Back to Journals » OncoTargets and Therapy » Volume 16
Oral Anlotinib Maintenance Therapy for an Advanced Malignant Peritoneal Mesothelioma Diagnosed by Laparoscopy After Initial Misdiagnosis to Obtain Longer Progression-Free Survival: Case Report and Literature Review
Authors Qu FJ , Zhou Y, Wang H
Received 9 August 2023
Accepted for publication 9 November 2023
Published 15 November 2023 Volume 2023:16 Pages 961—972
DOI https://doi.org/10.2147/OTT.S430190
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Arseniy Yuzhalin
Fan-Jie Qu,1,* Yi Zhou,1,* Hai Wang2
1Department of Oncology, Affiliated Dalian Third People’s Hospital of Dalian Medical University, Dalian, Liaoning Province, 116033, People’s Republic of China; 2Department of Pathology, Affiliated Dalian Third People’s Hospital of Dalian Medical University, Dalian, Liaonin Provinceg, 116033, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Fan-Jie Qu, Department of Oncology, Affiliated Dalian Third People’s Hospital of Dalian Medical University, No. 40 Qianshan Road, Dalian, Liaoning Province, 116033, People’s Republic of China, Email [email protected]
Abstract: Malignant peritoneal mesothelioma (MPeM) is a rare and highly invasive malignant tumor with a lack of specificity in clinical manifestations, which can easily lead to misdiagnosis and missed diagnosis. Due to the difficulty of early diagnosis, most patients are already in the advanced stage when diagnosed, and the prognosis is poor. At present, there is no standard treatment strategy, and the existing treatment methods are not effective, the duration of remission is short, which cannot meet the clinical needs. Here we describe a patient with advanced MPeM, initially misdiagnosed as ovarian cancer, who responded to treatment with bevacizumab in combination with albumin-bound paclitaxel and cisplatin. In preparation for cytoreductive surgery (CRS), MPeM was confirmed by laparoscopic peritoneal nodule biopsy combined with histological and immunohistochemical results. Subsequently, due to intolerable neurotoxicity after chemotherapy, she received oral anlotinib therapy on April 25, 2022, and remained stable disease (SD) with the medication, having achieved more than 14 months of progression-free survival (PFS) as of the date of our manuscript submission. The patient’s total treatment time was over 19 months. These treatments delayed tumor progression, reduced drug side effects, maintained a good quality of life, and further extended overall survival (OS). Our experience is that on the one hand, it is necessary to increase the clinician’s understanding of the disease, and make full use of tissue samples and immunohistochemical staining to reduce the occurrence of misdiagnosis. On the other hand, based on preliminary evidence, we found that oral anlotinib offers a viable maintenance treatment strategy for patients with advanced mesothelioma, which needs to be further explored in future studies.
Keywords: MPeM, misdiagnosis, anlotinib, maintenance therapy, progression-free survival
Introduction
Malignant peritoneal mesothelioma (MPeM) is a rare, highly malignant tumor with an incidence of 0.41 to 1.9 per 1 million. Early diagnosis is difficult, and most patients are already in the advanced stage at the time of diagnosis, with a poor prognosis.1 Most of the clinical symptoms of MPeM are non-specific, and the diversity of clinical manifestations mainly depends on the degree of intraperitoneal tumor spread. There are often abdominal fluid accumulation, abdominal mass, abdominal distension, abdominal pain and intestinal obstruction clinically,2,3 which are very similar to the clinical manifestations of abdominal metastasis of ovarian cancer and other malignant tumors, so it is easy to be misdiagnosed in daily work.4,5 The final diagnosis of MPeM depends on histopathological analysis and immunohistochemistry results.5–7
The treatment methods of MPeM include systemic chemotherapy, hyperthermic intraperitoneal chemotherapy (HIPEC), cytoreductive surgery (CRS), etc., but the current treatment strategy has not been unified. For operable patients, CRS combined with HIPEC can extend overall survival to more than 3 years.8,9 Unfortunately, the growth pattern of MPeM is different from that of other tumors in the abdominal cavity, and it often extensively involves the mesentery and parietal peritoneum. Even if the peritoneum is normal by naked eyes, it still has a pathologic positive rate of 54% under the optical microscope. Therefore, to achieve complete resection, extensive resection of the peritoneum and intestinal tube is required.10,11 At the same time, CRS/HIPEC combined treatment can lead to multiple complications such as intestinal leakage and bone marrow suppression, and not only the perioperative mortality is 1%-11%, but also the postoperative recurrence rate is as high as 35%.5,12 The overall prognosis of patients with postoperative recurrence and most patients who are already in the advanced stage when diagnosed is poor, with an average survival time of only 1 year and a 5-year survival rate of only 20%.7,13
Due to the rare occurrence and highly invasive nature of MPeM, it is difficult to conduct randomized clinical studies. Currently, there is no standard treatment plan, and most studies on the treatment of MPeM are extrapolated based on malignant pleural mesothelioma (MPlM).1 Some studies have shown that cisplatin combined with pemetrexed has the same efficacy as MPlM in the treatment of MPeM, and can be recommended as the first-line treatment for patients with unresectable MPeM.14,15 Due to the large adverse reaction and poor effect of systemic chemotherapy, local abdominal chemotherapy can be selected according to the patient’s condition. However, due to the lack of subsequent standard treatment, patients with drug resistance and adverse reactions have a poor prognosis.1,5
In recent years, molecularly targeted drugs and immunotherapy represented by immune checkpoint inhibitors (ICIs) have achieved great success in the field of cancer, and some treatments have also brought benefits to patients with malignant mesothelioma (MM), but their effectiveness and safety need to be repeatedly verified by further studies.16,17
This case is an elderly woman who was misdiagnosed with ovarian cancer based on imaging and cytology results at the initial diagnosis, and was finally diagnosed as epithelioid MPeM by laparoscopic peritoneal biopsy. There is currently no standard treatment option for advanced MPeM maintenance therapy. We evaluated the safety and efficacy of anlotinib maintenance in this advanced elderly MPeM patient who intolerable with chemotherapy and summarized the currently available evidence.
Case Presentation
A 66-year-old woman came to our hospital with abdominal distension for 1 month. About 1 month before the visit, the patient developed abdominal distension, which gradually worsened. Ultrasound examination revealed a large amount of abdominal fluid, and abdominal puncture and drainage were performed, and suspected cancer cells were detected in ascites.
Previous history: The patient underwent modified radical resection for right breast cancer on December 28, 2010 due to a right breast mass with a size of 1.5*1.0*1.0CM. The postoperative pathology was invasive lobular carcinoma with a histological grade of 2–3 and no axillary lymph node metastasis was found (0/12). Immunohistochemical results indicated ER (+), PR (-), HER2(-), Ki67(50%+). After 4 cycles of CE adjuvant chemotherapy, oral letrozole was taken for 5 years, and no signs of recurrence and metastasis were found during regular follow-up. She has a history of hypertension for more than 2 years. She denied any history of asbestos exposure or a family history of malignancy.
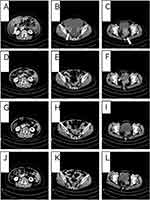
A baseline abdominal computed tomography (CT) (Figure 1A–C) examination of the patient revealed a large amount of fluid in the abdomen, cystic and solid mixed density masses in the perihepatic, intraperitoneal, pelvic, retroperitoneal, and bilateral adnexal areas, which were significantly enhanced after enhanced scanning, while the peritoneum was diffuse and thickened, and part of the greater omentum was cake shaped. After hospitalization, chest CT and gastroenteroscopy were completed and no signs of tumor were found. Assay CA125 greater than 1000u/mL.
On November 29, 2021, peritoneal puncture catheterization was performed, and the extracted ascites were sent for examination of shed cells several times, but only a small number of tumor cells were found in the ascites, and tumor cells were finally collected to prepare cell wax blocks. Immunohistochemical examination showed that HBME1(-) CK7(+) CEA (-) CK (+) WT-1(1% weak +) D2-40 (-) PAX-8(individual +), combined with immunohistochemical results, clinical images and tumor markers, reproductive origin tumors were considered, and mesothelial origin tumors were not considered. The patient was therefore diagnosed with extensive abdominal metastasis of ovarian cancer.
After the tumor was assessed as unresectable by a specialist in gynecologic oncology, we administered intraperitoneal chemotherapy combined with systemic chemotherapy combined with bevacizumab (bevacizumab 400mg IV on day 1+ albumin-bound paclitaxel 400mg IV on day 1+ Cisplatin 60mg intraperitoneal perfusion on day 1.8, q3w) on December 2, 2021. After 1 cycle of treatment, the patient’s abdominal distension was quickly relieved and ascites subsided significantly. CT reexamination after two cycles of treatment showed that ascites disappeared, abdominal and pelvic masses were significantly reduced, and we judged the efficacy as partial remission (PR) (Figure 1D–F). CA125 decreased significantly to 117 u/mL.
The gynecological oncologist was consulted again to evaluate the possibility of surgical resection and to prepare for cytoreductive surgery (CRS) after communicating with the patient. In the third cycle, bevacizumab was discontinued and cisplatin was adjusted to 30mg intravenous infusion on day 1–4. During chemotherapy, the patient developed grade 3 leukopenia, grade 2 gastrointestinal reactions, and grade 2 neurotoxicity. Laparoscopic exploration surgery was performed on February 21, 2022, during which uterine atrophy was observed with regular morphology, and no abnormalities were observed in the appearance of both fallopian tubes and ovaries, but multiple gray and white nodules were observed on the uterus, adnexa, mesentery, surface of liver, peritoneum, and surface of the omentum. The omentum was in the shape of cakes and was extensively attached to the bowel. After laparoscopic evaluation, it was found that the lesions were extensive, and surgical resection was difficult with little possible benefit. After communicating with the family again, cytoreductive surgery (CRS) was abandoned, and only peritoneal nodule biopsy was completed.
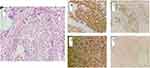
Postoperative pathology (Figure 2A) showed (peritoneum)malignant tumor. Hematoxylin and eosin staining showed that tumor cells were arranged like nests without obvious papillary structure. Meanwhile, tumor cells were epithelioid, a few were fusiform, the cytoplasm was hyaline, and the nucleus was obviously heterogeneous. Immunohistochemical results (Figure 2B–E) showed CK5/6 (+), Calretinin (-), HBME1 (+), PAX - 8 (+), P53 (+ 80%), WT - 1 (Scattered +), CEA (-), D2-40 (-), ER (+ 80%), Vim (+), Combined with histological findings, the patient was eventually diagnosed with malignant peritoneal mesothelioma (mainly epithelial differentiation).
Considering that the initial chemotherapy regimen was effective, the original regimen was continued for 2 cycles. CT reexamination after 5 cycles showed that the efficacy remained PR (Figure 1G–I) and CA125 8 u/mL. However, at this time, the patient developed severe hand and foot numbness with tingling and did not recover during intermittent periods, so the patient refused to continue chemotherapy and asked to try oral drugs for maintenance therapy.
Due to the lack of standard treatment options, our team initiated a strategy for oral anlotinib maintenance therapy after reviewing the literature and communicating with patients. Therefore, treatment with oral anlotinib [10 mg once a day (qd) on days 1–14 every 3wk] was administrated on April 25, 2022.Regular follow-up during maintenance treatment, multiple CT reviews showed that the intraperitoneal lesions remained stable, and the overall evaluation was stable disease (SD) (Figure 1J–L). Meanwhile, the main toxicity in patients during this period is high blood pressure, which can be well controlled. Up to the time of submission of this paper, the performance status (PS) score of the patient remained 0, and the PFS has been obtained for more than 14 months after monotherapy anlotinib maintenance treatment, and the overall OS has been obtained for more than 19 months.
However, CA125 began to rise again in January 2023. CA125 was significantly elevated when the patient was first diagnosed, and gradually decreased to the normal range after receiving chemotherapy. It had been stable for a while, but it seemed to be on the rise recently (Figure 3). It is suggested that CA125 may be used as a biomarker to evaluate efficacy and monitor recurrence in MPeM patients. As the patient remained stable on imaging and had no associated symptoms, we continued the current maintenance therapy with anlotinib.
 |
Figure 3 Dynamic evolution of CA125 during the course of disease. |
Patient responses to all medications were classified and graded according to the Solid Tumor Response Evaluation Criteria (RECIST), and adverse events were classified and graded using the National Cancer Institute Standard for Common Terminology for Adverse Events (NCI-CTCAE) version 4.0. All procedures carried out in studies involving human participants comply with the ethical standards of institutions and/or national research councils and are in accordance with the Declaration of Helsinki (as amended in 2013). Informed consent was obtained prior to each treatment in this case report. Patients also sign informed consent forms for the release of relevant clinical and imaging data from their cases, included consent for the publication of the case details.
Discussion
Malignant mesothelioma (MM) is a highly malignant tumor originating from mesothelial cells and involving pleura, peritoneum, pericardium, ovary and vaginae of testis, etc. The pleura cavity is the most common and the peritoneum is relatively rare.5 Malignant peritoneal mesothelioma (MPeM) originates in the peritoneal lining and usually appears diffuse, spreading widely throughout the abdomen, with rare metastases spreading beyond the abdominal cavity.5,13
The etiology of MM is not fully understood, the main risk factor is asbestos exposure, about 80% of pleural mesothelioma patients may be associated with it, but only 50% of peritoneal mesothelioma patients have a history of asbestos exposure. In addition, the etiology of MM may also be related to infection, some organic chemicals, minerals, radiation, Simian Virus 40 (SV40) and genetic factors.13,18
MM presents different clinical symptoms depending on the location of the disease, and the diagnosis mainly depends on imaging and pathological examination. Typical imaging findings are localized or diffuse pleural or peritoneal thickening, which may be accompanied by multiple nodules of varying sizes. If patients have pleural or abdominal effusion, some of them can be definitively diagnosed by cytological examination, but the final diagnosis is based on histopathological analysis combined with immunohistochemical examination results.5,7
Since the incidence of MPeM is rare, and most MPeM are asymptomatic or have no specific symptoms in the early stage of the disease, the onset of the disease is insidious, and the diagnosis is usually in the advanced stage.5,13 The diversity of clinical manifestations mainly depends on the degree of intraperitoneal tumor spread, and often presents with abdominal fluid accumulation, abdominal mass, abdominal distension, abdominal pain and intestinal obstruction,2,3 which is very similar to the clinical manifestations of intraperitoneal metastasis of ovarian and other malignant tumors, so it is easy to be misdiagnosed in daily work.4,5
First of all, imaging examination is the main cause of misdiagnosis in patients without pathological examination, because typical imaging findings of mesothelioma are rarely seen in practice. Among them, MPeM needs to be distinguished from peritoneal metastasis of female ovarian cancer, peritoneal metastasis of male gastric cancer, lymphoma and tuberculous peritonitis.4,5 At the same time, the pathological diagnosis of MPeM is also easy to fall into the diagnostic trap. Misdiagnosis or missed diagnosis of cytology is another important factor leading to clinical misdiagnosis. In terms of cytological diagnosis, 60% to 70% of mesothelioma patients with peritoneal effusion can be diagnosed by detecting exfoliative cytology in ascites. However, only a very small number of malignant cells can be detected in ascites, and the positive detection rate of tumor cells is less than 51%.3
Some experts believe that the diagnosis of mesothelioma by cytology must meet the requirements of tumor cell atypia and mesothelioma characteristics, and the tumor cells express mesothelial cell-derived immune markers. For patients who cannot obtain pleural or peritoneal lesion tissues, if the number of mesothelioma cells is sufficient and representative, the diagnosis of MM can be made by preparing cell wax blocks for immunohistochemical examination and fluorescence in situ hybridization analysis, combined with clinical manifestations and imaging examinations.19
This case was initially misdiagnosed as ovarian cancer with peritoneal metastasis. On the one hand, it was misdiagnosed as adenocarcinoma due to the small number of tumor cells found in the cytological examination of ascites shedding, and the lack of typical mesothelioma features, which cells presented as morula cells with vacuoles in the cytoplasm. On the other hand, when collecting tumor cells from patients to prepare cell wax blocks and conducting immunohistochemical analysis, CK (+) CK7(+) CEA (-) HBME1 (-) WT-1(1% weak +) D2-40 (-) PAX-8(individual +) was indicated, among which mesothelioma derived immune markers were mostly negative. Eventually, combined with imaging and tumor markers, it was mistaken for ovarian cancer with peritoneal metastasis.
However, the final diagnosis of MPeM relies on histopathological analysis combined with immunohistochemical examination. In terms of histopathologic diagnosis, MM is characterized by bidirectional differentiation and is divided into three types, namely epithelial type, sarcomatous type and mixed type.6 Among them, epithelial type is the most common, accounting for 70%, which is arranged into glandular tube, papillary, cord and nest. The tumor cells are diverse and have obvious atypia.Because the histopathological manifestations of mesothelioma are very similar to those of adenocarcinoma in hematoxylin-eosin staining, the pathologist’s lack of knowledge or experience in the pathological features of the disease can still lead to misdiagnosis.6
In particular, epithelioid mesothelioma with tubular, papillary structure is very similar to ovarian serous cancer, because some high-grade serous cancers also have a variety of papillary, adenoid, and solid growth patterns. Immunohistochemical detection is an indispensable part of the diagnosis of MM. Immunohistochemical staining is used in the diagnosis and differential diagnosis of MM to distinguish epithelioid MM from various cancers (lung, breast, ovary, gastric).5,6
MM does not have specific antibodies. Mesothelioma not only expresses epithelial-derived immune markers such as CKpan, CK7, CK8/18, etc., but also mesothelial derived markers. Combined detection of multiple antibodies is often required for comprehensive diagnosis. Useful markers for the diagnosis of mesothelioma include calretinin, Wilms1 (WT-1), cytokeratin 5/6, Vimentin and podoplanin (D2-40). However, the sensitivity and specificity of single indicators are not 100%, so when all clinical manifestations, imaging and histological features are consistent, experts recommend the use of at least 2 mesothelioma and 2 cancer markers to make the sensitivity and specificity greater than 80%.5,6,20 Diagnosis of MM can be challenging for pathologists, and it is important to correlate histological findings from sufficient biopsy samples with clinical and imaging features.
Our patient underwent laparoscopic biopsy of peritoneal masses. Postoperative pathology revealed (peritoneum) malignant tumor.Tumor cells were arranged in a nest-like manner without obvious papillary structure and they were epithelioid with a small number of fusiform cells. Immunohistochemistry also marked multiple mesothelial derived markers, among which HBME1(+), WT-1(scattered +) and Vim (+) were simultaneously positive, but CEA was negative. Combined with histomorphologic characteristics, the final diagnosis was MPeM (mainly epithelial differentiation). Therefore, the pathological manifestations of mesothelioma are diverse, and if only one mesothelial derived immune marker is selected during diagnosis, misdiagnosis is likely to occur after negative expression.
Therefore, in order to reduce the occurrence of clinical misdiagnosis, appropriate histological specimens are very important for pathological and immunohistochemical examination. Laparoscopy, exploratory laparotomy, CT or ultrasound guided puncture biopsy have become the main means to obtain specimens. At present, laparoscopy is recommended as the first choice for reasons such as less trauma, more abundant excision biopsy specimens, safer biopsy under visual conditions and higher success rate.1
In addition, with the further study of mesothelioma, some cytogenetic and molecular markers are helpful for diagnosis. CDKN2A/P16 deletion has been reported in up to 70% of primary epithelioid mesotheliomas and 90% to 100% of sarcomatoid mesotheliomas. The presence of this homozygous gene deletion is by far the best indicator of mesothelioma.20 For patients with economic conditions, high-throughput sequencing technology can also be used for genetic testing. Common genetic mutations in mesothelioma include BAP1, SETD2, NF2, CDKN2A/B, LASTS1/2, PBRM1 and SMARCC1, etc., especially for patients with histological diagnosis difficulties, through the results of combined genetic testing, it helps to make accurate pathological diagnosis. At the same time, high-throughput sequencing technology gene detection can not only assist in diagnosis, but also find the sites of targeted drugs and provide basis for genetic counseling.5
In summary, our patient was initially mistaken for peritoneal metastasis of ovarian cancer, which was related to our insufficient understanding of the disease. MM patients have low incidence, atypical clinical manifestations and lack of specificity. For imaging findings of atypical peritoneal mesothelioma, such as nodular thickening of the peritoneum, radiologists and clinicians need to include mesothelioma in the differential diagnosis and conduct histopathological examination as early as possible to avoid misdiagnosis, regardless of prior tumor history. Although laparoscopy has certain risks, it can observe the gross manifestations of the tumor in the serosal cavity, and can accurately clip or remove the tumor, through histopathological examination, combined with relevant immune markers, so as to more accurately diagnose mesothelioma. In patients with conditions, laparoscopy is strongly recommended when cytology is not definitive.
Due to the rarity and highly aggressive nature of MPeM, clinical diagnosis is usually at an advanced stage, when it is often manifested by abdominal complications caused by the spread of the disease, or manifested by starvation. Without treatment, life expectancy is less than one year.13 Although some progress has been made in the treatment of MPeM, the prognosis for MPeM remains poor. At present, there are few clinical trials evaluating systematic treatment for unresectable advanced MPeM, so there is no standard treatment protocol. Most studies on the treatment of MPeM are extrapolated based on pleural mesothelioma.1
Pemetrexed combined with cisplatin chemotherapy may be considered for patients who cannot be surgically resected.14,15 Although cisplatin combined with pemetrexed is the standard first-line chemotherapy regimen for unresectable malignant pleural mesothelioma (MPlM),21,22 the efficacy of this regimen in malignant peritoneal mesothelioma (MPeM) remains unclear. Some studies with a small sample size have shown that cisplatin combined with pemetrexed can benefit malignant peritoneal mesothelioma (MPeM), with a median progression-free survival of 7.1–11.0 months and a median overall survival of 15.4–15.8 months, which is consistent with the efficacy of MPlM and can be recommended as the first-line treatment for patients with unresectable MPeM.14,15
In terms of maintenance therapy, a previous Phase II study showed that pemetrexed maintenance therapy did not bring survival benefits to patients with MPlM.23 Due to the low concentration of drugs in the abdominal cavity during systemic chemotherapy, the therapeutic effect is limited and the side effects are large, so some studies have shown that local abdominal chemotherapy can be selected according to the patient’s situation. There is currently no second-line treatment option for peritoneal mesothelioma that has shown survival benefit as a relapsed or refractory disease once first-line treatment has developed resistance, so inclusion in clinical trials is considered.5
Recently, immune checkpoint inhibitors (ICIs) have shown some advantages in the treatment of various tumors. Monotherapy PD-1 inhibitors such as nivolumab, pembrolizumab, and PD-L1 inhibitors such as avelumab have shown good clinical efficacy and safety in multiple studies,17,24,25 providing new treatment options for patients with advanced mesothelioma. However, monotherapy immunotherapy has limited effect on overall prognosis.Dual immunotherapy is thought to be more effective. CheckMate 743 study also demonstrated for the first time that dual immunotherapy as a first-line treatment improves survival in patients with unresectable MPlM. Nivolumab combined with ipilimumab resulted in a significant OS benefit compared to pemetrexed combined with cisplatin/carboplatin standard chemotherapy (median OS 18.1 months vs 14.1 months; HR = 0.74, P = 0.002), and the 3-year overall survival benefit was 23% and 15%, respectively, indicating that dual immunotherapy can bring lasting survival benefit to patients.26 However, the above studies were carried out in MPlM, but there are no studies on MPeM at present.In the Phase II clinical study of CTLA-4 antibody tremelimumab and PD-L1 antibody durvalumab in the treatment of advanced MM, Calabrò et al included a total of 40 patients with unresectable pleural mesothelioma and peritoneal mesothelioma. The results showed that the patients’ median PFS and median OS were 5.7 months and 16.6 months, respectively, with good efficacy and safety.27
With the deepening of research on MM, molecular targeted therapy has attracted more and more attention. Studies have found that EGFR is overexpressed in 44 to 97% of MM patients and ALK rearrangement in 13% of MPeM patients, but the efficacy of tyrosine kinase inhibitors (TKIs) is not ideal.28,29 Angiogenesis plays a crucial role in the growth, progression and metastasis of malignant tumors.30 Vascular endothelial growth factor (VEGF)secreted by tumor cells can stimulate the proliferation of vascular endothelial cells, leading to changes in vascular permeability and angiogenesis.31 Vascular endothelial growth factor is also a key mitogen of malignant mesothelioma cells, and serum VEGF level is significantly negatively correlated with the survival rate of patients with MM, suggesting that VEGF production can not only promote tumor angiogenesis, but also directly stimulate tumor growth. VEGF may be a key regulatory factor of MM growth. It is related to the pathogenesis of malignant mesothelioma.32 Studies have shown that drugs that target VEGF or its receptors may be effective.
There is increasing evidence that inhibition of angiogenic pathways can be used as a strategy for the treatment of MM. A Phase III clinical study showed that bevacizumab combined with pemetrexed plus cisplatin significantly improved OS in MPlM patients, extending the median OS by 2.7 months (18.8 months vs 16.1 months). This study established the first-line treatment status of bevacizumab combined with pemetrexed plus cisplatin.16 However, considering the small survival advantage of bevacizumab and the increased adverse reactions after combination therapy,16 many countries have not approved the treatment of bevacizumab.
A multicenter, double-blind, randomized Phase II clinical trial (RAMES Study)33 explored the efficacy and safety of gemcitabine plus ramucirumab as a second-line treatment for patients with MPlM, following pemetrexed plus a platinum regimen. The results of this study suggest that gemcitabine combined with ramumab compared with gemcitabine alone significantly improves OS (13.8 months vs 7.5 months) and is a viable second-line treatment option for advanced MPlM, regardless of patient age, histological type, and time to disease progression after first-line treatment.
In addition to the anti-vascular targeting drugs of these macromolecular monoclonal antibodies, small-molecule multi-target anti-vascular tyrosine kinase inhibitors have also been studied. In a Phase II clinical study,34 the efficacy of first-line anti-vascular TKI nintedanib combined with chemotherapy was observed. The median PFS in patients treated with nintedanib combined with chemotherapy was 9.7 months compared with placebo (HR: 0.49, P=0.006). The median OS was 20.6 months (HR: 0.70, P=0.197), indicating some efficacy. Unfortunately, in the subsequent phase III clinical study of LUME-Meso, it was not observed that nintedanib combined with chemotherapy improved patient survival.35 Other small-molecule multi-target anti-angiotyrosine kinase inhibitors, including axitinib, sorafenib and imatinib, failed to benefit patients in the treatment of MPlM.24
It can be seen that although the overall efficacy of anti-angiogenesis drugs is limited, it may also bring some improvement in survival for some MPlM patients. Therefore, the selection of effective anti-vascular targeted drugs for MM patients is still being explored, including its application in MPeM. Yang et al evaluated the anti-tumor activity and safety of vascular endothelial growth factor receptor 2(VEGFR-2) inhibitor Apatinib in MPeM in vitro and in vivo, and the results confirmed that Apatinib can effectively inhibit the proliferation and metastasis of MPeM.36
Anlotinib is a novel multi-target TKI independently developed in China, which simultaneously blocks vascular endothelial growth factor receptor (VEGFR), fibroblast growth factor receptor (FGFR), platelet-derived growth factor receptor (PDGFR), c-Kit and Ret, which are involved in tumorigenesis and tumor angiogenesis, and it has shown antitumor effects on a variety of tumor models in vitro and in vivo.37–39 Importantly, according to preclinical studies, effect of anlotinib is superior to that of sunitinib, sorafenib, and nintedanib, which are the three major clinical antiangiogenic drugs.37,38
Researchers evaluated the safety and antitumor activity of anlotinib in patients with a variety of advanced refractory solid tumors, including non-small cell lung cancer, colon cancer, renal cell carcinoma (RCC), thyroid medulla cancer, and soft tissue sarcoma. The results of the study showed that anlotinib has a broad spectrum of anti-tumor potential and controlled toxic.40 Results from Phase II /III clinical trials as well as the results of a retrospective study indicate that anlotinib has demonstrated encouraging clinical activity in a variety of solid tumors, including non-small cell lung cancer, liver cancer, RCC, and soft tissue sarcoma.41–43 Anlotinib was approved by the CFDA in 2018 as a third-line treatment for patients with advanced NSCLC. Anlotinib had a significantly lower incidence of grade 3 or higher side effects compared to Sunitinib.41,43
These findings suggest that anlotinib, which targets vascular pathways that promote tumor growth, has shown encouraging clinical activity and is well tolerated in a variety of epithelial malignancies and soft tissue sarcomas, and it is reasonable to speculate that anlotinib may be a reasonable option for advanced malignant mesothelioma where treatment options are relatively sparse, although its effect on MM has not been studied.
Here, we report a patient with advanced MPeM who achieved a prolonged remission with anlotinib maintenance therapy. Anlotinib monotherapy kept tumors stable for more than 14 months, providing a targeted treatment option for MPeM patients. Since only one MPeM patient was observed in this report, the clinical data are very limited, and further observation and accumulation of more experience are needed. We want to encourage researchers to focus on patients with advanced MPeM, and well-designed trials are needed in the future to obtain better evidence to validate the findings in our report.
In this case, we also continuously monitored the dynamic changes of her CA125. Currently, circulating tumor markers have not been extensively studied in MPeM management. Some researchers explored the clinical significance of CA125, CEA and CA19.9 in MPeM patients, and found that CA125 had the highest baseline diagnostic sensitivity rate, which was 53.3%. CA125 can turn negative in patients treated with appropriate cytoreductive surgery (CRS) and hyperthermic intraperitoneal chemotherapy (HIPEC), while CA125 remains elevated in patients with persistent macro disease. This study suggests that further studies are needed to evaluate the role of CA125 in MPeM management.44
Our patient had a significant increase in CA125 at the initial diagnosis, and after response to chemotherapy, CA125 gradually decreased to the normal range with the remission of the disease, and remained stable for a time, suggesting that CA125 may be used as a biological indicator to evaluate efficacy and monitor recurrence in MPeM patients. Unfortunately, the patient’s CA125 has recently shown an upward trend, which seems to indicate the possibility of the disease progressing again. As the patient remained stable on imaging and asymptomatic, we continued maintenance therapy with anlotinib.
Although there are no routine predictive biomarkers for identifying patients who may benefit from MM treatment, a deeper understanding of the biology of MM could reveal more molecular mechanisms of MM in the future, offering the possibility of implementing individualized treatment.
Conclusion
In summary, the onset of MPeM is hidden and the clinical manifestations lack specificity, which is easy to cause misdiagnosis and missed diagnosis. At the same time, the existing treatment methods of MPeM are not effective and the prognosis is poor. Actively carrying out puncture biopsy or laparoscopy to obtain tissue samples and applying multiple immune markers in pathological diagnosis can reduce the probability of misdiagnosis of malignant mesothelioma. With the deepening of the research and understanding of MPeM, the continuous development of molecular targeting and immunotherapy will bring a new dawn for the diagnosis and treatment of MPeM.
Abbreviations
MPeM, Malignant peritoneal mesothelioma; CRS, cytoreductive surgery; SD, stable disease; PFS, progress free survival; OS, overall survival; HIPEC, hyperthermic intraperitoneal chemotherapy; MPlM, malignant pleural mesothelioma; immune checkpoint inhibitors (ICIs); MM, malignant mesothelioma; CT, Computed tomography; PR, partial remission; PS, Performance status; TKIs, tyrosine kinase inhibitors; VEGF, vascular endothelial growth factor; VEGFR-2, vascular endothelial growth factor receptor-2; FGFR, fibroblast growth factor receptor; PDGFR, platelet-derived growth factor receptor; RCC, renal cell carcinoma.
Data Sharing Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Ethics Approval and Consent to Participate
This study was approved by the Ethics Committee of Affiliated Dalian Third People’s Hospital of Dalian Medical University.Informed consent was obtained prior to each treatment in this case report. Written informed consent has been obtained from the patient for the release of relevant clinical and imaging data from their cases, included consent for the publication of the case details.
Acknowledgments
The authors have read the CARE Checklist (2016), and the manuscript was prepared and revised according to the CARE Checklist (2016).
Author Contributions
The authors listed in this article have made significant contributions to the work of the report, whether in concept, research design, execution, data acquisition, analysis and interpretation, or in all of these areas, and meet the following criteria. All authors participated in drafting or writing, or substantially revised or critically reviewed the article, and have agreed on the journal in which the article will be submitted. Finally, all authors agree to take responsibility and be accountable for the contents of the article.
Funding
No funding was received for this study.
Disclosure
The authors declare that there are no conflicts of interest regarding the publication of this paper.
References
1. Kusamura S, Kepenekian V, Villeneuve L, et al. Peritoneal mesothelioma: PSOGI/EURACAN clinical practice guidelines for diagnosis, treatment and follow-up. Eur J Surg Oncol. 2021;47(1):36–59. PMID: 32209311. doi:10.1016/j.ejso.2020.02.011
2. Zhao J, Zuo TT, Zheng RS, et al. Epidemiology and trend analysis on malignant mesothelioma in China. Chin J Cancer Res. 2017;29(4):361–368. PMID: 28947868. doi:10.21147/j.issn.1000-9604.2017.04.09
3. Manzini VDP, Recchia L, Cafferata M, et al. Malignant peritoneal mesothelioma: a multicenter study on 81 cases. Ann Oncol. 2010;21(2):348–353. PMID: 19635740. doi:10.1093/annonc/mdp307
4. Grzankowski KS, Brightwell RM, Kasznica JM, et al. Malignant peritoneal mesothelioma without asbestos exposure: an ovarian cancer imitator. Gynecol Oncol Rep. 2014;17(11):10–12. PMID: 26076085. doi:10.1016/j.gore.2014.11.002
5. Boussios S, Moschetta M, Karathanasi A, et al. Malignant peritoneal mesothelioma: clinical aspects, and therapeutic perspectives. Ann Gastroenterol. 2018;31(6):659–669. PMID: 30386115. doi:10.20524/aog.2018.0305
6. Husain AN, Colby TV, Ordóñez NG, et al. Guidelines for pathologic diagnosis of malignant mesothelioma 2017 update of the consensus statement from the international mesothelioma interest group. Arch Pathol Lab Med. 2018;142(1):89–108. PMID: 28686500. doi:10.5858/arpa.2017-0124-RA
7. Gudmundsson E, Labby Z, Straus CM, et al. Dynamic contrast - enhanced CT for the assessment of tumour response in malignant pleural mesothelioma: a pilot study. Eur Radiol. 2019;29(2):682–688. PMID: 29967955. doi:10.1007/s00330-018-5533-9
8. Helm JH, Miura JT, Glenn JA, et al. Cytoreductive surgery and hyperthermic intraperitoneal chemotherapy for malignant peritoneal mesothelioma: a systematic review and meta ⁃ analysis. Ann Surg Oncol. 2015;22(5):
9. Baratti D, Kusamura S, Cabras AD, et al. Diffuse malig⁃ nant peritoneal mesothelioma: long⁃term survival with complete cy⁃ toreductive surgery followed by hyperthermic intraperitoneal chemo⁃ therapy (HIPEC. Eur J Cancer. 2013;49(15):3140–3148. PMID: 23831335. doi:10.1016/j.ejca.2013.05.027
10. García-Fadrique A, Mehta A, Mohamed F, et al. Clinical presentation, diagnosis, classification and management of peritoneal mesothelioma: a review. J Gastrointest Oncol. 2017;8(5):915–924. PMID: 29184697. doi:10.21037/jgo.2017.08.01
11. Sugarbaker PH. Update on the management of malignant peritoneal mesothelioma. Transl Lung Cancer Res. 2018;7(5):599–608. PMID: 30450299. doi:10.21037/tlcr.2018.08.03
12. Alexander HR, Li CY, Kennedy TJ. Current management and future opportunities for peritoneal metastases: peritoneal mesothelioma. Ann Surg Oncol. 2018;25(8):2159–2164. PMID: 29423664. doi:10.1245/s10434-018-6337-5
13. Kim J, Bhagwandin S, Labow DM, et al. Malignant peritoneal mesothelioma: a review. Ann Transl Med. 2017;5(11):236. PMID: 28706904. doi:10.21037/atm.2017.03.96
14. Fujimoto E, Kijima T, Kuribayash K, et al. First ⁃line che⁃ motherapy with pemetrexed plus cisplatin for malignant peritoneal mesothelioma. Expert Rev Anticancer Ther. 2017;17(9):865–872. PMID: 28594258. doi:10.1080/14737140.2017.1340157
15. Nagata Y, Sawada R, Takashima A, et al. Efficacy and safety of pemetrexed plus cisplatin as first⁃line chemotherapy in advanced malignant peritoneal mesothelioma.Jpn. J Clin Oncol. 2019;49(11):1004–1008. PMID: 31287877. doi:10.1093/jjco/hyz104
16. Zalcman G, Mazieres J, Margery J, et al. Bevacizumab for newly diagnosed pleural mesothelioma in the mesothelioma avastin cisplatin pemetrexed study (MAPS): a randomised, controlled, open⁃label, Phase 3 trial. Lancet. 2016;387(10026):1405–1414. PMID: 26719230. doi:10.1016/S0140-6736(15)01238-6
17. Alley W, Lopez J, Santoro A, et al. Clinical safety and ac⁃ tivity of pembrolizumab in patients with malignant pleural mesothe⁃ lioma (KEYNOTE ⁃ 028): preliminary results from a non ⁃ ran⁃ domised, open⁃label, phase 1b trial. Lancet Oncol. 2017;18(5):623–630. PMID: 28291584. doi:10.1016/S1470-2045(17)30169-9
18. Bridda A, Padoan I, Mencarelli R, et al. Peritoneal mesothelioma: a review. Med Gen Med. 2007;9(2):32. PMID: 17955087 PMCID: PMC1994863.
19. Sheaff M. Guidelines for the cytopathologic diagnosis of epithelioid and mixed-type malignant mesothelioma: complementary statement from the international mesothelioma interest group, also endorsed by the international academy of cytology and the papanicolaou society of cytopathology. A proposal to be applauded and promoted but which requires updating. Diagn Cytopathol. 2020;48(10):877–879. PMID: 31976625. doi:10.1002/dc.24318
20. Arif Q, Husain AN. Malignant mesothelioma diagnosis. Arch Pathol Lab Med. 2015;139(8):978–980. PMID: 26230591. doi:10.5858/arpa.2013-0381-RA
21. Vogelzang NJ, Rusthoven JJ, Symanowski J, et al. Phase III study of pemetrexed in combination with cisplatin versus cisplatin alone in patients with malignant pleural mesothelioma. J Clin Oncol. 2003;21(14):
22. Shukuya T, Takahashi T, Imai H, et al. Comparison of cisplatin plus pemetrexed and cisplatin plus gemcitabine for the treatment of malignant pleural mesothelioma in Japanese patients. Respir Investig. 2014;52(2):101–106. PMID: 24636265. doi:10.1016/j.resinv.2013.07.002
23. Dudek AZ, Wang X, Gu L, et al. Randomized study of maintenance pemetrexed versus observation for treatment of malignant pleural mesothelioma: CALGB 30901. Clin Lung Cancer. 2020;21:553–561e1. PMID: 32727707. doi:10.1016/j.cllc.2020.06.025
24. Scherpereel A, Wallyn F, Albelda SM, et al. Novel therapies for malignant pleural mesothelioma. Lancet Oncol. 2018;19(3):e161–e172. PMID: 29508763. doi:10.1016/S1470-2045(18)30100-1
25. Hotta K, Fujimoto N. Current evidence and future perspectives of immune-checkpoint inhibitors in unresectable malignant pleural mesothelioma. J Immunother Cancer. 2020;8(1):e000461. PMID: 32098830. doi:10.1136/jitc-2019-000461
26. Peters S, Scherpereel A, Cornelissen R, et al. First-line nivolumab plus ipilimumab versus chemotherapy in patients with unresectable malignant pleural mesothelioma: 3-year outcomes from CheckMate 743. Ann Oncol. 2022;33(5):488–499. PMID: 35124183. doi:10.1016/j.annonc.2022.01.074
27. Calabrò L, Morra A, Giannarelli D, et al. Tremelimumab combined with durvalumab in patients with mesothelioma (NIBIT-MESO-1): an open-label, non-randomised, Phase 2 study. Lancet Respir Med. 2018;6(6):451–460. PMID: 29773326. doi:10.1016/S2213-2600(18)30151-6
28. Chia PL, Scott AM, John T. Epidermal growth factor receptor (EGFR) ⁃targeted therapies in mesothelioma. Expert Opin Drug Deliv. 2019;16(4):
29. Hung YP, Dong F, Watkins JC, et al. Identification of ALK rearrangements in malignant peritoneal mesothelioma. JAMA Oncol. 2018;4(2):
30. Kerbel RS. Tumor angiogenesis. N Engl J Med. 2008;358:2039–2049. doi:10.1056/NEJMra0706596
31. Dvorak HF. Vascular permeability factor/vascular endothelial growth factor: a critical cytokine in tumor angiogenesis and a potential target for diagnosis and therapy. J Clin Oncol. 2002;20(21):4368–4380. doi:10.1200/JCO.2002.10.088
32. Strizzi L, Catalano A, Vianale G, et al. Vascular endothelial growth factor is an autocrine growth factor in human malignant mesothelioma. J Pathol. 2001;193(4):468–475. PMID: 11276005. doi:10.1002/path.824
33. Pinto C, Zucali PA, Pagano M, et al. Gemcitabine with or without ramucirumab as second-line treatment for malignant pleural mesothelioma (RAMES): a randomised, double-blind, placebo-controlled, phase 2 trial. Lancet Oncol. 2021;22(10):1438–1447. PMID: 34499874. doi:10.1016/S1470-2045(21)00404-6
34. Grosso F, Steele N, Novello S, et al. Nintedanib plus pemetrexed/ cisplatin in patients with malignant pleural mesothelioma: phase ii results from the randomized, placebo-controlled LUMEMeso trial. J Clin Oncol. 2017;35(31):3591–3600. PMID: 28892431. doi:10.1200/JCO.2017.72.9012
35. Scagliotti GV, Gaafar R, Nowak AK, et al. Nintedanib in combination with pemetrexed and cisplatin for chemotherapy‐naive patients with advanced malignant pleural mesothelioma (LUME-Meso): a double-blind, randomised, placebo-controlled phase 3 trial. Lancet Respir Med. 2019;7(7):569–580. PMID: 31103412. doi:10.1016/S2213-2600(19)30139-0
36. Yang ZR, Chen ZG, Du XM, et al. Apatinib mesylate inhibits the proliferation and metastasis of epithelioid malignant peritoneal mesothelioma in vitro and in vivo. Front Oncol. 2020;10:585079. PMID: 33365269 PMCID: PMC7750508. doi:10.3389/fonc.2020.585079
37. Lin B, Song X, Yang D, et al. Anlotinib inhibits angiogenesis via suppressing the activation of VEGFR2, PDGFRβ and FGFR1. Gene. 2018;654(654):77–86. PMID: 29454091. doi:10.1016/j.gene.2018.02.026
38. Xie C, Wan X, Quan H, et al. Preclinical characterization of anlotinib, a highly potent and selective vascular endothelial growth factor receptor-2 inhibitor. Cancer Sci. 2018;109(4):1207–1219. PMID: 29446853 PMCID: PMC5891194. doi:10.1111/cas.13536
39. Chen XZ. Anlotinib for refractory advanced non‐small cell lung cancer in China. JAMA Oncol. 2019;5(1):
40. Sun Y, Niu W, Du F, et al. Safety, pharmacokinetics, and antitumor properties of anlotinib, an oral multi-target tyrosine kinase inhibitor, in patients with advanced refractory solid tumors. J Hematol Oncol. 2016;9:105. doi:10.1186/s13045-016-0332-8
41. Shen G, Zheng F, Ren D, et al. Anlotinib: a novel multi-targeting tyrosine kinase inhibitor in clinical development. J Hematol Oncol. 2018;11(1):120. PMID: 30231931 PMCID: PMC6146601. doi:10.1186/s13045-018-0664-7
42. Chi Y, Fang Z, Hong X, et al. Safety and efficacy of anlotinib, a multikinase angiogenesis inhibitor, in patients with refractory metastatic soft‐tissue sarcoma. Clin Cancer Res. 2018;24(21):
43. Yao W, Du X, Wang J, Wang X, Zhang P, Niu X. Long-term efficacy and safety of anlotinib as a monotherapy and combined therapy for advanced sarcoma. Onco Targets Ther. 2022;15:669–679. doi:10.2147/OTT.S365506
44. Baratti D, Kusamura S, Martinetti A, et al. Circulating CA125 in patients with peritoneal mesothelioma treated with cytoreductive surgery and intraperitoneal hyperthermic perfusion. Ann Surg Oncol. 2007;14(2):500–508. PMID: 17151789. doi:10.1245/s10434-006-9192-8
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.