Back to Journals » Journal of Pain Research » Volume 13
Oral Spirulina Platensis Attenuates Hyperglycemia and Exhibits Antinociceptive Effect in Streptozotocin-Induced Diabetic Neuropathy Rat Model
Authors Abdel-Daim MM, Shaaban Ali M , Madkour FF, Elgendy H
Received 26 June 2020
Accepted for publication 5 August 2020
Published 15 September 2020 Volume 2020:13 Pages 2289—2296
DOI https://doi.org/10.2147/JPR.S267347
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Robert B. Raffa
Mohamed M Abdel-Daim,1 Mohamed Shaaban Ali,2 Fedekar F Madkour,3 Hamed Elgendy2,4
1Pharmacology Department, Faculty of Veterinary Medicine, Suez Canal University, Ismailia 41522, Egypt; 2Anesthesia Department, Assiut University Hospitals, Assiut, Egypt; 3Marine Science Department, Faculty of Science, Port Said University, Port Said 42526, Egypt; 4Anesthesia Department, HAMAD Medical Corporation & Weill Cornell Medicine Qatar & Qatar University, Doha, Qatar
Correspondence: Hamed Elgendy
Anesthesia Department, HAMAD Medical Corporation, P.O. Box 3050, Doha, Qatar
Tel +974- 4011 4271
Fax +974- 4439 1511
Email [email protected]
Introduction: Diabetic neuropathy is a common consequence of diabetes. Hyperalgesia is one of the main symptoms of diabetic neuropathy. In response to noxious stimuli, streptozotocin (STZ)-induced diabetic rats show exaggerated hyperalgesic behavior, while Spirulina platensis has anti-inflammatory, antioxidant, and insulin-like effects. To assess the antinociceptive effect of oral Spirulina platensis (SP) powder on formalin-induced nociceptive responses in STZ-induced diabetic rats.
Methods: Sixty mature male albino rats were randomly allocated into six equal groups (10 in each group). Group 1 (control non-diabetic group) received 0.9% saline; group 2 was given oral pure SP powder-treated as a non-diabetic control group, group 3 was sodium salicylate-treated rats and used as a positive non-diabetic control group, group 4 managed as vehicle-treated diabetic rats, group 5 considered as SP-treated-diabetic group, and sodium salicylate-treated-diabetic rats used as a diabetic positive control group (group 6). STZ-diabetic rats were orally given SP in a dose of 500 mg kg/day for 1 month. The formalin test was implemented in two phases: the early phase in the first 10-min post-formalin injection, and the late phase was considered in the 15– 60 min post-formalin injection time interval.
Results: Pain scores were increased in the diabetic groups during both phases of the experiment. Blood glucose was significantly reduced in diabetic rats that received oral SP, P < 0.01. Besides, SP-treated rats had lower pain scores during both phases of the experiment than untreated diabetic ones. However, in the sodium salicylate group, the pain score was reduced only during the second phase. An exaggerated nociceptive response occurred in diabetic rats after the formalin test. A significant antinociceptive effect appeared in SP-treated control and diabetic rats.
Discussion: The findings suggest that oral Spirulina platensis could have a potential therapeutic role for managing induced painful diabetic neuropathy in rats.
Keywords: antinociceptive, Spirulina platensis, diabetes, neuropathy, rat
Introduction
Diabetes is a common chronic disabling disease resulting in chronic hyperglycemia that produces perturbation of cellular metabolism. It disturbs the metabolic pathways, leads to excessive free radical production and oxidative stress. Hyperglycemia, either acute or chronic, leads to oxidative stress and microvascular consequences in the peripheral nerves and initiates diabetic neuropathy. Diabetic neuropathy is a common microvascular disease that estimated recently, approximately 10–20% in diabetic subjects who exhibit painful neuropathy.1
Oxidative stress process occurs when a cellular system fails to get rid of the accumulated oxygen free radicals produced during metabolism. These radicals attack and destruct proteins, lipids, and nucleic acids, contributing to disturbance of energy metabolism, cell signaling, and transport activities. These accumulated oxygen-free radicals lead to cell death through programmed cell death pathway.2
Acute hyperglycemia in type-2 diabetes is also closely linked to reactive oxygen species-mediated oxidative stress.3 Numerous dietary supplements were tried in different countries as a traditional food that thought to be beneficial and activates the total radical antioxidant potentials in diabetic patients.4
Spirulina platensis (SP) is naturally growing freshwater blue-green algae, enriched with proteins and essential nutrients. The use of SP has been found to reduce high serum lipids5 and decrease high blood pressure.5,6 Moreover, it demonstrated neuroprotective and reactive oxygen species (ROS) scavenging actions observed in various experimental studies.7,8 A biliprotein, C-phycocyanin, extracted from SP, had anti-inflammatory activity.9 Antioxidant properties of SP were described before.10,11
Jung et al extracted insulin-like protein from SP with the same molecular mass, immunoreactivity, as that of bovine insulin. It has been successfully decreased diabetic complications by increasing body weight and markedly reducing high blood glucose and glycosylated hemoglobin in diabetic rats.12 It showed improved glycemic control as consistent with another experimental study.13
Up to our knowledge, Spirulina platensis is not investigated before in the treatment of painful diabetic neuropathy. Therefore, we initiated our rat model of formalin-induced painful diabetic neuropathy. To evaluate the oral administration of 500 mg/kg SP for 1 month for attenuation of diabetes and antinociceptive effect on painful diabetic neuropathic rat model.
Materials and Methods
We have the approval of our Institutional Board Ethical Committee for animal research at the Faculty of Veterinary Medicine, Suez Canal University, on 06-11-2018 (Registration no. 14–186). We performed all the procedures, and rats were treated in conformity with and followed the principles on ethical animal research outlined in the ethical guidelines by the International Council for Laboratory Animal Science.
Chemicals
Spirulina platensis powder in a pure premium form was obtained from (Herba Force, UK). The strain of Spirulina platensis was purchased from CFTRI Mysore, India, as used in a previous study.14
Animals
Mature male albino rats weighing 180–230 g were purchased from the animal house of National Research Centre, Dokki-Cairo. The animals were housed individually in cages, kept on a 12 h light/dark cycle, at a comfortable temperature (21 ± 1°C), humidity (55 ± 5%), and free access to food and water at all times. We demonstrated our experimental model in Figure 1. Sixty mature male albino rats were randomly allocated into six equal groups (10 in each group). Group 1 (control non-diabetic group) received 0.9% saline; group 2 was given oral pure SP powder-treated as the non-diabetic control group, group 3 was sodium salicylate-managed rats and used as a positive non-diabetic control group, group 4 managed as vehicle-treated diabetic rats, group 5 considered as SP-treated-diabetic group, and sodium salicylate-treated- diabetic animals used as a diabetic positive control group (group 6). STZ-diabetic rats were orally given SP using a stomach tube in a dose of 500 mg kg/day for 1 month. We tested this dose before in a previous study of our group.14
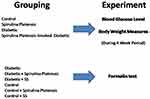 |
Figure 1 Algorithm of the study design. |
Induction of Diabetes
To ensure that streptozotocin (STZ) have injured islets of langerhans, 30 rats were kept on overnight fasting before the STZ injection, as fasting could induce quick stress injury to islets as reported by Zhang et al.15 STZ was freshly prepared by dissolving 50 mM sodium citrate (pH 4.5) solution containing 150 mM NaCl, which was administered as a subcutaneous injection at the dose of 60 mg/kg body weight. The administration of STZ should be done immediately after fresh preparation. Blood glucose level was measured by electronic glucometer. After the STZ injection by 2 weeks, we confirmed that those animals developed maintained hyperglycemia above 400 mg/dL and then started to perform data analysis.16
Formalin Test
For pain assessment, the formalin test was performed, which was described elsewhere.17 A summary, before starting the test, each rat was acclimatized to the observation box.
Fifty microlitre of 2.5% formalin was injected subcutaneously, in the planter surface of right-hand paw, using a 25-gauge syringe needle. Immediately, each experimental animal was placed in a Plexiglas box. First, we began determining nociceptive responses by placing each animal in the box and continued observations for 60 min. We could determine the nociceptive score for each 5 min block during that period by time measurement, which was spent in each of the four behavioral categories: 0 nociceptive score when the position and posture of the injected hind paw are indistinguishable from the contralateral paw. Level 1 determined when the injected paw has little or no weight placed on it. Level number 2 could be distinguished when the injected paw is elevated and is not in contact with any surface. The third nociceptive score level could be determined when the injected paw is licked, bitten, or shaken. We have measured a weighted nociceptive score, which ranged from 0 to 3, was calculated by multiplying the time spent in each category by the category weight, summing these products, and dividing by the total time for each 5 min block of time. Two phases were determined; the early (first) phase in the first 10-min post-formalin injection, and the late (second) phase was considered in the time interval 15–60-min post-formalin injection.18
Study Design
Spirulina platensis powder was administered orally using a stomach tube; we used 500 mg/kg for 1 month starting from the day 15th after STZ administration for the second and fifth groups. Sodium salicylate was administered orally for the third and sixth groups at a dose of 200 mg/kg 60 min before the formalin test. Body weight and serum blood glucose levels for all rats were monitored at the beginning and the end of the experiment. After 8 h of fasting, serial blood samples were collected by tail nipping and assessed for blood glucose by an electronic portable glucometer.
Data and Statistical Analysis
The normality of data was checked by Shapiro–Wilk’s test. Data on blood glucose level and pain score in formalin test showed normal distribution P-value >0.05; hence, data were expressed as mean ± SD and groups were compared using student’s paired t-test. While data of weight showed P-value <0.05 using Shapiro–Wilk’s test (did not show the normality of data) and expressed as mean ± S.E.M. and analyzed using nonparametric test; Wilcoxon Signed Ranks Test. The probability level at P ≤ 0.05 is considered Significant. Analysis was done on SPSS (IBM Inc., the USA) version 21.0.
Results
Figure 1 demonstrates the algorithm of the study design. Besides, serial blood glucose measurements before and after treatment are described in Table 1.
 |
Table 1 Effect of Spirulina on Blood Glucose Level |
The first and second groups showed average blood glucose values below 100 mg/dl before and after a month of treatment. The third vehicle-treated diabetic group showed a significant rise in blood glucose after 1 month above 250 mg/dl (P ˂ 0.001). However, the fourth Spirulina platensis oral powder-treated diabetic group revealed better control of blood sugar to below 150 mg/dl (P ˂ 0.01), as shown in Table 1.
During the blood glucose measuring experiment, we observed a significant decrease in blood glucose in diabetic rats with Spirulina compared with the diabetic non-treated group, as shown in Table 1.
Regarding Body weight gain in our examined rats, the Spirulina platensis-treated diabetic group had gained weight after 4 weeks, (P = 0.066), as well values of the control Spirulina platensis-treated group showed (P = 0.028). However, the third vehicle-treated diabetic group showed insignificant weight gain (P = 0.332) as shown in Table 2.
 |
Table 2 Effect of Spirulina on Weight Gain |
During the formalin test experiment, we observed that the diabetic rats’ group showed an elevated pain score in both phases. We observed a reduced level of pain score for both formalin test phases in Spirulina platensis - received diabetic rats in comparison to untreated-diabetic animals, P < 0.01. Also, SP-treated rats had lower pain scores during both phases of the experiment than untreated diabetic ones.
However, in the sodium salicylate (positive control) group, the pain score was reduced only during the second phase. At the same time, an exaggerated nociceptive response occurred in diabetic rats after the formalin test. A significant antinociceptive effect appeared in SP-treated control and diabetic rats, as shown in Figure 2.
Discussion
The present study revealed that the administration of Spirulina platensis orally could attenuate diabetes with improved blood glucose levels and weight gain. Implementation of the formalin test demonstrated a pronounced antinociceptive response in control and diabetic rats at both phases of that test.
Spirulina platensis has many health benefits: antioxidant advantages,10 anti-inflammatory activity,9 anti-diabetic actions,12,13 and hypocholesterolemic.19
Pain is categorized depending on its cause, as the first type of nociceptive, second type as neuropathic or mixed of both forms.20 Different modalities of treating diabetic neuropathy were described in animal models. Anticonvulsants, especially gabapentin and carbamazepine, and opioids were ultimately used.21
Regarding its anti-inflammatory characteristics, Shih et al9 had reported that C-phycocyanin, which was an extract of Spirulina platensis, revealed anti-inflammatory and anti-hyperalgesic effects. Another experimental study demonstrated a significant decrease in inflammatory mediators’ activities, for instance, IL-6 and IL-1β caused by Spirulina platensis. A mouse model of zymosan-induced arthritis explored this anti-inflammatory activity.22
Chen et al23 also demonstrated: the microglial cells exhibited enormous anti-inflammatory activity of phycocyanin material and Spirulina. They concluded that both materials significantly inhibited the release of lipopolysaccharide-induced lactate dehydrogenase. They suppressed inflammatory mediators’ expression, for instance, iNOS, IL-6, COX-2, and TNF-α.
Regarding the anti-diabetic effect of Spirulina platensis, we think that the insulin-like effect of Spirulina platensis may be responsible for diabetic state attenuation of the diabetic treated rats as consistent with previous studies.5,13 Li et al discovered that Spirulina protects pancreatic Beta cells, which could explain blood glucose control.24 Moreover, Spirulina’s active materials include immunoreactive insulin; therefore, diabetic subjects may get a crucial benefit.25 In our model attenuation of diabetic state, we observed weight gain in Spirulina platensis-treated diabetic rats.
Spirulina platensis antioxidant effect was reported before.11 Usage of Spirulina as a nutritional supplement a long time ago was attributed to its structure as filamentous cyanobacteria.26 Phycocyanin is one of the significant constituents of Spirulina platensis. The tetrapyrrole chromophore gives its deep blue color of phycocyanin. Moreover, phycocyanobilin is covalently connected to the apoprotein. The phycocyanin conveys inhibition of oxygen free radical production. Spirulina platensis attributes its promising antioxidant properties to phycocyanobilin.26
Spirulina antioxidant properties can be activated through various pathways. First is through activation of the scavenging system, and secondly, it is by preventing DNA damage and lipid peroxidation. Moreover, it augments the activity of superoxide dismutase, catalase, and suppresses reactive oxygen species.27 It was proved that Spirulina organizes the IκB signaling as well the p38 pathways to exhibit its free radicals scavenging, anti-inflammatory and immunomodulatory properties.28
Treatment with Spirulina significantly inhibits oxygen free radicals as many experimental studies reported it. These protective effects may be attributed to phycocyanins, β-carotene, and other substances included within Spirulina.14
One study exposed neuroblastoma cells to oxidative stress injury and explored Spirulina platensis suppress lipid peroxidation. Bermejo-Bescóset et al discussed the impact of Spirulina antioxidant activity on glutathione reduction and peroxidation enzymes.11
Interestingly, when additional environmental stress factor is excited, the protective ability of Spirulina platensis could be boosted.27 Therefore, these promising algae could be crucial agents for the management of disorders aggravated by reactive oxygen species and the creation of novel treatment evolution.
Moreover, Spirulina safeguards against neurotoxicity in rats by inhibiting oxygen free radicals.14 Neurotoxicity rat – model was induced using 6-hydroxydopamine and revealed the antagonizing effect of Spirulina. As consistent with our study, which showed a reduction in diabetes mellitus induced neuropathy in a painful rat model.29
Schmeichel30 et al demonstrated that an imbalance between reactive oxygen species and its detoxification in chronic hyperglycemia could be crucial for neuropathy in animal models. Moreover, neuronal cell damage in diabetes is guided by programmed cell death through the unifying mechanism by supporting glial cells.31
Production of oxygen free radicals influenced mitochondrial dysfunction, which contributes markedly to neuropathy.32 On the other hand, programmed cell death of the peripheral neurons results from sudden glycemic deprivation and is expected to be through oxidative stress mechanism.33
Chronic hyperglycemia does not only induce neuron injury but impairs the small-caliber neurons to grow and regenerate. Diabetic neuropathy in those subjects is an enormously dynamic process and moves simultaneously towards both edges of degeneration and regeneration. Moreover, targets of any therapeutic regimens for neuropathy disorder are creating equilibrium on the side of regeneration and avoid dysregulation of this balance.34,35 Russell et al31 have encouraged this hypothesis, including the measurement of ROS mediators in neurons of sensory origin. He illustrated using antioxidants in the prevention of neuron injury. Moreover, Spirulina and C-phycocyanin inhibit toxic effects and inflammatory genes expression of neuronal supporting cells which explains the reduction of neurotoxicity in neuronal cells.23 We observed the anti-nociception effect of Spirulina platensis in our diabetic neuropathic rat model.
Regarding treating neurotoxicity, a study of Hwang et al demonstrated36 that salicylate administration results in a marked rise in IL-1β mRNAs expression, TNF-α as well as NMDA receptor in the inner ear neurons of mice. Use of Spirulina as a dietary supplementation significantly decreased the toxicity-induced tinnitus. Moreover, it suppresses the mRNAs expression of the previous inflammatory mediators in their mouse model.
Further proof comes from the dietary supplementation of mice with a hyperglycemic diet. In this stressful condition, the animals develop hyperglycemia that induces oxygen-free radicals production and impairs scavenging.37 This poor glycemic control worsens the disease progression and augments consequences.38
If we can use nutritional supplement potential like Spirulina platensis, which attenuate elevated blood glucose levels,5 potentiates anti-inflammatory,7 antihyperalgesic,9 and induce antioxidants effects against oxygen free radicals.11 It will be our most promising approach by using these microalgae to fight neuropathy, attenuate diabetic state, as well as other miserable consequences in diabetes as neuropathy.
In the field of Spirulina researches, there are enormous experimental studies, hoverer, few human studies have been established to examine its anti-inflammatory abilities or antioxidant properties.
A volunteer human study in the elderly populations revealed the impact of antioxidant activities on their immunological parameters.39 Park et al demonstrated that using dietary supplementation of Spirulina evoked a striking increase in the level of interleukin-2, while simultaneously decreasing IL-6 levels. Moreover, researchers observed a progressive increase in superoxide dismutase enzyme activity is proportional to a time-dependent change in the antioxidant level. Spirulina was tested in Diabetes Mellitus Type II patients, in terms of lowering lipid profile. However, in those patients, oral Spirulina did not affect glucose.
On the other hand, another clinical study investigated diabetic patients and followed their HbA1C and serum lipids, which revealed a marked reduction of these variables after Spirulina’s consumption for a few months.5 Shetty et al, when used Spirulina as an adjuvant antioxidant and implement its use in patients with oral submucous fibrosis, succeed in getting improvement.40
Other studies revealed that muscle destruction produced by oxidative stress could be inhibited after Spirulina administration.41,42 Their results demonstrated that MDA levels were markedly inhibited, while superoxide dismutase enzyme levels were significantly enhanced in those subjects who had taken Spirulina.
Because of Spirulina dietary supplementation is not completely established as a potential therapy, more clinical trials are warranted. Moreover, the antioxidant advantages of painful diabetic neuropathy were not touched, as we examined in our experimental model.
One of our limitations in this experimental study that we did not measure reactive species in neurons of either subject with diabetes treated and diabetic non-treated rats with Spirulina platensis. Therefore, we recommend a future comprehensive study, including serum and neuronal level of ROS after administration of Spirulina to our painful neuropathy rat model as a preliminary step before the human study.
Conclusion
The results of the present study demonstrated attenuated diabetic status and antinociceptive effect of orally administrated Spirulina platensis for 1 month to the painful diabetic neuropathic rat model. The biochemical measurement of ROS in a future study is warranted.
Acknowledgments
We appreciate the great effort of Dr. Daniel Mandell, Anesthesiology Dept., University of Pittsburgh Medical Center, USA, for his English editing of our manuscript. Authors thank Dr Brian Gunn for his efforts in revising our manuscript and Dr Hanaa HEGO for her statistical help. Authors acknowledge Open Access funding provided by the Qatar National Library. Previous Presentation in Conferences: Part of the study results was presented at European society of anesthesia (ESA), Stockholm, Sweden, June 2014.
Authorship
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval of the version to be published; and agree to be accountable for all aspects of the work.
Disclosure
The authors did not receive any grant or financial support for this study. The authors report no conflicts of interest for this work.
References
1. Rudroju N, Bansal D, Talakokkula ST, et al. Comparative efficacy and safety of six antidepressants and anticonvulsants in painful diabetic neuropathy: a network meta-analysis. Pain Physician. 2013;16:E705–E714.
2. Rosen P, Nawroth PP, King G, Moller W, Tritschler HJ, Packer L. The role of oxidative stress in the onset and progression of diabetes and its complications: a summary of a congress series sponsored by UNESCO-MCBN, the American Diabetes Association and the German Diabetes Society. Diabetes Metab Res Rev. 2001;17:189–212.
3. Sampson MJ, Gopaul N, Davies IR, Hughes DA, Carrier MJ. Plasma F2 isoprostanes: direct evidence of increased free radical damage during acute hyperglycemia in type 2 diabetes. Diabetes Care. 2002;25:537–541. doi:10.2337/diacare.25.3.537
4. Kulshreshtha A, Zacharia AJ, Jarouliya U, Bhadauriya P, Prasad GB, Bisen PS. Spirulina in health care management. Curr Pharm Biotechnol. 2008;9:400–405. doi:10.2174/138920108785915111
5. Parikh P, Mani U, Iyer U. Role of spirulina in the control of glycemia and lipidemia in type 2 diabetes mellitus. J Med Food. 2001;4:193–199. doi:10.1089/10966200152744463
6. Ichimura M, Kato S, Tsuneyama K, et al. Phycocyanin prevents hypertension and low serum adiponectin level in a rat model of metabolic syndrome. Nutr Res. 2013;33:397–405. doi:10.1016/j.nutres.2013.03.006
7. Romay C, Gonzalez R, Ledon N, Remirez D, Rimbau V. C-phycocyanin: a biliprotein with antioxidant, anti-inflammatory and neuroprotective effects. Curr Protein Pept Sci. 2003;4:207–216. doi:10.2174/1389203033487216
8. Reddy CM, Bhat VB, Kiranmai G, Reddy MN, Reddanna P, Madyastha KM. Selective inhibition of cyclooxygenase-2 by C-phycocyanin, a biliprotein from Spirulina platensis. Biochem Biophys Res Commun. 2000;277:599–603. doi:10.1006/bbrc.2000.3725
9. Shih CM, Cheng SN, Wong CS, Kuo YL, Chou TC. Antiinflammatory and antihyperalgesic activity of C-phycocyanin. AnesthAnalg. 2009;108:1303–1310.
10. Benedetti S, Benvenuti F, Pagliarani S, Francogli S, Scoglio S, Canestrari F. Antioxidant properties of a novel phycocyanin extract from the blue-green alga Aphanizomenonflos-aquae. Life Sci. 2004;75:2353–2362. doi:10.1016/j.lfs.2004.06.004
11. Pinero Estrada JE, Bermejo Bescos P, Villar Del Fresno AM. Antioxidant activity of different fractions of Spirulina platensis protean extract. Farmaco. 2001;56:497–500. doi:10.1016/S0014-827X(01)01084-9
12. Jung M, Park M, Lee HC, Kang YH, Kang ES, Kim SK. Antidiabetic agents from medicinal plants. Curr Med Chem. 2006;13:1203–1218. doi:10.2174/092986706776360860
13. Muthuraman P, Senthilkumar R, Srikumar K. Alterations in beta-islets of Langerhans in alloxan-induced diabetic rats by marine Spirulina platensis. J Enzyme Inhib Med Chem. 2009;24:1253–1256. doi:10.3109/14756360902827240
14. Abdel-Daim MM, Abuzead SM, Halawa SM. Protective role of Spirulina platensis against acute deltamethrin-induced toxicity in rats. PLoS One. 2013;8:e72991. doi:10.1371/journal.pone.0072991
15. Zhang S, Xu H, Yu X, Wu Y, Sui D. Metformin ameliorates diabetic nephropathy in a rat model of low-dose streptozotocin-induced diabetes. Exp Ther Med. 2017;14:383–390. doi:10.3892/etm.2017.4475
16. Takeda Y, Shimomura T, Asao H, Wakabayashi I. Relationship between immunological abnormalities in rat models of diabetes mellitus and the amplification circuits for diabetes. J Diabetes Res. 2017;2017:4275851. doi:10.1155/2017/4275851
17. Dubuisson D, Dennis SG. The formalin test: a quantitative study of the analgesic effects of morphine, meperidine, and brain stem stimulation in rats and cats. Pain. 1977;4:161–174. doi:10.1016/0304-3959(77)90130-0
18. Coderre TJ, Fundytus ME, McKenna JE, Dalal S, Melzack R. The formalin test: a validation of the weighted-scores method of behavioural pain rating. Pain. 1993;54:43–50. doi:10.1016/0304-3959(93)90098-A
19. Nagaoka S, Shimizu K, Kaneko H, et al. A novel protein C-phycocyanin plays a crucial role in the hypocholesterolemic action of Spirulina platensis concentrate in rats. J Nutr. 2005;135:2425–2430. doi:10.1093/jn/135.10.2425
20. Mackey S, Carroll I, Emir B, Murphy TK, Whalen E, Dumenci L. Sensory pain qualities in neuropathic pain. J Pain. 2012;13:58–63. doi:10.1016/j.jpain.2011.10.002
21. Wallace MS. Diagnosis and treatment of neuropathic pain. CurrOpinAnaesthesiol. 2005;18:548–554.
22. Remirez D, Gonzalez R, Merino N, Rodriguez S, Ancheta O. Inhibitory effects of Spirulina in zymosan-induced arthritis in mice. Mediators Inflamm. 2002;11:75–79. doi:10.1080/09629350220131917
23. Chen JC, Liu KS, Yang TJ, Hwang JH, Chan YC, Lee IT. Spirulina and C-phycocyanin reduce cytotoxicity and inflammation-related genes expression of microglial cells. Nutr Neurosci. 2012;15:252–256.
24. Li XL, Xu G, Chen T, et al. Phycocyanin protects INS-1E pancreatic beta cells against human islet amyloid polypeptide-induced apoptosis through attenuating oxidative stress and modulating JNK and p38 mitogen-activated protein kinase pathways. Int J Biochem Cell Biol. 2009;41:1526–1535. doi:10.1016/j.biocel.2009.01.002
25. Khursheed S, Anwer R, Zutshi S, Fatma T. Screening of photosynthetic O2 -evolving prokaryotes for an insulin-like antigen(1). J Phycol. 2012;48:243–245. doi:10.1111/j.1529-8817.2011.01086.x
26. Wu Q, Liu L, Miron A, Klimova B, Wan D, Kuca K. The antioxidant, immunomodulatory, and anti-inflammatory activities of Spirulina: an overview. Arch Toxicol. 2016;90:1817–1840. doi:10.1007/s00204-016-1744-5
27. Abdelkhalek NK, Ghazy EW, Abdel-Daim MM. Pharmacodynamic interaction of Spirulina platensis and deltamethrin in freshwater fish Nile tilapia, Oreochromis niloticus: impact on lipid peroxidation and oxidative stress. Environ Sci Pollut Res Int. 2015;22:3023–3031. doi:10.1007/s11356-014-3578-0
28. Khan M, Varadharaj S, Ganesan LP, et al. C-phycocyanin protects against ischemia-reperfusion injury of heart through involvement of p38 MAPK and ERK signaling. Am J Physiol Heart Circ Physiol. 2006;290:H2136–H2145. doi:10.1152/ajpheart.01072.2005
29. Tobon-Velasco JC, Palafox-Sanchez V, Mendieta L, et al. Antioxidant effect of Spirulina (Arthrospira) maxima in a neurotoxic model caused by 6-OHDA in the rat striatum. J Neural Transm (Vienna). 2013;120:1179–1189. doi:10.1007/s00702-013-0976-2
30. Schmeichel AM, Schmelzer JD, Low PA. Oxidative injury and apoptosis of dorsal root ganglion neurons in chronic experimental diabetic neuropathy. Diabetes. 2003;52:165–171. doi:10.2337/diabetes.52.1.165
31. Russell JW, Sullivan KA, Windebank AJ, Herrmann DN, Feldman EL. Neurons undergo apoptosis in animal and cell culture models of diabetes. Neurobiol Dis. 1999;6:347–363. doi:10.1006/nbdi.1999.0254
32. Russell JW, Golovoy D, Vincent AM, et al. High glucose-induced oxidative stress and mitochondrial dysfunction in neurons. FASEB J. 2002;16:1738–1748. doi:10.1096/fj.01-1027com
33. Honma H, Podratz JL, Windebank AJ. Acute glucose deprivation leads to apoptosis in a cell model of acute diabetic neuropathy. J Peripher Nerv Syst. 2003;8:65–74. doi:10.1046/j.1529-8027.2003.03009.x
34. Polydefkis M, Griffin JW, McArthur J. New insights into diabetic polyneuropathy. JAMA. 2003;290:1371–1376. doi:10.1001/jama.290.10.1371
35. Apfel SC. Nerve regeneration in diabetic neuropathy. Diabetes Obes Metab. 1999;1:3–11.
36. Hwang JH, Chen JC, Chan YC. Effects of C-phycocyanin and Spirulina on salicylate-induced tinnitus, expression of NMDA receptor and inflammatory genes. PLoS One. 2013;8:e58215. doi:10.1371/journal.pone.0058215
37. Folmer V, Soares JC, Rocha JB. Oxidative stress in mice is dependent on the free glucose content of the diet. Int J Biochem Cell Biol. 2002;34:1279–1285. doi:10.1016/S1357-2725(02)00065-1
38. Tsai EC, Hirsch IB, Brunzell JD, Chait A. Reduced plasma peroxyl radical trapping capacity and increased susceptibility of LDL to oxidation in poorly controlled IDDM. Diabetes. 1994;43:1010–1014. doi:10.2337/diab.43.8.1010
39. Park HJ, Lee YJ, Ryu HK, Kim MH, Chung HW, Kim WY. A randomized double-blind, placebo-controlled study to establish the effects of spirulina in elderly Koreans. Ann Nutr Metab. 2008;52:322–328.
40. Shetty P, Shenai P, Chatra L, Rao PK. Efficacy of spirulina as an antioxidant adjuvant to corticosteroid injection in management of oral submucous fibrosis. Indian J Dent Res. 2013;24:347–350. doi:10.4103/0970-9290.118001
41. Lu HK, Hsieh CC, Hsu JJ, Yang YK, Chou HN. Preventive effects of Spirulina platensis on skeletal muscle damage under exercise-induced oxidative stress. Eur J Appl Physiol. 2006;98:220–226. doi:10.1007/s00421-006-0263-0
42. Kalafati M, Jamurtas AZ, Nikolaidis MG, et al. Ergogenic and antioxidant effects of spirulina supplementation in humans. Med Sci Sports Exerc. 2010;42:142–151. doi:10.1249/MSS.0b013e3181ac7a45
 © 2020 The Author(s). This work is published by Dove Medical Press Limited, and licensed under a Creative Commons Attribution License.
The full terms of the License are available at http://creativecommons.org/licenses/by/4.0/.
The license permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
© 2020 The Author(s). This work is published by Dove Medical Press Limited, and licensed under a Creative Commons Attribution License.
The full terms of the License are available at http://creativecommons.org/licenses/by/4.0/.
The license permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.

