Back to Journals » Cancer Management and Research » Volume 15
Osimertinib for an Advanced NSCLC Patient with Two Common EGFR Mutations and a Concomitant MET Exon 14 Skipping Mutation: A Case Report
Authors Liu Z, Song P, Zhou L, Ji D , Shen H, Dong H, Feng X
Received 12 March 2023
Accepted for publication 20 June 2023
Published 12 July 2023 Volume 2023:15 Pages 645—650
DOI https://doi.org/10.2147/CMAR.S412199
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Antonella D'Anneo
Zhicong Liu,1,* Pengtao Song,2,* Lingyan Zhou,1 Dongxiang Ji,1 Hui Shen,1 Hui Dong,1 Xueren Feng1
1Department of Respiratory Medicine, Huzhou Central Hospital, Affiliated Central Hospital Huzhou University, Huzhou, People’s Republic of China; 2Department of Pathology, Huzhou Central Hospital, Affiliated Central Hospital Huzhou University, Huzhou, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Hui Dong; Xueren Feng, Department of Respiratory Medicine, Huzhou Centre Hospital, Affiliated Centre Hospital Huzhou University, Huzhou, People’s Republic of China, Email [email protected]; [email protected]
Background: Lung cancer remains the leading cause of cancer-related mortality. Studies have revealed that a combination of crizotinib and EGFR tyrosine kinase inhibitors (TKIs) could be an effective treatment option for patients with sensitizing EGFR mutations and de novo or acquired MET amplification. Until now, there have been few reports of the response in patients harboring three mutations.
Case Presentation: A patient was diagnosed with advanced lung adenocarcinoma harboring EGFR Del19, L858R mutation and METex14. She received osimertinib, and repeated imaging revealed further tumor progression. Sixty-six days later, combined treatment with osimertinib and crizotinib was initiated. Unfortunately, the patient succumbed to death at home after 17 days.
Conclusion: This report firstly provided a lung adenocarcinoma patient with two common EGFR mutations (Del19 and L858R) and METex14. Our case raises a reminder about the tolerance and safety of combination therapy, especially in older peoples.
Keywords: lung adenocarcinoma, compound mutations, osimertinib, crizotinib, combination therapy, case report
Background
Lung cancer is ranked as the most common cause of cancer-related death and threatens human health worldwide.1 Based on modern molecular diagnostics techniques, the molecular classification of non-small-cell lung cancer (NSCLC), which is the prevalent subtype of lung cancer, is increasingly accurate. In recent years, diverse oncogenic drivers have been identified in NSCLC, including epidermal growth factor receptor (EGFR), mesenchymal-epithelial transition (MET) and anaplastic lymphoma kinase (ALK).2,3 Selective targeting of oncogenic mutations has revolutionized the treatment of genome-defined subtypes of NSCLC. Currently, TKIs are the standard first-line treatment options for advanced NSCLC patients with an identified driver mutation.4 Some examples of the more well-established treatments include alectinib, which is a potent ALK inhibitor, and osimertinib, which is a potent EGFR inhibitor.
EGFR mutations detected as deletions in exon 19 (Del19) and the L858R point mutation in exon 21 are the most common EGFR mutations and account for approximately 10–15% of NSCLC cases in individuals of European descent and ~30% of NSCLC cases in individuals of East Asian descent.5 Some EGFR-TKIs are currently approved as first-line therapies for EGFR mutation-positive NSCLC. These include first-generation, second-generation and third-generation TKIs: erlotinib and gefitinib, afatinib and dacomitinib and osimertinib, respectively.6 Osimertinib (AZD9291) is an oral, third-generation, irreversible and selective EGFR-TKI that inhibits the EGFR-TKI-sensitizing mutation and T790M resistance mutation.7 On the basis of the results of the AURA clinical program and FLAURA trial, osimertinib is approved by the FDA as a first-line drug for common sensitive EGFR mutations or a second-line drug for acquired resistance to first-generation EGFR-TKIs via the T790M mutation,8–11 especially in NSCLC patients with central nervous system metastases.12 Additionally, another Phase III trial (ADAURA) also supported adjuvant osimertinib as a highly effective therapy in patients with resected EGFR-mutated stage IB-IIIA NSCLC.13,14
The prevalence of MET exon 14 skipping mutation (METex14) in lung adenocarcinoma has been reported to be approximately 3%.15 It appears that this alteration is more common in older nonsmoking women.16 Several studies have suggested that there are two classes of MET-targeting therapeutics for METex14 NSCLC in clinical development. Crizotinib (XALKORI) is the first targeted therapy to demonstrate antitumor efficacy in METex14 NSCLC.17 Many case studies have reported the efficacy of crizotinib in patients with METex14 alterations in lung cancer.18,19 In addition to crizotinib, other inhibitors, such as capmatinib, tepotinib, and savolitinib, have been proposed for the treatment of adult patients with metastatic NSCLC whose tumors harbor METex14.20
Herein, we present the case of an NSCLC patient harboring three EGFR mutations, including EGFR Del19, L858R and METex14, who experienced exceptional rapid disease progression following EGFR TKI osimertinib and crizotinib treatment. We present the following case in accordance with the CARE reporting checklist.
Case Presentation
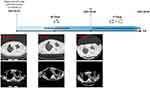
In March 2021, an 82-year-old, Asian, non-smoker and no second-hand smoker woman was admitted to our hospital for chest pain and cough. Chest CT (computed tomography) scanning showed a 4.3*3.7 cm mass beside the mediastinum of the left lung and a small amount of fluid accumulated in the left thoracic cavity (Figure 1). A biopsy specimen taken by CT-guided fine-needle aspiration (FNA) showed lung adenocarcinoma with positive TTF-1 and Napsin A and negative P40 and CK5/6 (Figure 2). Magnetic resonance imaging (MRI) of the brain showed no obvious malignant metastases. In addition, abdominal ultrasound revealed no obvious abnormalities. On the basis of the results of FNA and chest CT, the patient was diagnosed with lung adenocarcinoma (cT2bNxM1a, stage IV, ECOG-PS 1). Due to pleural effusion and advanced age, the patient subsequently underwent mutational analysis. The PCR results identified two common EGFR mutations (Del19 and L858R) and METex14 (c.2888–192891del), which was verified by NGS analysis (forty-eight gene panel). To our knowledge, lung adenocarcinoma harboring three mutations has not been reported thus far. Considering the weak positive antinuclear antibody staining and advanced age of the patient, combination therapy might have induced severe treatment-related adverse events (AEs), especially increased risks of interstitial pneumonia and a cardiac event. Therefore, monotherapy with the EGFR inhibitor osimertinib was administered in April 2021. A chest CT scan was reexamined in the outpatient department one month later, and the result revealed a similar local lesion (4.8*4.3 cm) and a decrease in pleural effusion, which was confirmed as SD (Stable Disease) according to RECIST criteria (Figure 1). After 2 months, radiological progression was demonstrated with an increase in size (5.9*5.5 cm) and in pleural effusion. Rebiopsy or liquid biopsy was suggested to explore the resistance mechanism of osimertinib, but the patient refused because of advanced age and high price. Therefore, she received a combination of osimertinib 80 mg once daily and crizotinib 250 mg twice a day (Figure 1). Unfortunately, the patient died at home suddenly 17 days later. The cough and chest tightness were appeared after combination therapy but the cause of death remains unknown.
 |
Figure 2 Representative images of H&E staining and immunohistochemistry. (A) H&E staining of the lung biopsy showed NSCLC. (B and C) Immunohistochemistry showed positive TTF-1 (B) and Napsin A (C). |
Discussion and Conclusions
Despite recent advances in anticancer drugs, the prognosis of advanced NSCLC, especially in patients with nonclassical or compound mutations, remains poor. Susumu Kobayashi et al analyzed the EGFR mutation pattern of 79 NSCLC cases harboring EGFR mutations.21 They found that compound EGFR mutations comprised 14% of all mutations in their cohort. In addition to an atypical mutation (EGFR-L858R+A871G), most patients with EGFR TKI-sensitizing mutations (G719X, exon 19 deletion, L858R, and L861Q) responded to EGFR TKIs.
MET activation mutations have been identified in multiple cancer types and play an important role in tumor initiation, development, and therapeutic resistance.22 Genomic alterations in MET include MET gene amplification, MET protein overexpression, and METex14 and MET gene point mutations.20 The MET pathway is a well-known mechanism of resistance to EGFR-TKIs in NSCLC.23,24 Some studies have suggested that inhibition of both EGFR and MET is required to obtain tumor regression. Marjorie Aubanel reported that the combination of a first- or third-generation EGFR TKI and crizotinib can achieve durable disease control in EGFR-mutated lung adenocarcinoma patients harboring MET amplification.25 In addition to MET amplification, Kauffmann-Guerrero et al identified METex14 as an important factor mediating acquired resistance to EGFR TKI in patients with sensitizing EGFR mutations, and the combination of an EGFR TKI and MET inhibitor could be an effective treatment option.26
In our case, three mutations were detected in an NSCLC patient who experienced disease progression on EGFR TKI osimertinib treatment. Re-biopsy and molecular analysis are important in case of tumor progression. Moreover, liquid biopsy is an important complement to tissue biopsy in some specific situations.27,28 Unfortunately, the patient refused rebiopsy or liquid biopsy in our case and died at home after one month of osimertinib combined with crizotinib administration. Although the exact mechanisms involved remain unclear, we reasoned three possible explanations for the death of this patient. 1) Tumor lysis syndrome (TLS) is an acute, life-threatening disease that is associated with the initiation of cytopenia therapy for malignant tumors. TLS occurs in invasive hematologic malignancies and most solid tumors. A pattern of metabolic disruption occurs as a result of large amounts of intracellular contents entering the systemic circulation. Its characteristics include hyperuricemia, hyperphosphatemia, hyperkalemia, hypocalcemia, and uremia, all of which can lead to arrhythmias, seizures, renal failure, and sudden death.29 Monoclonal antibodies and tyrosine kinase inhibitors are two examples of widely used targeted therapies that have been shown to induce TLS, either as monotherapy or in combination with conventional chemotherapy. TLS usually occurs within 12 to 72 hours of the initiation of cytotoxic therapy, although it rarely appears before treatment (known as spontaneous TLS) and may occur more than 72 hours after the start of treatment. A cascade of pathophysiological events resulting from extensive tumor lysis can lead to multiple organ failure, of which renal failure and heart failure are the most common, or sudden death.30 2) Treatment-related AEs that frequently occur after crizotinib treatment include abnormal vision, diarrhea, nausea, vomiting, constipation, elevated aminotransferase levels, edema, upper respiratory tract infections, dyslexia, and dizziness. Grade ≥3 AEs, including neutropenia, elevated aminotransferase levels, fatigue, interstitial lung disease (ILD), pneumonia, and prolonged electrocardiogram QTc, have also been reported.31–33 Severe adverse events can be fatal. 3) The incidence of venous thromboembolism (VTE) in lung cancer varies from 5 to 14%.34,35 One study found that thromboses occurred in 12% of patients with EGFR mutations and 15.3% of patients with MET mutations.36,37 Treatment options for patients with embolism are limited, and survival is poor.38
Conclusion
To our knowledge, this is the first report of a NSCLC patient with EGFR Del19, L858R and METex14. The patient experienced progression during Osimertinib treatment which suggests METex14 might affect the efficacy of EGFR targeted therapy in NSCLC patients. The combination of EGFR TKI and MET inhibitor could provide a potential treatment for these patients. However, the tolerance and safety of combination therapy deserved our attention.
Data Sharing Statement
The data that support this case report are available from the corresponding author on reasonable request, since respecting the Ethics Committee to protect patient confidentiality.
Ethics Approval and Consent to Participate
The article was reviewed and approved by the by the research ethics committee of Huzhou Central Hospital, Affiliated Central Hospital of Huzhou University. Written informed consent was signed by the patient.
Consent for Publication
Written informed consent was obtained from the participant’s next to kin (son) for publication of this case report and accompanying clinical data.
Acknowledgments
We apologize to all researchers whose relevant contributions were not cited due to space limitations.
Author Contributions
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval of the version to be published; and agree to be accountable for all aspects of the work.
Funding
This work was supported by grants from the Project of Zhejiang Basic Public Benefit Research of Zhejiang Province (No. LGD21H010001 to LZC) and the Public Benefit Research of Huzhou Science and Technology Bureau (NO. 2022GZB01 to FXR). The foundation supported gene mutational analysis.
Disclosure
The authors declare that they have no competing interests in this work.
References
1. Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer statistics, 2021. CA Cancer J Clin. 2021;71(1):7–33. doi:10.3322/caac.21654
2. Pirker R, Filipits M. Personalized treatment of advanced non-small-cell lung cancer in routine clinical practice. Cancer Metastasis Rev. 2016;35(1):141–150.
3. Rocco G, Morabito A, Leone A, Muto P, Fiore F, Budillon A. Management of non-small cell lung cancer in the era of personalized medicine. Int J Biochem Cell Biol. 2016;78:173–179.
4. Gelatti ACZ, Drilon A, Santini FC. Optimizing the sequencing of tyrosine kinase inhibitors (TKIs) in epidermal growth factor receptor (EGFR) mutation-positive non-small cell lung cancer (NSCLC). Lung Cancer. 2019;137:113–122.
5. Rosell R, Moran T, Queralt C, et al. Screening for epidermal growth factor receptor mutations in lung cancer. N Engl J Med. 2009;361(10):958–967.
6. Igawa S, Fukui T, Kasajima M, et al. First-line osimertinib for poor performance status patients with EGFR mutation-positive non-small cell lung cancer: a prospective observational study. Invest New Drugs. 2021:564.
7. Igawa S, Ono T, Kasajima M, et al. Impact of EGFR genotype on the efficacy of osimertinib in EGFR tyrosine kinase inhibitor-resistant patients with non-small cell lung cancer: a prospective observational study. Cancer Manag Res. 2019;11:4883–4892.
8. Goss G, Tsai CM, Shepherd FA, et al. Osimertinib for pretreated EGFR Thr790Met-positive advanced non-small-cell lung cancer (AURA2): a multicentre, open-label, single-arm, Phase 2 study. Lancet Oncol. 2016;17(12):1643–1652.
9. Yang JC, Ahn MJ, Kim DW, et al. Osimertinib in Pretreated T790M-Positive Advanced Non-Small-Cell Lung Cancer: AURA Study Phase II Extension Component. J Clin Oncol. 2017;35(12):1288–1296.
10. Soria J-C, Ohe Y, Vansteenkiste J, et al. Osimertinib in Untreated EGFR -Mutated Advanced Non–Small-Cell Lung Cancer. N Engl J Med. 2018;378(2):113–125. doi:10.1056/NEJMoa1713137
11. Mok TS, Wu YL, Ahn MJ, et al. Osimertinib or Platinum-Pemetrexed in EGFR T790M-Positive Lung Cancer. N Engl J Med. 2017;376(7):629–640.
12. Goss G, Tsai CM, Shepherd FA, et al. CNS response to osimertinib in patients with T790M-positive advanced NSCLC: pooled data from two phase II trials. Ann Oncol. 2018;29(3):687–693.
13. Herbst RS, Wu YL, John T, et al. Adjuvant Osimertinib for Resected EGFR-Mutated Stage IB-IIIA Non-Small-Cell Lung Cancer: updated Results From the Phase III Randomized ADAURA Trial. J Clin Oncol. 2023;41(10):1830–1840.
14. Wu YL, Herbst RS, Mann H, Rukazenkov Y, Marotti M, Tsuboi M. ADAURA: Phase III, Double-blind, Randomized Study of Osimertinib Versus Placebo in EGFR Mutation-positive Early-stage NSCLC After Complete Surgical Resection. Clin Lung Cancer. 2018;19(4):e533–e536.
15. Lee CC, Yamada KM. Identification of a novel type of alternative splicing of a tyrosine kinase receptor. Juxtamembrane deletion of the c-met protein kinase C serine phosphorylation regulatory site. J Biol Chem. 1994;269(30):19457–19461.
16. Vuong HG, Ho ATN, Altibi AMA, Nakazawa T, Katoh R, Kondo T. Clinicopathological implications of MET exon 14 mutations in non-small cell lung cancer - A systematic review and meta-analysis. Lung Cancer. 2018;123:76–82.
17. Hong L, Zhang J, Heymach JV, Le X. Current and future treatment options for MET exon 14 skipping alterations in non-small cell lung cancer. Ther Adv Med Oncol. 2021;13:1758835921992976.
18. Drilon A, Clark JW, Weiss J, et al. Antitumor activity of crizotinib in lung cancers harboring a MET exon 14 alteration. Nat Med. 2020;26(1):47–51.
19. Awad MM, Leonardi GC, Kravets S, et al. Impact of MET inhibitors on survival among patients with non-small cell lung cancer harboring MET exon 14 mutations: a retrospective analysis. Lung Cancer. 2019;133:96–102.
20. Drusbosky LM, Dawar R, Rodriguez E, Ikpeazu CV. Therapeutic strategies in METex14 skipping mutated non-small cell lung cancer. J Hematol Oncol. 2021;14(1):129.
21. Kobayashi S, Canepa HM, Bailey AS, et al. Compound EGFR mutations and response to EGFR tyrosine kinase inhibitors. J Thorac Oncol. 2013;8(1):45–51.
22. Tovar EA, Graveel CR. MET in human cancer: germline and somatic mutations. Ann Transl Med. 2017;5(10):205.
23. Sequist LV, Waltman BA, Dias-Santagata D, et al. Genotypic and histological evolution of lung cancers acquiring resistance to EGFR inhibitors. Sci Transl Med. 2011;3(75):75ra26.
24. Bean J, Brennan C, Shih JY, et al. MET amplification occurs with or without T790M mutations in EGFR mutant lung tumors with acquired resistance to gefitinib or erlotinib. Proc Natl Acad Sci U S A. 2007;104(52):20932–20937.
25. Aubanel M, Swalduz A, Avrillon V, et al. Combining EGFR and MET Inhibition With Crizotinib in EGFR-mutated Lung Adenocarcinoma Harboring MET Amplification: a Brief Report. Clin Lung Cancer. 2020;21(6):e601–e606.
26. Kauffmann-Guerrero D, Kahnert K, Kumbrink J, Syunyaeva Z, Tufman A, Huber RM. Successful Treatment of a Patient With NSCLC Harboring an EGFR Mutation and a Concomitant Met Exon 14 Skipping Mutation Combining Afatinib and Crizotinib. Clin Lung Cancer. 2019;20(1):59–62.
27. Oscorbin IP, Smertina MA, Pronyaeva KA, et al. Multiplex Droplet Digital PCR Assay for Detection of MET and HER2 Genes Amplification in Non-Small Cell Lung Cancer. Cancers. 2022;14(6):786.
28. Rossi E, Aieta M, Tartarone A, et al. A fully automated assay to detect the expression of pan-cytokeratins and of EML4-ALK fusion protein in circulating tumour cells (CTCs) predicts outcome of non-small cell lung cancer (NSCLC) patients. Translational Lung Cancer Res. 2021;10(1):80–92.
29. Abu-Alfa AK, Younes A. Tumor lysis syndrome and acute kidney injury: evaluation, prevention, and management. Am J Kidney Dis. 2010;55(5 Suppl 3):S1–S13.
30. Williams SM, Killeen AA. Tumor Lysis Syndrome. Arch Pathol Lab Med. 2019;143(3):386–393.
31. Camidge DR, Bang YJ, Kwak EL, et al. Activity and safety of crizotinib in patients with ALK-positive non-small-cell lung cancer: updated results from a Phase 1 study. Lancet Oncol. 2012;13(10):1011–1019.
32. Shaw AT, Ou SH, Bang YJ, et al. Crizotinib in ROS1-rearranged non-small-cell lung cancer. N Engl J Med. 2014;371(21):1963–1971.
33. Shaw AT, Kim DW, Nakagawa K, et al. Crizotinib versus chemotherapy in advanced ALK-positive lung cancer. N Engl J Med. 2013;368(25):2385–2394.
34. Tagalakis V, Levi D, Agulnik JS, Cohen V, Kasymjanova G, Small D. High risk of deep vein thrombosis in patients with non-small cell lung cancer: a cohort study of 493 patients. J Thorac Oncol. 2007;2(8):729–734.
35. Walker AJ, Baldwin DR, Card TR, Powell HA, Hubbard RB, Grainge MJ. Risk of venous thromboembolism in people with lung cancer: a cohort study using linked UK healthcare data. Br J Cancer. 2016;115(1):115–121.
36. Liu Y, Wang W, Wu F, et al. High discrepancy in thrombotic events in non-small cell lung cancer patients with different genomic alterations. Transl Lung Cancer Res. 2021;10(3):1512–1524.
37. Chiari R, Ricciuti B, Landi L, et al. ROS1-rearranged Non-small-cell Lung Cancer is Associated With a High Rate of Venous Thromboembolism: analysis From a Phase II, Prospective, Multicenter, Two-arms Trial (METROS). Clin Lung Cancer. 2020;21(1):15–20.
38. Mahajan A, Brunson A, White R, Wun T. The epidemiology of cancer-associated venous thromboembolism: an update. Semin Thromb Hemost. 2019;45(4):321–325.
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.