Back to Journals » Clinical Epidemiology » Volume 10
Oral metronidazole use and risk of acute pancreatitis: a population-based case-control study
Authors Barbulescu A, Oskarsson V , Lindblad M , Ljung R , Brooke HL
Received 13 December 2017
Accepted for publication 11 May 2018
Published 25 October 2018 Volume 2018:10 Pages 1573—1581
DOI https://doi.org/10.2147/CLEP.S159702
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Irene Petersen
Andrei Barbulescu,1 Viktor Oskarsson,2 Mats Lindblad,3,4 Rickard Ljung,1 Hannah L Brooke1
1Unit of Epidemiology, Institute of Environmental Medicine, Karolinska Institutet, Stockholm, Sweden; 2Unit of Nutritional Epidemiology, Institute of Environmental Medicine, Karolinska Institutet, Stockholm, Sweden; 3Division of Surgery, Department of Clinical Science Intervention and Technology, Karolinska Institutet, Stockholm, Sweden; 4Centre for Digestive Diseases, Karolinska University Hospital, Stockholm, Sweden
Objective: Oral metronidazole used in combined regimens for Helicobacter pylori eradication has been associated with an increased risk of acute pancreatitis; however, it is less clear whether a similar association exists for single-regimen metronidazole. We, therefore, examined the association of single and combined regimens of oral metronidazole with risk of acute pancreatitis.
Methods: In this population-based case-control study, all individuals in Sweden (aged 40–84 years) hospitalized with acute pancreatitis between January 2006 and December 2008 were identified from a national hospital register (n=5,996). Controls, matched for calendar year, age, and sex, were randomly sampled from a national population register (n=60,681). Data on oral metronidazole and covariates were extracted from national health and prescription registers. Odds ratios (ORs) of acute pancreatitis, according to timing of the latest metronidazole prescription before hospitalization, were estimated using logistic regression models. Confounding by indication was examined by contrasting the main results with the association when amoxicillin was used as exposure. The robustness of results was examined by calculating incidence rate ratios using a self-controlled case series approach.
Results: After adjustment for potential confounders, there was a substantially increased risk of acute pancreatitis within 30 days of oral metronidazole exposure, both for single (OR: 4.06; 95% confidence interval [CI]: 1.90–8.64) and combined (OR: 11.80; 95% CI: 6.86–20.28) regimens, compared to nonexposure. In contrast, the adjusted OR was 1.79 (95% CI: 1.25–2.54) for current use of amoxicillin compared to nonexposure. These results were supported by the self-controlled cases series analysis (incidence rate ratio: 3.30; 95% CI: 2.69–4.06, for single and combined regimens of oral metronidazole pooled). There was no strong association between oral metronidazole and acute pancreatitis more than 30 days after exposure.
Conclusion: There was an increased risk of acute pancreatitis within 30 days of exposure to single and combined regimens of oral metronidazole. While reverse causality and confounding by indication cannot be entirely excluded, they are unlikely to fully explain the association. These results warrant an increased awareness among physicians.
Keywords: case–control studies, Helicobacter infections, drug therapy, metronidazole, adverse effects, pancreatitis, epidemiology, Sweden
Introduction
Acute pancreatitis is a painful condition associated with a mortality of up to 30%.1 Cholelithiasis and alcohol abuse account for approximately 70% of all cases, while drugs are thought to be responsible for up to 6% of all cases.2–4 To date, more than 500 drugs have been positively associated with risk of acute pancreatitis.5,6 However, most of these associations are based on individual case reports, with little evidence coming from clinical trials or observational studies.7
Metronidazole, a common antimicrobial drug used against several anaerobic bacteria and protozoan organisms,8 has the potential to penetrate the pancreas due to its high bioavailability after oral administration.9 Case reports have suggested that metronidazole might induce acute pancreatitis,10–13 with latency ranging from 12 hours to 38 days. Clinical outcomes and laboratory parameters improved following metronidazole discontinuation in all case reports, and some of them described recurrence of acute pancreatitis after rechallenge.10,11,13 In contrast, among 6,485 patients with metronidazole prescriptions recorded in US pharmacy databases, there were no hospitalizations for acute pancreatitis within 1 month of taking the drug.14 In the largest and most well-controlled study to date, a population-based case–control study from Denmark (n=3,083 cases of acute pancreatitis), the risk of acute pancreatitis in current users (<31 days) of oral metronidazole was 3 times the risk in those unexposed.15 However, in stratified analyses, only metronidazole used in combined regimens for Helicobacter pylori (H. pylori) eradication was associated with acute pancreatitis.15 Since this association could be attributed to the other drugs in the combinations or to the indication itself, the authors concluded that the role of metronidazole required further investigation.
The aim of this study was to further assess the association of oral metronidazole with risk of acute pancreatitis according to 1) timing of the latest metronidazole prescription before hospitalization and 2) single and combined metronidazole regimens.
Materials and methods
Study design
A nested case–control study, previously described in detail,16 was conducted within a dynamic source population, which consisted of all individuals aged 40–84 years living in Sweden during the study period, 1st January 2006 and 31st December 2008. The study was based on deidentified data from national registers. All individuals in the source population hospitalized with a first diagnosis of acute pancreatitis were included as cases. Ten controls for each case were randomly sampled from the same source population using frequency-based density sampling, matched for calendar year, sex, and age. Individuals with malignant neoplasms (except nonmelanoma skin cancer; as recorded in the Swedish Cancer Register) or pancreatic diseases (International Statistical Classification of Diseases and Related Health Problems [ICD]-10 codes K85, K86, K87 or ICD-9 code 577; as recorded in the Swedish National Patient Register) prior to the study were excluded. Follow-up was until the first episode of acute pancreatitis, diagnosis of malignant neoplasm as above, end of the study period, death, or emigration, whichever occurred first. For cases, the index date was the date of hospital admission for their first episode of acute pancreatitis. For controls, the index date was a random date from the matched calendar year.
Data sources
The National Patient Register, which has had an almost complete coverage of all hospitalized patients in Sweden since 1987, was used to identify diagnoses of acute pancreatitis and comorbid conditions.17 The nationwide Prescribed Drugs Register, which started in July 2005, was used to identify prescriptions for oral metronidazole, concomitant drugs, and drugs used as proxies for comorbid conditions.18 The Total Population Register was used to determine sex, age, country of birth, civil status, and date of emigration.19 The National Education Register was used to determine the highest level of education (as a proxy for socioeconomic position).20 The Cancer Register and the Cause of Death Register were used to identify diagnoses of malignant neoplasms and date of death, respectively.21,22
Outcome
The outcome was defined as a primary or secondary diagnosis of acute pancreatitis (ICD-10 code K85) in the National Patient Register. In previous work, we have shown that the National Patient Register has a high positive predictive value for diagnoses of acute pancreatitis, ranging from 83% (definitive acute pancreatitis) to 98% (probable acute pancreatitis).23
Exposure
Exposure was measured by dispensed prescriptions containing oral metronidazole (Anatomic Therapeutic Classification [ATC] code P01AB01) in the Prescribed Drugs Register. If a person had collected several metronidazole prescriptions, the latest prescription before index date was used. Metronidazole exposure was classified according to timing before index date as 1) reference – no prescription; 2) current – latest prescription within 30 days; 3) recent – latest prescription between 31 and 180 days; 4) past – latest prescription between 181 and 365 days; and 5) former – latest prescription earlier than 365 days.
Combined metronidazole regimens for the treatment of H. pylori were defined as at least 1 prescription of proton pump inhibitors (PPIs) (ATC code A02BC) in combination with amoxicillin (ATC code J01CA04), tetracycline (ATC code J01AA07), or macrolide (ATC code J01FA) antibacterials, or a fixed combination of these drugs (ATC code A02BD06), within 15 days before or after the latest metronidazole prescription. Combined metronidazole regimens for the treatment of intra-abdominal infections was defined as at least 1 prescription of β-lactam (ATC code J01C or J01D), aminoglycoside (ATC code J01G), quinolone (ATC code J01M), vancomycin (ATC code J01XA01), or combinations of sulfonamides and trimethoprim (ATC code J01EE) antibacterials within 15 days before or after the latest metronidazole prescription. Use of metronidazole outside such combinations was considered single regimen.
Statistical analysis
The association of oral metronidazole with risk of acute pancreatitis was estimated by odds ratios (ORs) using multivariate unconditional logistic regression models. The crude model contained only the matching variables, that is, sex, age at index date (5-year categories), and calendar year at index date. A second model additionally included the variables adjusted for in the previous case–control study on this topic,15 that is, diagnosis of inflammatory bowel disease, diagnosis of alcohol-related disease or treatment of alcohol dependence, diagnosis of cholelithiasis, and use of thiopurine or sulfasalazine drugs (Table S1 for all utilized ICD and ATC codes). A third model additionally included civil status (categorized as married, never married, or widowed/divorced), highest education (categorized as primary [≤9 years], secondary [10–12 years], or tertiary [>12 years]), country of birth (categorized as Sweden or other), diagnosis of diabetes or use of antidiabetic treatment, diagnosis of chronic obstructive pulmonary disease, diagnosis of gastrointestinal ulcer, and use of PPIs, H2-receptor blockers, nonsteroidal anti-inflammatory drugs, or opioid analgesics (Table S1 for all utilized ICD and ATC codes). A polypharmacy score (categorized as ≤4, 5–9 or ≥10 drugs) was also included in the third model as a proxy for other comorbid conditions. This score was created by summing the number of unique classes of drugs (first 4 positions of the ATC code), excluding those listed in Table S1, dispensed to each subject within 180 days before index date.24 All drug treatments, except the main exposure and those used in combination with ICD codes to define a comorbid condition, refer to prescriptions dispensed within 180 days before index date.
In supplementary analyses, the individuals with former exposure (>365 days) to metronidazole were reclassified as unexposed, replicating the previously published case–control study.15 A further analysis was conducted in a subsample of participants with index dates between January 2007 and December 2008, since this allowed for drug exposure to be correctly classified up to 548 days before index date. Separate analyses were performed in 1) individuals with single or multiple metronidazole prescriptions and 2) men and women. Also, to examine the possibility of confounding by indication, the analyses were repeated using a different antibacterial (amoxicillin, ACT codes J01CA04 and J01CR02) as the exposure.
Finally, the data were reanalyzed using a self-controlled case series design to examine the robustness of the study findings.25,26 With this approach, only the cases are included and act as their own controls, meaning that both measured and unmeasured confounding by time-invariant factors, are accounted for by the study design. We considered 30 days after a metronidazole prescription (both single and combined regimens) as the exposed period; all other periods were considered unexposed. Initially, all acute pancreatitis diagnoses during the study period were included, except those diagnosed within 7 days of an earlier diagnosis, since they were considered to be part of the first episode. However, since recurrent events may not be independent and the occurrence of the first event is rare, the analysis was also run using just the first event for each individual.27 Poisson regression models stratified by 5-year age groups and conditional on each individual were used to calculate incidence rate ratios (IRRs).27 An offset for the duration of each exposed or unexposed period was also included in the models, as was an indicator of the monthly average number of PPI prescriptions dispensed within each exposed or unexposed period. Since this method does not account for confounding by indication, which involves time varying factors, these analyses were also repeated using amoxicillin as the exposure.
All analyses were conducted using Stata 14.2 (StataCorp, College Station, TX, USA).
Results
After excluding 169 cases and 969 controls with incomplete information on covariates, a total of 5,996 cases of acute pancreatitis and 60,681 controls were included in the study. Just over half of the cases were men, with the largest proportion being 60–64 years old (Table 1).
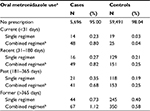
Compared with controls, a smaller proportion of cases were university educated, married, and born in Sweden (Table 1). However, cases were prescribed more drugs and had more comorbid conditions than controls. Overall, 5% of cases and 2% of controls were exposed to oral metronidazole before index date, of which 1% of cases and 0.07% of controls had been exposed within 30 days (Table 2).
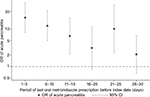
The odds of acute pancreatitis among current users of oral metronidazole (<31 days before index date) were 8 times the odds of acute pancreatitis among unexposed individuals (adjusted OR: 8.22; 95% confidence interval [CI]: 5.32–12.68) (Table 3). The association was more modest for recent (31–180 days), past (181–365 days), and former (>365 days) oral metronidazole use (adjusted ORs [95% CIs]: 1.38 [1.02–1.87], 1.62 [1.20–2.20] and 1.44 [1.15–1.79], respectively). As shown in Figure 1, the odds among current users were highest 1–5 days after exposure (OR: 20.47; 95% CI: 8.24–50.85) and decreased thereafter with some random variation.
Compared with unexposed individuals, the odds of acute pancreatitis was higher in individuals exposed to both single regimen (adjusted OR for current use: 4.06; 95% CI: 1.90–8.64) and, especially, combined regimens of oral metronidazole (adjusted OR for current use: 11.80; 95% CI: 6.86–20.28) (Table 4). Similar to the main analysis, there was only a modest association more than 30 days after metronidazole exposure both for single and combined regimens.
The association between current use of oral metronidazole and risk of acute pancreatitis did not change substantially in the supplementary analyses in which 1) individuals with former exposure to metronidazole were reclassified as unexposed; 2) only individuals with index dates between January 2007 and December 2008 were analyzed; and 3) individuals with single or multiple metronidazole prescriptions were analyzed separately (Table S2). However, the OR of acute pancreatitis was almost twice as large in men (adjusted OR: 11.39; 95% CI: 6.28–20.65) as in women (adjusted OR: 5.61; 95% CI: 2.93–10.73). In contrast to the 8-fold increased odds of acute pancreatitis for current use of oral metronidazole, the adjusted OR was 1.79 (95% CI: 1.25–2.54) for current use of amoxicillin compared to nonexposure.
Finally, using the self-controlled cases series approach, the risk of acute pancreatitis was 3 times higher within periods of exposure to oral metronidazole (<31 days of prescription) compared to the risk within periods of nonexposure (IRR: 3.30; 95% CI: 2.69–4.06). This estimate was slightly reduced by just including the first event for each individual (IRR: 2.47; 95% CI: 1.88–2.25). When amoxicillin was used as the exposure, the magnitude of the association was half that of metronidazole (IRR: 1.65; 95% CI: 1.31–2.07).
Discussion
In this large population-based case–control study, there was a substantially increased risk of acute pancreatitis within 30 days of exposure to oral metronidazole, both for single and combined regimens.
The results of the current study broadly support the findings of the only previous well-controlled study.15 However, the magnitude of the association for current exposure to oral metronidazole was more than 2 times higher in our study. The earlier study classified those exposed to oral metronidazole for more than 1 year before index date as unexposed, while we had them in a separate exposure category. Nonetheless, replicating that strategy did not result in matching effect sizes. In the previous study, the association between oral metronidazole and risk of acute pancreatitis was restricted to combined metronidazole regimens for H. pylori eradication. As such, it was not clear if the association was driven by metronidazole itself, other drugs in the combination, confounding by indication (eg, gastrointestinal ulcers leading to pancreatitis by penetration), detection bias (ie, patients being more frequently examined by gastroenterologists), or reverse causality (ie, metronidazole being prescribed for symptoms of acute pancreatitis that were misinterpreted as gastritis or diverticulitis). In contrast, we observed an association with acute pancreatitis that was not limited to combined metronidazole regimens. This finding is consistent with the possibility that metronidazole itself, at least in part, is responsible for the association. We cannot entirely rule out the presence of reverse causality, which may occur if treatment is empirical and not based on radiology and/or blood tests (eg, in a primary health care setting). Nonetheless, it seems unlikely that reverse causality explains the full magnitude of the observed association, especially considering the substantial increase in risk seen beyond the first 5 days after exposure. We hypothesized that confounding by indication would be more likely in combined oral metronidazole regimens, used for H. pylori and intra-abdominal infections, than in single regimens. As such, our finding of an association for both single and combined regimens reduces the possibility that confounding by indication is the sole explanation for the association. Nonetheless, since there was a modest association with acute pancreatitis when amoxicillin was used as the exposure, confounding by indication is still a possibility, assuming there is no true association with amoxicillin. However, the magnitude of the association with oral metronidazole was substantially larger than that with amoxicillin, especially in the case–control analysis, indicating that confounding by indication is unlikely to fully explain the association.
The risk of acute pancreatitis more than 30 days after exposure to oral metronidazole was modest, even though ORs larger than 1 were observed throughout the study period. This may be due to misclassification of confounder variables, unmeasured confounding, or random variation. However, since the estimates were close to the null value, the magnitude of confounding bias was most likely low and should not explain the strong association of current exposure to oral metronidazole with risk of acute pancreatitis.
Using a self-controlled case series approach supported the main findings from the case–control analyses of an increased risk of acute pancreatitis after exposure to oral metronidazole. The advantage of this approach is that both measured and unmeasured confounding by time-invariant factors are accounted for by the study design. However, as discussed, confounding by indication may partly explain the association of oral metronidazole with acute pancreatitis since indications are time-varying and thus not controlled by either method. Nonetheless, this is unlikely to be the only explanation.
The biological mechanisms behind an association between oral metronidazole and risk of acute pancreatitis are unknown, but an idiosyncratic immune-mediated reaction has been speculated.28,29 We observed that the association with current use of oral metronidazole differed by sex, which, in theory, could be the consequence of interactions between metronidazole and other risk factors that are unequally distributed between sexes. However, further research is required to understand these results in depth.
The strengths of the current study include the large sample size, which allowed for more precise estimation than previous studies, and the use of nationwide register-based data, which removed the possibility of selection and recall bias and led to good internal and external validity. In addition, by sampling controls randomly from the same source population as cases throughout the whole follow-up period, the ORs are fair approximations of IRRs. Nevertheless, some limitations of the study need to be acknowledged. First, despite the high positive predictive value of a recorded diagnosis of acute pancreatitis in the Swedish Patient Register, we had no access to medical charts, indicating potential outcome misclassification. Second, the specificity of the exposure measurement is expected to be reduced by imperfect adherence to prescriptions, while its sensitivity was inevitably reduced by the lack of information on in-hospital use of metronidazole.18 However, adherence to antimicrobial treatments, such as metronidazole, is, in general, good.30 Moreover, any such misclassification was most likely nondifferential between cases and controls in this prospective setting. Importantly, the ORs obtained using a subsample of individuals with index dates between 2007 and 2008, where drug exposure could be correctly classified within 548 days before index date, were consistent with those obtained using the whole sample. This indicates that the impact of exposure misclassification as a result of proximity to the start of the Swedish Prescribed Drugs Register was negligible.
Conclusion
In conclusion, in this large population-based case–control study, there was an increased risk of acute pancreatitis within 30 days of exposure to oral metronidazole, which was supported by a self-controlled case series analysis. The observed association withstood adjustment for a large number of potential confounders and was not limited to metronidazole used in combined regimens. While reverse causality and confounding by indication cannot be entirely excluded, they are unlikely to fully explain the association. These findings contribute to the evidence of a positive association between oral metronidazole and risk of acute pancreatitis, which warrants an increased awareness among physicians.
Ethics approval
The study was approved by the Regional Ethical Review Board in Stockholm, Sweden (ethical approval number 2008/1360-31/4).
Disclosure
Rickard Ljung is employed at the Swedish Medical Products Agency, Uppsala, Sweden; the views expressed in this paper do not necessarily represent the views of the agency. Rickard Ljung has received consultancy fees from Pfizer in 2016, not related to this project. The authors report no other conflicts of interest in this work.
References
Forsmark CE, Vege SS, Wilcox CM. Acute pancreatitis. N Engl J Med. 2016;375(20):1972–1981. | ||
Jones MR, Hall OM, Kaye AM, Kaye AD. Drug-induced acute pancreatitis: a review. Ochsner J. 2015;15(1):45–51. | ||
Vinklerová I, Procházka M, Procházka V, Urbánek K. Incidence, severity, and etiology of drug-induced acute pancreatitis. Dig Dis Sci. 2010;55(10):2977–2981. | ||
Vidarsdottir H, Möller PH, Vidarsdottir H, Thorarinsdottir H, Björnsson ES. Acute pancreatitis: a prospective study on incidence, etiology, and outcome. Eur J Gastroenterol Hepatol. 2013;25(9):1068–1075. | ||
Bergholm U, Langman M, Rawllins M, et al. Drug-induced acute pancreatitis. Pharmacoepidemiol Drug Safety. 1995;4(6):329–334. | ||
Nitsche C, Maertin S, Scheiber J, Ritter CA, Lerch MM, Mayerle J. Drug-induced pancreatitis. Curr Gastroenterol Rep. 2012;14(2):131–138. | ||
Tenner S. Drug induced acute pancreatitis: does it exist? World J Gastroenterol. 2014;20(44):16529–16534. | ||
Löfmark S, Edlund C, Nord CE. Metronidazole is still the drug of choice for treatment of anaerobic infections. Clin Infect Dis. 2010;50 (Suppl 1): S16–S23. | ||
Lamp KC, Freeman CD, Klutman NE, Lacy MK. Pharmacokinetics and pharmacodynamics of the nitroimidazole antimicrobials. Clin Pharmacokinet. 1999;36(5):353–373. | ||
O’Halloran E, Hogan A, Mealy K. Metronidazole-induced pancreatitis. HPB Surg. 2010;2010:523468. | ||
Wong K, Nabeel M. A rare culprit in pancreatitis. Am J Gastroenterol. 2015;110(Suppl 1):S64. | ||
Cabrera R, Caverzagie K. Metronidazole-induced pancreatitis in a patient treated for acute diverticulitis. J Gen Intern Med. 2011;26:S496. | ||
Yilmaz M, Kinikoglu O, Ceyla B, Arslan F, Mert A. Recurrent pancreatitis induced by metronidazole re-exposure and a review of the current literature. Acta Gastroenterol Belg. 2016;79(3):389–390. | ||
Friedman GD, Selby JV. How often does metronidazole induce pancreatitis? Gastroenterology. 1990;98(6):1702–1703. | ||
Nørgaard M, Ratanajamit C, Jacobsen J, Skriver MV, Pedersen L, Sørensen HT. Metronidazole and risk of acute pancreatitis: a population-based case-control study. Aliment Pharmacol Ther. 2005;21(4):415–420. | ||
Ljung R, Lagergren J, Bexelius TS, Mattsson F, Lindblad M. Increased risk of acute pancreatitis among tetracycline users in a Swedish population-based case-control study. Gut. 2012;61(6):873–876. | ||
Ludvigsson JF, Andersson E, Ekbom A, et al. External review and validation of the Swedish national inpatient register. BMC Public Health. 2011;11:450. | ||
Wettermark B, Hammar N, Fored CM, et al. The new Swedish Prescribed Drug Register – opportunities for pharmacoepidemiological research and experience from the first 6 months. Pharmacoepidemiol Drug Saf. 2007;16(7):726–735. | ||
Ludvigsson JF, Almqvist C, Bonamy AK, et al. Registers of the Swedish total population and their use in medical research. Eur J Epidemiol. 2016;31(2):125–136. | ||
Swedish Register of education. Joint Programming Initiative. Available from: http://www.jpi-dataproject.eu/Home/Database/348?topicId=4. Accessed March 29, 2017. | ||
Barlow L, Westergren K, Holmberg L, Talbäck M. The completeness of the Swedish Cancer Register: a sample survey for year 1998. Acta Oncol. 2009;48(1):27–33. | ||
Brooke HL, Talbäck M, Hörnblad J, et al. The Swedish cause of death register. Eur J Epidemiol. 2017;32(9):765–773. | ||
Razavi D, Ljung R, Lu Y, Andrén-Sandberg A, Lindblad M. Reliability of acute pancreatitis diagnosis coding in a National Patient Register: a validation study in Sweden. Pancreatology. 2011;11(5):525–532. | ||
Razavi D, Lindblad M, Bexelius T, Oskarsson V, Sadr-Azodi O, Ljung R. Polypharmacy and risk of acute pancreatitis. Pharmacoepidemiol Drug Saf. 2016;25(11):1337–1341. | ||
Petersen I, Douglas I, Whitaker H. Self controlled case series methods: an alternative to standard epidemiological study designs. BMJ. 2016;354:i4515. | ||
Lawlor DA, Tilling K, Davey Smith G. Triangulation in aetiological epidemiology. Int J Epidemiol. 2016;45(6):1866–1886. | ||
Whitaker HJ, Farrington CP, Spiessens B, Musonda P. Tutorial in biostatistics: the self-controlled case series method. Stat Med. 2006;25(10):1768–1797. | ||
Badalov N, Baradarian R, Iswara K, Li J, Steinberg W, Tenner S. Drug-induced acute pancreatitis: an evidence-based review. Clin Gastroenterol Hepatol. 2007;54(6):648–661. | ||
Ksiądzyna D. Drug-induced acute pancreatitis related to medications commonly used in gastroenterology. Eur J Intern Med. 2011;22(1):20–25. | ||
Jin J, Sklar GE, Min Sen Oh V, Chuen Li S. Factors affecting therapeutic compliance: a review from the patient’s perspective. Ther Clin Risk Manag. 2008;4(1):269–286. |
Supplementary materials
 © 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.