively analyzed to assess the outcomes and risk factors associated with PB development.
Results: This study included 97 girls and 224 boys with influenza virus pneumonia with a median age of 42 months. Among them, 36 patients (11.2%) were categorized as having PB based on bronchoscopy findings. PB patients had significantly longer fever durations (p=0.010) and higher risks of developing severe conditions including respiratory failure (p< 0.001), acute respiratory distress syndrome (p< 0.001), and air-leak syndrome (p< 0.001) compared to non-PB patients. Conventional treatment including the use of neuraminidase inhibitors and antibiotics did not differ between the PB and non-PB patients, but PB patients required more anti-inflammatory treatment (p=0.019) and ventilator support (p< 0.001). Combined univariate and multivariate analyses suggested that radiographic findings, including mediastinal emphysema (p=0.012) and lung consolidation (p=0.012), as well as increased levels of neutrophils (p=0.026), aspartate aminotransferase (p=0.004), and lactate dehydrogenase (p< 0.001), were identified as risk factors for PB development in patients with influenza virus pneumonia. Although PB patients required more intensive care and had longer hospital stays, they all recovered well after treatment.
Conclusion: Influenza virus infection is linked to PB development in children. Identifying risk factors and early intervention such as bronchoscopy can improve the prognosis of children with PB.
Keywords: influenza virus, plastic bronchitis, bronchoscopy, pneumonia, pediatric
Introduction
Plastic bronchitis (PB), also known as cast bronchitis, is a rare lung disease characterized by the formation of thick or gummy casts in the endobronchial system, causing partial or complete airway obstruction, resulting in severe respiratory distress and life-threatening respiratory and circulatory complications. In children, PB can occur at any age but is more prevalent in children aged 2 to 12 years.1 The number of patients diagnosed with PB has increased in the recent years, probably because there are more and more methods to identify PB such as the use of bronchoscopy. The common symptoms of PB include coughing, wheezing, and shortness of breath. The etiology of PB remains unclear, however, it has been linked to respiratory infections caused by Mycoplasma pneumoniae, influenza virus (Inf-V), adenovirus, and other non-infectious conditions such as lymphatic disorders, congenital heart diseases, severe asthma, cystic fibrosis, sickle cell anemia, and smoking2–6 in both adults and children. Recent studies have suggested three possible mechanisms for PB development: airway inflammation leading to increased mucus secretion;7 various infections leading to necrotic detachment of respiratory epithelial cells and mucosal edema; and leakage of lymphatic fluid from the airways.7–9 However, the exact mechanism and pathophysiology of PB remain unclear.
INF-V infection plays an important role in PB pathogenesis.10 Patients with influenza pneumonia have high morbidity and mortality influenza pneumonia and the severity of pneumonia may be related to PB development.10–12 A study from Japan identified PB patients within 66 patients that were hospitalized with influenza virus associated lower respiratory tract infections.13 However, the exact rate of PB in pediatric patients with influenza pneumonia remains uncertain because most studies were reports with sporadic cases or a series of small number of cases and lack systematic summary of their clinical features. Furthermore, it is also important for clinicians to identify PB early for prompt treatment to avoid devastating outcomes. In this study, we retrospectively analyzed 321 children with INF-V pneumonia who underwent bronchoscopy examination and compared the differences in clinical features and outcomes between patients with and without PB.
Patients and Methods
Patients
We reviewed and analyzed the clinical data, including patient characteristics, medical history, clinical manifestations, laboratory and radiological evaluations, treatment and outcomes, of 321 patients aged between 1 month and 16 years of age who were hospitalized and diagnosed with severe INF-V pneumonia and underwent bronchoscopy examination at Shenzhen Children’s Hospital between 1st January, 2009 and 31st December, 2020. This study was conducted in accordance with the Declaration of Helsinki and was approved by the Ethics Committee of Shenzhen Children’s Hospital. Written consent was obtained from the patient’s parents or legal guardians.
Diagnostic Criteria and Treatment
INF-V infection was confirmed by the detection of the influenza A and/or influenza B in nasopharyngeal swab, sputum, or bronchoalveolar lavage fluid (BALF) by enzyme-linked immunosorbent assay (ELISA), colloidal gold assay, polymerase chain reaction (PCR) or real-time PCR methods. The diagnosis of INF-V pneumonia was made according to the Chinese expert consensus on diagnosis and treatment of influenza in children (2020 Edition) published by the China National Clinical Research Center for Respiratory Diseases. Influenza pneumonia patients with lung consolidation was identified by radiological examination of solid lesions or atelectasis in one or more lung segments.14 Severe pneumonia was considered when the patient had one or more of the following manifestations: 1) Patients with symptoms of hypoxemia, tachypnea, or Hoover’s sign, 2) Extrapulmonary complications; and 3) Admission to the intensive care unit (ICU), requiring mechanical ventilation, or death. Airway obstruction was suspected if the patient had developed any of the following symptoms: 1) Difficulty in breathing, 2) Decreased blood oxygen saturation, and 3) Coughing up tube-like casts.
All patients were enrolled and treated according to the standard care pathway. Bronchoscopy examination and bronchoalveolar lavage (BAL) therapy were performed when patients had persistent or worsening respiratory symptoms or when airway obstruction was suspected after conventional treatment according to the standard pathway. PB was confirmed by the presence of thick or rubbery casts causing obstruction of the bronchi by bronchoscopic examination. Patient outcomes were evaluated at the time of discharge. A good prognosis was considered when the patients had resolved or recovered from clinical symptoms. A poor prognosis was considered if the patients had persistent or worsened symptoms, discontinued treatment, or died.
Statistical Analysis
Statistical analyses were performed using SPSS version 25.0 software. Continuous variables between the groups were examined using the Mann–Whitney U-test. Categorical variables between the groups were assessed using the Fisher’s exact test and the Pearson’s χ2 test. Statistical significance was set at p<0.05. Univariate analyses were performed using logistic regression. Variables with a p value less than 0.02 were selected as candidates for the multivariate analysis to identify risk factors associated with the development of PB.
Results
Patient Characteristics
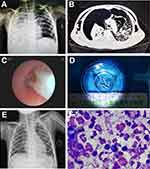
Patient characteristics are presented in Table 1. A total of 97 girls and 224 boys with a median age of 42 months were included in this study. Patients with INF-V pneumonia were divided into PB and non-PB (NPB) groups based on whether the patients had PB observed by bronchoscopy (Figure 1). There were 36 patients (11.2%) in the PB group and 285 patients (88.8%) in the non-PB group. Notably, in the PB group, 30 patients had influenza A infection (83.3%), 5 patients had influenza B infection (13.9%), and 1 patient (2.8%) had co-infection with both viruses. Patients in the PB group were significantly older than non-PB group (51 months vs 42 months; Mann–Whitney U-test, p=0.014). PB patients also had a longer hospital stay than non-PB patients (12 days vs 9 days; Mann–Whitney U-test, p=0.003). No significant differences were observed between the two groups in terms of history of previous illness and allergies.
 |
Table 1 Patient Characteristics |
The patients’ clinical manifestations were summarized and their differences between the PB and non-PB groups were compared (Table 1). The main symptoms of pneumonia including fever (86.3%) and cough (97.8%) were observed in most of the patients. However, a significantly longer duration of fever was observed in the PB group than in the non-PB group (8 days vs 6 days; Mann–Whitney U-test, p=0.003). The proportion of patients who developed pulmonary symptoms including tachypnea (83.3% vs 47.7%; Fisher’s exact test; p<0.001), dyspnea (63.9% vs 24.9%; Fisher’s exact test; p<0.001), and wheezing (50.0% vs 30.2%; Fisher’s exact test; p=0.017) was significantly higher in the PB group than in the non-PB group. Extrapulmonary symptoms, such as abdominal pain (19.4% vs 7.4%; Fisher’s Exact test, p=0.035) and agitation (30.6% vs 14.0%; Fisher’s exact test, p=0.011), were also more frequently observed in PB patients than in the non-PB patients. More importantly, the proportion of patients who developed severe systemic diseases, including respiratory failure (58.3% vs 20.4%; Fisher’s exact test, p<0.001), acute respiratory distress syndrome (25.0% vs 1.4%; Fisher’s exact test, p<0.001), and air-leak syndrome (36.1% vs 8.4%; Fisher’s exact test; p<0.001), was significantly higher in PB patients than in the non-PB patients.
Laboratory and Radiological Evaluations
Laboratory findings of the patients were examined (Table 2). No significant differences were observed between the PB and non-PB groups in terms of complete blood counts, inflammatory markers and biochemical assessments, or co-infection with other pathogens such as bacteria, mycoplasma, Epstein-Barr virus, and adenovirus. Radiological findings were evaluated, and radiological abnormalities were more observed more frequently observed in the PB group than in the non-PB group. The proportion of patients with pleural effusion (52.7% vs 33.7%; Fisher’s exact test, p=0.029), pneumothorax (11.1% vs 1.4%; Fisher’s exact test, p=0.003), mediastinal emphysema (25.0% vs 2.5%; Fisher’s exact test, p<0.001), lung consolidation (77.8% vs 34.3%; Fisher’s exact test; p<0.001), and atelectasis (41.7% vs 24.6%; Fisher’s exact test; p=0.028), was significantly higher in the PB group than in the non-PB group.
 |
Table 2 Laboratory and Radiological Examinations of Patients with Severe Influenza Pneumonia |
Treatment and Patient Outcomes
Patient treatment details are summarized in Table 3. All patients received treatment with neuraminidase inhibitor. The timing of prescribing a neuraminidase inhibitor (less than 48 h after the onset of symptoms) did not differ significantly between the PB and non-PB groups. Most patients received antibiotics (91.0%); however, no differences were observed between the PB and non-PB groups (94.4% vs 90.5%). Patients with persistent fever received intravenous immunoglobulin (IVIG) and methylprednisolone (1–2mg/kg/day for 3–5 days) to reduce the inflammatory response. There were significant differences between the proportion of patients in the two groups receiving IVIG (66.7% vs 42.3%; Fisher’s exact test, p=0.006) and methylprednisolone (66.7% vs 46.0%; Fisher’s exact test, p=0.019). The percentage of patients who required intensive care (61.1% vs 28.4%; Fisher’s exact test, p<0.001) and mechanical ventilation (52.8% vs 16.5%; Fisher’s exact test, p<0.001) was also significantly higher in the PB group than in the non-PB group. None of the patients died during the observational period. All patients showed good recovery after discharge.
 |
Table 3 Treatment of Patients with Severe Influenza Pneumonia |
Logistic Regression Analysis of Patients with and without PB
To study the risk factors associated with PB formation in patients with INF-V pneumonia, a univariate logistic regression analysis was performed (Table 4). We observed that the duration of fever; symptoms including tachypnea, dyspnea, wheezing, and agitation; presence of acute respiratory distress syndrome; and radiological findings, including pleural effusion, pneumothorax, mediastinal emphysema, lung consolidation, and atelectasis, and lactate dehydrogenase levels were risk factors associated with the formation of PB. Factors with a p value less than 0.2 were included in the multivariate logistic regression analysis (Table 5). We observed that mediastinal emphysema, lung consolidation, and levels of aspartate aminotransferase (AST), lactate dehydrogenase (LDH), and neutrophils were the independent risk factors associated with PB development.
 |
Table 4 Univariate Logistic Regression Analysis of Patients with Plastic Bronchitis |
 |
Table 5 Multivariate Analysis of Patients with Plastic Bronchitis |
Discussion
PB was first described as a rare respiratory disease characterized by airways filled with mucus-tube-like casts, leading to complete or partial obstruction of the bronchi or bronchial trees.15 The etiology of PB remains unclear. The currently accepted classification system divides PB into two subtypes based on the pathological features.16 Type I PB occurs mainly in respiratory infectious diseases and is characterized by a large infiltration of inflammatory cells on pathohistological analysis.17 Type II PB occurs mainly in cases of congenital heart defects and is characterized by few or no inflammatory cells. In this study, all PB patients underwent pathohistological analysis and were characterized as having type I PB.
Respiratory viruses including influenza are a major cause of PB.18,19 However, the exact incidence of PB in children with infected influenza infection has not yet been fully studied. Influenza belongs to the enveloped Orthomyxoviridae viruses and can be divided into types A, B, C and D. Both influenza A and B viruses can be further divided into subtypes based on the differential expression of the two antigens against hemagglutinin (H) and neuraminidase (N).20 In this study, we included a relatively large cohort of pediatric patients and observed that 11% of the patients with INF-V pneumonia developed PB. Furthermore, most INF-V patients who developed PB were of the H1N1 subtype, which is consistent with the previous reports.13 Historically, the development of children with INF-V infection, particularly H1N1 patient has been associated with severe diseases and a higher mortality rate. However, no patients died, and patient outcomes did not differ between the PB and non-PB groups, probably because of the early intervention of bronchoscopy.
Direct removal of bronchial PB via bronchoscope is the gold standard for diagnosing PB.21 It is also an important therapeutic tool to remove PB to eliminate bronchial blockage.10 However, it is sometimes difficult to determine when to perform bronchoscopy in children with early stage PB.5,22,23 On the one hand, the lack of specific clinical manifestations of PB can lead to misdiagnosis of the disease and delay in treatment.5 On the other hand, bronchoscopy is an invasive procedure, and excessive medical treatment can increase the risk of bronchoscopy-related complications, especially in young children.24 Therefore, it is important to identify the risk factors associated with PB without overtreating the patients. In this study, there was no significant sexual predilection for PB development in children with INF-V pneumonia. Most patients in this study younger than 6 years of age. Interestingly, patients who developed PB were significantly older than those who did not develop PB. This is likely because older children tend to have more a developed immune system, which may exhibit a greater inflammatory response after INF-V infection. Previous studies have suggested that children with underlying conditions are more likely to develop PB and have higher mortality rates after INF-V infection.25 However, more than 80% of the children in the PB group had no underlying disease, suggesting that PB can occur in previously healthy children after INF-V infection and should alert physicians to early diagnosis and treatment.
Currently, at our center, we are aiming for early bronchoscopy to avoid treatment delays in patients with PB. Bronchoscopic evaluation is recommended for patients with pneumonia who have persistent or worsening respiratory symptoms or are suspected to have airway obstruction. During the study period, we compared patients with and without PB to examine the risk factors associated with PB development and the treatment outcomes. Fever was one of the main symptoms in both PB and non-PB patients. Generally, the incubation period after INF-V infection is 1 to 4 days, and peaks within 24 h after infection,26 with hyperthermia lasting 5 to 8 days or longer in severely ill patients.12 We observed that PB patients had a significantly longer duration of fever at a median of 8 days than the non-PB patients. Consistent with the findings of previous reports, patients with PB had a significantly higher incidence of manifestations suggestive of airway obstruction, including wheezing, gasping, tachypnea, and dyspnea. In addition, we observed that patients were more likely to have extrapulmonary symptoms, such as abdominal pain and agitation. Patients with PB often lack specific laboratory findings. Previous studies have suggested that PB patients may have a higher expression of LDH, probably because of hypoxia caused by PB-induced bronchial blockage.27,28 Consistent with the findings of these reports, we also observed a significantly elevated LDH levels in the PB group compared with those in the non-PB group. In the present study, we also observed that low AST levels, and low neutrophil counts were independent risk factors for PB development.29 However, the exact cut-off to predict PB development requires further examination because of the small number of PB patients with PB in this study. On radiological examinations, PB patients had a higher incidence of pleural effusion, pneumothorax, mediastinal emphysema, lung consolidation, and atelectasis than non-PB patients. Although radiological findings may not be specific during the early stage of PB, early radiological examinations remain crucial to identify severe pneumonia caused by INF-V and to avoid treatment delay.
Conventional treatment, including the use of neuraminidase inhibitor (NAI) and antibiotics, did not differ between the PB and non-PB patients. However, the proportion of patients requiring systemic treatment, including glucocorticoids and IVIG was significantly higher in the PB group. PB patients are also at a higher risk of developing severe complications, such as respiratory failure, ARDS, and air-leak syndrome.30 Consequently, patients with PB are more likely to require critical care and ventilatory support. Although PB patients required more intensive treatment compared with non-PB patients, they can be quickly weaned off the ventilator and dyspnea can be reduced after PB removal by bronchoscopy.23 All patients recovered well with no sequelae except that some of them were discharged home with continued oral NAI and antibiotic treatment.
This study had several limitations. This was a single-center study. Although the hospital is a third-tertiary hospital that attracts patients from many neighboring cities, in the future, a multicenter study with a larger sample size is needed to better represent the characteristics and outcomes of INF-V patients with PB development in the general population. Furthermore, because of the relatively short study period, we did not analyze the differences between INF-V patients who developed PB across different influenza epidemics. This may be crucial for studying the link between viral virulence and PB development and requires further investigation.
In conclusion, we observed that approximately 11% of patients with INF-V-associated pneumonia may developed PB. Although early stage PB patients may not have specific symptoms, or laboratory and radiological findings, early bronchoscopic evaluation and removal of PB can be effective in resolving airway obstructions and preventing severe disease. Therefore, patients with INF-V pneumonia and PB can recover well after proper treatment.
Ethical Approval and Consent for Participate
Written consents in approval for publication of this manuscript were obtained from the parents or legal guardians for the participation of this study. This study was approved by the Ethics Committee of Shenzhen Children’s Hospital.
Consent for Publication
Written consents were obtained from the parents or legal guardians of the patients for the collection of clinical data and the publication of these data in this manuscript.
Author Contributions
Wenjian Wang and Uet Yu are shared corresponding authors. All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
Shenzhen Fundamental Research Program (JCYJ20190809170007587), Shenzhen Fund for Guangdong Provincial High-level Clinical Key Specialties (SZGSP012), Shenzhen Key Medical Discipline Construction Fund (SZXK032), Shenzhen Science and Technology Innovation Commission (RCBS20200714114858018), Shenzhen High-level Hospital Construction Fund.
Disclosure
The authors have no conflict of interests to declare.
References
1. Rubin BK. Plastic bronchitis. Clin Chest Med. 2016;37(3):405–408. doi:10.1016/j.ccm.2016.04.003
2. Dori Y, Keller MS, Rome JJ, et al. Percutaneous lymphatic embolization of abnormal pulmonary lymphatic flow as treatment of plastic bronchitis in patients with congenital heart disease. Circulation. 2016;133(12):1160–1170. doi:10.1161/circulationaha.115.019710
3. Lu S, Liu J, Cai Z, Shuai J, Huang K, Cao L. Bronchial casts associated with Mycoplasma pneumoniae pneumonia in children. J Int Med Res. 2020;48(4):300060520911263. doi:10.1177/0300060520911263
4. Kirito Y, Matsubayashi T, Ohsugi K. Plastic bronchitis: three cases caused by influenza B virus Yamagata lineage. Pediatr Int. 2019;61(4):421–423. doi:10.1111/ped.13799
5. Yuan L, Huang JJ, Zhu QG, Li MZ, Zhuo ZQ. Plastic bronchitis associated with adenovirus serotype 7 in children. BMC Pediatr. 2020;20(1):268. doi:10.1186/s12887-020-02119-4
6. Sharma VJ, Iyengar AJ, Zannino D, et al. Protein-losing enteropathy and plastic bronchitis after the Fontan procedure. J Thorac Cardiovasc Surg. 2021;161(6):2158–2165.e4. doi:10.1016/j.jtcvs.2020.07.107
7. Jaramillo AM, Azzegagh Z, Tuvim MJ, Dickey BF. Airway mucin secretion. Ann Am Thorac Soc. 2018;15(Suppl 3):S164–S170. doi:10.1513/AnnalsATS.201806-371AW
8. Demont P, Fehr T, Geiser T, Ott SR. Bronchial cast formation in plastic bronchitis. Respiration. 2016;91(4):325–326. doi:10.1159/000445441
9. Maleux G, Storme E, Cools B, et al. Percutaneous embolization of lymphatic fistulae as treatment for protein-losing enteropathy and plastic bronchitis in patients with failing Fontan circulation. Catheter Cardiovasc Interv. 2019;94(7):996–1002. doi:10.1002/ccd.28501
10. Terano C, Miura M, Fukuzawa R, et al. Three children with plastic bronchitis associated with 2009 H1N1 influenza virus infection. Pediatr Infect Dis J. 2011;30(1):80–82. doi:10.1097/INF.0b013e3181f10fff
11. Yu H, Huang J, Huai Y, et al. The substantial hospitalization burden of influenza in central China: surveillance for severe, acute respiratory infection, and influenza viruses, 2010–2012. Influenza Other Respir Viruses. 2014;8(1):53–65. doi:10.1111/irv.12205
12. Cao B, Li XW, Mao Y, et al. Clinical features of the initial cases of 2009 pandemic influenza A (H1N1) virus infection in China. N Engl J Med. 2009;361(26):2507–2517. doi:10.1056/NEJMoa0906612
13. Uda K, Shoji K, Koyama-Wakai C, et al. Clinical characteristics of influenza virus-induced lower respiratory infection during the 2015 to 2016 season. J Infect Chemother. 2018;24(6):407–413. doi:10.1016/j.jiac.2018.01.002
14. Cavallazzi R, Ramirez JA. Influenza and viral pneumonia. Clin Chest Med. 2018;39(4):703–721. doi:10.1016/j.ccm.2018.07.005
15. Healy F, Hanna BD, Zinman R. Pulmonary complications of congenital heart disease. Paediatr Respir Rev. 2012;13(1):10–15. doi:10.1016/j.prrv.2011.01.007
16. Seear M, Hui H, Magee F, Bohn D, Cutz E. Bronchial casts in children: a proposed classification based on nine cases and a review of the literature. Am J Respir Crit Care Med. 1997;155(1):364–370. doi:10.1164/ajrccm.155.1.9001337
17. Lu Z, Zheng Y. Plastic bronchitis associated with adenovirus infection. Lancet Infect Dis. 2018;18(4):474. doi:10.1016/s1473-3099(18)30095-1
18. Wang Y, An S. Plastic bronchitis associated with influenza A virus in children with asthma. J Int Med Res. 2021;49(12):3000605211065370. doi:10.1177/03000605211065370
19. Murata Y, Ishihara S, Sato Y, Ohta T. Plastic bronchitis associated with influenza: an adult case. Intern Med. 2021;60(10):1647–1648. doi:10.2169/internalmedicine.5313-20
20. Sullivan SJ, Jacobson RM, Dowdle WR, Poland GA. 2009 H1N1 influenza. Mayo Clin Proc. 2010;85(1):64–76. doi:10.4065/mcp.2009.0588
21. Li Y, Williams RJ, Dombrowski ND, et al. Current evaluation and management of plastic bronchitis in the pediatric population. Int J Pediatr Otorhinolaryngol. 2020;130:109799. doi:10.1016/j.ijporl.2019.109799
22. Yoshida M, Funata K, Koinuma G, Miyairi I. Plastic bronchitis associated with influenza. J Pediatr. 2021;238:336–337. doi:10.1016/j.jpeds.2021.06.021
23. Zhang J, Kang X. Plastic bronchitis associated with influenza virus infection in children: a report on 14 cases. Int J Pediatr Otorhinolaryngol. 2015;79(4):481–486. doi:10.1016/j.ijporl.2015.01.002
24. Vielkind M, Wolter-Warmerdam K, Jackson A, et al. Airway obstruction and inflammation on combined bronchoscopy in children with down syndrome. Pediatr Pulmonol. 2021;56(9):2932–2939. doi:10.1002/ppul.25573
25. Jain S, Kamimoto L, Bramley AM, et al. Hospitalized patients with 2009 H1N1 influenza in the United States, April–June 2009. N Engl J Med. 2009;361(20):1935–1944. doi:10.1056/NEJMoa0906695
26. Bell D, Nicoll A, Fukuda K, et al. Non-pharmaceutical interventions for pandemic influenza, international measures. Emerg Infect Dis. 2006;12(1):81–87. doi:10.3201/eid1201.051370
27. Xu Q, Zhang L, Hao C, et al. Prediction of bronchial mucus plugs formation in patients with refractory mycoplasma pneumoniae pneumonia. J Trop Pediatr. 2017;63(2):148–154. doi:10.1093/tropej/fmw064
28. Hu EC, He JG, Liu ZH, et al. High levels of serum lactate dehydrogenase correlate with the severity and mortality of idiopathic pulmonary arterial hypertension. Exp Ther Med. 2015;9(6):2109–2113. doi:10.3892/etm.2015.2376
29. Cho WH, Kim YS, Jeon DS, et al. Outcome of pandemic H1N1 pneumonia: clinical and radiological findings for severity assessment. Korean J Intern Med. 2011;26(2):160–167. doi:10.3904/kjim.2011.26.2.160
30. Valente T, Lassandro F, Marino M, Squillante F, Aliperta M, Muto R. H1N1 pneumonia: our experience in 50 patients with a severe clinical course of novel swine-origin influenza A (H1N1) virus (S-OIV). Radiol Med. 2012;117(2):165–184. doi:10.1007/s11547-011-0734-1
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.