Back to Journals » ClinicoEconomics and Outcomes Research » Volume 9
Optimizing choice of oral interferon-free treatment for genotype 1 hepatitis C virus using testing for NS5A resistance: a cost-utility analysis from the perspective of the Italian National Health Service
Authors Westerhout KY, Bouwmeester W, Duchesne I, Pisini M, Piena MA, Damele F, Gueron B, Treur M, Belsey J
Received 19 July 2016
Accepted for publication 27 October 2016
Published 27 February 2017 Volume 2017:9 Pages 163—172
DOI https://doi.org/10.2147/CEOR.S117650
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Giorgio Colombo
Kirsten Y Westerhout,1 Walter Bouwmeester,1 Inge Duchesne,2 Marta Pisini,2 Marjanne A Piena,1 Francesco Damele,3 Beatrice Gueron,2 Maarten Treur,1 Jonathan Belsey4
1Pharmerit BV, Marten Meesweg, Rotterdam, the Netherlands; 2Janssen EMEA, Turnhoutseweg, Beerse, Belgium; 3Janssen-Cilag SpA, Via Michelangelo Buonarroti, Cologno Monzese, Italy; 4JB Medical Ltd, Old Brickworks, Little Cornard, United Kingdom
Background: Patients with genotype-1 hepatitis C virus infection who have failed to respond to standard therapy or who relapse following treatment may be considered for an interferon-free regimen incorporating a nonstructural protein 5A (NS5A) inhibitor. Sustained virologic response (SVR) with these regimens is typically >90%, but this is reduced in patients with NS5A resistance. European Association for Study of the Liver guidelines recommend simeprevir + sofosbuvir ± ribavirin (SMV+SOF±R) for re-treating patients failing an NS5A inhibitor-containing regimen. An alternative strategy would be to test for NS5A resistance prior to treatment, with therapy optimized based on the results. This study investigates the cost-effectiveness of this strategy.
Materials and methods: A Markov model was used to estimate disease progression for treatment-experienced genotype 1 patients with severe fibrosis or compensated cirrhosis. Targeted treatment with either SMV+SOF±R or sofosbuvir + ledipasvir ± ribavirin (SOF+LDV±R) based on pretreatment NS5A resistance testing was compared to routine SOF+LDV±R without testing. Treatment duration was 12 or 24 weeks for patients with severe fibrosis or compensated cirrhosis (Metavir F3/F4). SVR data for the treatment options were based on the results of published clinical trials. The analysis was carried out from the perspective of the Italian National Health Service.
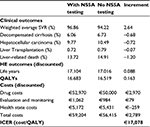
Results: Optimized treatment using NS5A resistance testing yielded 0.163 additional QALYs and increased costs of €2,789 per patient versus no testing. The incremental cost-effectiveness ratio (ICER) was €17,078/QALY. Sensitivity analysis identified the SVR attributable to each of the treatment regimens as the most sensitive determinant of ICER (range: €10,055/QALY–€43,501/QALY across plausible range). Probabilistic sensitivity analysis demonstrated that, at a willingness-to-pay threshold of €30,000/QALY, the probability that NS5A-directed treatment will be cost-effective is 81.4%.
Conclusion: Optimizing therapy with either SMV+SOF±R or SOF+LDV±R based on pretreatment NS5A resistance testing was cost-effective from the perspective of the Italian National Health Service, in treatment-experienced patients with severe fibrosis or compensated cirrhosis.
Keywords: hepatitis C, NS5A inhibitor, simeprevir, sofosbuvir, ledipasvir, health economics
Introduction
The objective of management of chronic hepatitis C virus (HCV) infection is to prevent progression from the asymptomatic viremic stage toward hepatic fibrosis, cirrhosis, and hepatocellular carcinoma (HCC). If untreated, ~15%–30% of infected patients will progress to cirrhosis over a 20 year period following which ~2%–4% per year will go on to develop malignancy.1–4 The use of specific antiviral therapy has the potential to prevent further hepatic injury. If HCV is rendered undetectable in the peripheral blood within the first 12–24 weeks following completion of therapy the likelihood of recurrence is very low. Achievement of sustained virologic response (SVR) within this time frame, therefore, constitutes a standard measure of treatment success for clinical trials of antiviral therapy. Treatment regimens involving multiple direct acting antiviral agents have been demonstrated to achieve extremely high SVR rates provided resistant viral strains do not emerge.5
The choice of components for a multi-agent regimen hinges on inhibiting a range of independent targets within the virus. Until recently, standard treatment involved the use of the nucleotide analog ribavirin in combination with the cytokine peginterferon alfa. Although this achieved moderately successful SVR,6 the addition of a serine protease inhibitor (e.g., simeprevir) has been shown to significantly improve viral eradication.5 The protease inhibitor targets two nonstructural proteins within the virus (NS3/4A) thereby inhibiting viral replication. More recently, regimens involving an RNA-dependent RNA-polymerase inhibitor sofosbuvir, which targets the NS5B protein, have been shown to improve SVR still further. An additional treatment option – an NS5A inhibitor (e.g., ledipasvir) – offers further therapeutic opportunities.7 Recently published phase III randomized controlled trials have shown that the combination of an NS5A inhibitor and an NS5B inhibitor, with or without ribavirin, can achieve SVR rates of close to 100% in patients with genotype 1 HCV infection and in the presence of severe fibrosis or cirrhosis.8,9
Given the large number of patients potentially undergoing treatment for HCV, the selection of an appropriate therapy combination will inevitably hinge not only on clinical performance but also on health economic drivers. Although antiviral treatment incurs significant expenditure, the cost consequences of chronic HCV infection itself are considerable. A recent analysis from the perspective of the Italian National Health Service estimated that for each patient a mean annual direct expenditure of €1,647 is incurred, with a further €3,052 annually attributable to indirect costs.10 The vast majority of these costs are attributable to the later stage complications of cirrhosis, HCC, and liver transplantation. If effective treatment can reliably eradicate the virus and prevent progression to these problems, the potential for offsetting the costs of treatment are considerable.
Achievement of both favorable clinical and economic results, however, is dependent on the infecting virus being sensitive to the treatment chosen. In an analysis of patients participating in clinical trials carried out using the combination of the NS5A inhibitor, ledipasvir with the NS5B inhibitor, sofosbuvir (LDV+SOF), an overall SVR of 97% was achieved in treatment-naïve patients and 91% in treatment-experienced patients. However, ~13.5% of the patients in the studies exhibited mutations to NS5A that conferred resistance to LDV. Among these patients SVR was 96% in treatment naïve reduced to 65% in treatment experienced.8,9 This not only has implications for the outcome of the initial treatment regimen, but additionally, by selecting out the resistant viral sub-population the persistent infection has a much higher risk of resistance to future treatment with further NS5A inhibitors. Another analysis of 41 patients who had failed to respond to 8–12 weeks of treatment with LDV+SOF within the phase II/III clinical trials programme showed that in 30 of these patients there were one or more detectable NS5A resistance-associated variants (RAVs).11 When these patients were re-treated with 24 weeks LDV+SOF, all eleven patients without RAVs achieved SVR, while this was only achieved in 18 (60%) of those with detectable RAVs. Among those with two or more RAVs detected, the SVR declined to 50%.
Consequently, in patients who exhibit NS5A RAVs, the use of an alternative regimen which would not be susceptible to the NS5A inhibitor resistance mutation is likely to offer the best opportunity to achieve SVR. One such regimen combining simeprevir and sofosbuvir (SMV+SOF) demonstrated in two clinical trials an overall SVR of 95% in treatment-naïve patients and 93% in treatment-experienced patients, based on 12 weeks of treatment duration for Metavir F3 and 24 weeks for Metavir F4 patients.12,13 As expected, the presence of the NS5A resistance mutation did not appear to impact the achievement of SVR for SMV+SOF.12,14
A potential solution to obtain the maximum SVR for all patients would be to test for NS5A resistance prior to the initiation of treatment and to treat those with significant resistance with SMV + SOF. At this stage, however, it is unclear whether this would represent a cost-effective strategy. In order to evaluate this, this paper presents the results of a cost-utility analysis exploring the economic impact of two alternative strategies in the most challenging patient group: those with severe fibrosis or compensated cirrhosis (Metavir F3/F4) who have previously been unsuccessfully treated using an interferon-containing regimen. The two strategies compared were as follows:
Blind treatment of all patients with LDV+SOF regardless of NS5A resistance status.
Pretreatment screening for NS5A resistance followed by either LDV+SOF or SMV+SOF depending on the test results.
Methods
A Markov state transition cost-utility model was developed to explore the cost-effectiveness of NS5A RAVs testing for determining optimum therapy in previously treated patients with genotype 1 HCV infection and either severe fibrosis or compensated cirrhosis (Metavir F3/F4).
A previously developed cost-utility model of HCV progression was used,15 with selected inputs adjusted to reflect the perspective of the Italian National Health Service (Servizio Sanitario Nazionale [SSN]). The model is composed of two phases. The first “treatment” phase relates to the initial antiviral treatment period, which includes 12–24 weeks of treatment followed by a 12-week posttreatment period, at which point viral response is assessed; the 24-week treatment being applied to patients with compensated cirrhosis (Metavir F4). Based on the outcome of the treatment phase, patients move into a second “posttreatment” Markov phase of the model, which captures long-term outcomes over the patients’ remaining lifespan. Patients may progress from F3 to F4 and from there to states of decompensated cirrhosis, HCC, and thence to liver transplant and/or liver-related death, with specific transition probabilities being determined by whether SVR has been achieved (Table 3).
Cycle length in the Markov phase is 1 year, with a lifetime horizon. Half cycle correction is applied. Both costs and benefits are discounted at 3% per year and are assessed from the perspective of the SSN.
Patient population
The patient population was defined as adults chronically infected with genotype 1 HCV, who had previously been treated with a regimen including peg-interferon with or without ribavirin and with or without a protease inhibitor, who had experienced either a null or a partial response, or who had relapsed after treatment completion. Patients with a Metavir score of F0–F2 were excluded.
Information on gender distribution and mean age and weight and the relative distribution of patients between Metavir F3 and F4 were determined from a cohort of 128 Italian HCV patients within a global dataset; 83% of whom were treatment experienced.16,17 Table 1 summarizes the values used for all baseline patient characteristics.
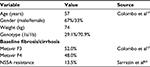  | Table 1 Baseline characteristics of patient population Abbreviation: NS5A, nonstructural protein 5A; Metavir F3, severe fibrosis; Metavir F4, compensated cirrhosis. |
The prevalence of NS5A resistance in the treated population was estimated at 13.5%, which reflects the results obtained from a combined European/US clinical trials dataset, incorporating 511 patients with genotype 1 HCV and compensated cirrhosis (F4).8,9 Assays were carried out using the MiSeq deep sequencing platform (Illumina® Inc., San Diego, CA, USA). For all cutoffs, the most frequent NS5A RAVs in GT1b subjects were Y93H and L31M. In GT1a subjects, the most frequent NS5A RAVs with 1% cutoff were K24R>L31M>Q30H>M28T>Y93H>Q30R. With 5%, 10%, 15%, and 20% cutoffs, Q30H and L31M were most frequent. No significant differences in SVR rates were seen for the different cutoffs.
Treatment strategies
All treatments were modeled in accordance with their European Medicines Agency (EMA) licensed dosage and recommended duration. Two strategies were evaluated based on the use or not of NS5A resistance testing:
All patients treated with LDV+SOF for 12 or 24 weeks for F3 and F4 patients.
All patients tested for NS5A baseline resistance:
NS5A-resistant patients treated with SMV+SOF for 12 weeks (F3) or 24 weeks (F4).
NS5A-sensitive patients treated with LDV+SOF for 12 weeks (F3) or 24 weeks (F4).
Treatment efficacy
SVR data for LDV+SOF were derived from pooled analyses of phase II and III clinical trials.8,9 SVR data for SMV+SOF were derived from the Optimal Treatment with a sIMeprevIr and Sofosbuvir Therapy (OPTIMIST-1) study for F3 patients12 and the Combination Of SiMeprevir and sOfosbuvir in HCV genotype 1 infected patientS (COSMOS) study for F4.13 For both treatment regimens, patients with severe fibrosis (F3) were treated for 12 weeks, while patients with compensated cirrhosis (F4) were treated for 24 weeks. Data from the OPTIMIST-2 study, which also evaluated SMV+SOF in F4 patients, were not used, as the treatment duration in this study was only 12 weeks,14 and therefore did not reflect the EMA licensed dosage. Values used are presented in Table 2.
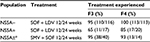  | Table 2 SVR values applied in the model Note: *NS5A resistance assumed not to impact on SVR with SMV/SOF regimen.11,13 Abbreviations: LDV, ledipasvir; NS5A, nonstructural protein 5A; SMV, simeprevir; SOF, sofosbuvir; SVR, sustained virologic response. |
The treatment-experienced patients in the OPTIMIST-1 and COSMOS studies had all previously received interferon-based regimens, with or without ribavirin. In the SOF + LDV treatment-experienced group, patients could also have received a protease inhibitor (principally boceprevir or telaprevir).
Health state transitions
Transition probabilities reflecting progression among advanced liver disease (ALD) were sourced from the literature. The majority of results were drawn from a recent comprehensive systematic review of the economic literature in the field, reflecting values used in 34 economic models published between 2000 and 2011.18 Where data were lacking, they were sourced from individual published papers. Values used are listed in Table 3.
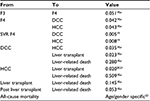  | Table 3 Annual transition probabilities Notes: aReprinted from Value Health, 14(8), Townsend R, McEwan P, Kim R, Yuan Y, Structural frameworks and key model parameters in cost-effectiveness analyses for current and future treatments of chronic hepatitis C, 1068–1077, Copyright 2011, with permission from Elsevier. doi: 10.1016/j.jval.2011.06.006.18 Abbreviations: DCC, decompensated cirrhosis; HCC, hepatocellular carcinoma; SVR, sustained virologic response. |
Utilities
Health state utilities
Utilities associated with posttreatment health states were derived from an established dataset23 that has been widely used in the world literature to evaluate the cost-effectiveness of treatments for HCV,24–31 particularly for those analyses carried out from the perspective of the Italian healthcare system.27–31 This choice was made to ensure comparability with other evaluations of alternative treatment strategies.
This utility set assigns a value of 1.00 to the SVR state for both F3 and F4. Given that the utility of a healthy age-matched population would be unlikely to reach this level, the impact of assigning a lower value for these two health states was explored in a scenario analysis.
On-treatment utility
Although oral interferon-free regimens have a much improved tolerability profile compared with older regimens, the potential for a decrease in utility while taking treatment still exists. The on-treatment utility decrement of −0.03 used in the model is that cited in a recently published cost-utility analysis of oral therapy, which was based on unpublished observations.32 Table 4 lists all utility values used in the model.
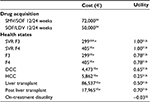  | Table 4 Utilities and costs used in the model Note: *Only applied for the year after treatment. aMarcellusi A, Viti R, Capone A, Mennini F. The economic burden of HCV-induced diseases in Italy. A probabilistic cost of illness model. Eur Rev Med Pharmacol Sci. 2015;19(9):1610–1620.10 bPetta S, Cabibbo G, Enea M, et al. Cost-effectiveness of sofosbuvirbased triple therapy for untreated patients with genotype 1 chronic hepatitis C. Hepatology. 2014;59(5):1692–1705, with permission from John Wiley and Sons.31 Abbreviations: DCC, decompensated cirrhosis; HCC, hepatocellular carcinoma; LDV, ledipasvir; SMV, simeprevir; SOF, sofosbuvir; SVR, sustained virologic response. |
Costs
Antiviral treatment-related costs
Current Italian list prices were used for all drug costs, based on the licensed doses. As a flat-pricing structure is applied in Italy, treatment costs were identical for both 12- and 24-week treatment regimens: €50,000 for LDV+SOF and €72,000 for SMV+SOF.
Treatment monitoring schedules were based on guidance from the Italian Liver Association33 with costings allocated according to the prices quoted by the Italian National Agency for Regional Healthcare Services. Screening cost for NS5A was estimated as €79.02.34
Health state costs
On-going annual costs associated with HCV-infected patients in each health state were obtained from a recently published burden of disease analysis that assessed the cost impact of HCV on the Italian SSN.10
All costs were inflated to 2015 values, using the Organisation for Economic Co-operation and Development (OECD) consumer prices index for Italy.
Sensitivity analysis
Deterministic and probabilistic sensitivity analyses were carried out to determine the influence of uncertainty surrounding input parameters. All variables listed in Tables 2–4 were tested in the sensitivity analyses. Values for parameter estimates were varied within the uncertainty distributions that best reflected the nature and uncertainty of each specific parameter. The standard error was assumed to vary by ±20% in cases where uncertainty information on variance was lacking. Results were expressed as tornado diagrams, showing the 15 variables in each case that exerted the greatest effect on the resulting incremental cost-effectiveness ratio (ICER).
In the multivariate probabilistic sensitivity analysis (PSA), 3,000 simulations were processed to represent the uncertainty of model results by varying the parameters by random draws from their assumed distributions. Based on the simulations, a scatterplot and an acceptability curve were drawn to estimate the probability of NS5A-targeted treatment being considered cost-effective versus untargeted treatment across various willingness-to-pay (WTP) thresholds per QALY gained.
Four scenario analyses were also carried out:
To assess the impact of restriction of NS5A-targeted treatment to patients with compensated cirrhosis (Metavir F4) only.
To assess the impact of restriction of NS5A-targeted treatment to patients with severe fibrosis (Metavir F3) only.
To assess the impact of reduction of the utility assigned to the SVR (F3) and SVR (F4) health states from 1.00 to 0.90.
To assess price sensitivity, three global discount values of 40%, 50% and 60% were applied in turn to all drug acquisition costs in the model.
Results
For the base case analysis, the strategy of testing-directed therapy yielded improved clinical outcomes (incremental QALY=0.163) at an additional per patient expenditure of €2,789. The resulting ICER in favor of the testing strategy was €17,078/QALY (Table 5). Testing-directed therapy was more effective (weighted average SVR 96.9% versus 94.2%), reducing the risk of progression to ALD and death. QALY gains are achieved as reaching SVR is associated with a higher utility value. Prevention of future ALD cases and ALD-related death is also reflected in the higher number of QALYs; the relative impact of this element on the total incremental QALYs is limited, however, as only a minority of patients will eventually progress to develop ALD. The higher costs for the testing-directed therapy were mainly due to the costs of SMV + SOF treatment. Cost savings resulting from a lower incidence of ALD in patients receiving testing-directed therapy were limited, as only a few patients eventually develop ALD.
Sensitivity analyses
Univariate deterministic sensitivity analysis demonstrates that the most sensitive driver of the ICER is the SVR attributable to each of the treatment regimens evaluated (Figure 1). The ICERs demonstrated across the values explored for SVR ranged from €10,055/QALY to €43,501/QALY. The results were robust to the remaining assumptions, with testing of no other single variable yielding an ICER below €13,495/QALY or above €20,013/QALY.
Probabilistic sensitivity analysis demonstrated that, at a WTP threshold of €30,000/QALY, the probability that NS5A-directed treatment will be cost-effective is 81.4%. At a WTP threshold of €50,000/QALY, the corresponding probability is 91.9%. A cost-effectiveness acceptability plot is presented in Figure 2.
  | Figure 2 Probabilistic sensitivity analysis: two-way cost-effectiveness acceptability curve. Abbreviations: LDV, ledipasvir; NS5A, nonstructural protein 5A; SMV, simeprevir; SOF, sofosbuvir. |
Scenario analyses
Table 6 shows the results for each of the scenario analyses. In each case the incremental values are reported from the perspective of NS5A testing versus no testing. The ICER for patients with compensated cirrhosis is considerably higher than that for the severe fibrosis group, reflecting the lower incremental difference for SVR achieved for the two testing/treatment regimens evaluated (Table 2). There was a substantial reduction in ICER at all levels of drug cost discounting explored, with the result falling below €10,000/QALY in each scenario.
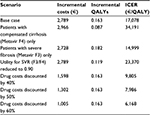  | Table 6 Results of scenario analyses Abbreviations: ICER, incremental cost-effectiveness ratio; SVR, sustained virologic response. |
Discussion
Clinical trial data relating to the clinical performance of oral interferon-free treatment regimens have only recently become available and, so far as we are aware, this is the first published economic analysis that compares the cost-effectiveness of the use of NS5A testing to determine treatment choice. The high levels of SVR potentially achievable make these regimens clinically attractive, but the relatively high costs involved mean that efficient targeting of treatment is essential. Given that resistance to NS5A inhibitors affects around one in eight patients and this resistance persists for long periods, consideration of this factor is relatively important in the selection of an appropriate treatment regimen.
We have demonstrated that, in treatment-experienced patients with severe fibrosis or compensated cirrhosis, prior assessment of NS5A sensitivity followed by targeted therapy with either LDV+SOF or SMV+SOF may be a cost-effective strategy. When considered from the perspective of the Italian National Health Service, testing-targeted therapy was associated with an ICER of €17,078/QALY versus untargeted therapy, well below conventionally accepted WTP thresholds. If treatment is limited to patients with established cirrhosis, the ICER rises to €34,191/QALY, reflecting the somewhat reduced incremental SVR benefit in patients of this type. Sensitivity analyses demonstrated the conclusions of the base case to be robust to a wide range of parameter variation, with only values of achievable SVR for both treatment groups at the lower end of the range tested yielding ICERs in excess of €30,000/QALY. Probabilistic sensitivity analysis demonstrated an 81% probability of a testing-targeted therapy being cost-effective at this WTP threshold.
These results are predicated on an assumption that drug acquisition costs will reflect the list prices. In situations where discounts are available, the differential price of the two regimens will decrease commensurately. At discount levels of 40% or greater, the ICER falls below €10,000/QALY.
The analysis is based on previous economic models that adopted a similar approach to the underlying disease progression model that has been used for a wide range of previous health economic assessments.15,20,35–38 This approach has been developed over a number of years and is considered both robust and representative of the disease. Additionally, the methodology complies with economic analysis critical appraisal guidance recommended for use by the UK National Institute of Health and Clinical Excellence.39
Limitations
Sensitivity analysis has demonstrated the most critical determinant of the ICER estimates in this model to be the SVR values used. The SVR estimates we have chosen are based on the best current estimates for the two treatment regimens considered but are subject to a number of limitations:
The SVR estimates for LDV+SOF are based on data presented at clinical conferences,8,9 as the final results of the relevant studies had not been published in full at the time the analysis was carried out.
There are no direct comparative studies available for LDV+SOF versus SMV+SOF and, because several of the studies from which data are drawn are non-comparative it has not been possible to carry out a network meta-analysis. Additionally, as characteristics of patients for which the AOF + LDV SVR data were published were not available, it has not been possible to generate a matching adjusted indirect treatment comparison, which would otherwise have been the preferred approach in these circumstances. The parallel (naïve) comparison methodology that we adopted instead may consequently be prone to undocumented patient and disease confounders.
Some of the data used are based on small subgroups of patients drawn from a larger clinical trial population. Small numbers, combined with the post hoc nature of these analyses may result in bias in the estimates.
The SVR data for LDV+SOF presented by Sarrazin et al8,9 were chosen for the model, as they best represented the population under evaluation, being broken down by Metavir score (F3 and F4) and by NS5A RAV status. For the F3 patients, prior interferon-based treatment history was also defined. For F3 treatment-experienced patients, this yielded an SVR of 65%, while for F4 patients (mixed treatment naïve and experienced) SVR was 85%. Given that treatment-naïve patients generally respond better to treatment than treatment experienced, the latter figure probably somewhat overstates the SVR likely to be seen in a pure treatment-experienced group.
An alternative analysis has also been presented,11 based on re-treatment of patients who had previously failed short course (8–12 weeks) LDV + SOF. In this analysis, those with a single NS5A RAV demonstrated an SVR of 69%, while those with two or more RAVs had an SVR of 50%. While not strictly applicable to our evaluation population, in that prior treatment was not with an interferon-containing regimen, the magnitude of failed SVR in the NS5A resistant patients was at least comparable to the values derived from the Sarrazin analyses, providing circumstantial support for our SVR estimates.
The model did not take into account specific costs associated with treatment-related adverse events (AEs). However, by eliminating peg-interferon and ribavirin, both the treatment regimens evaluated here have AE profiles that are significantly better than that conventionally seen with HCV treatment. In the largest trial of SMV+SOF in treatment-experienced patients (OPTIMIST-112), 6.5% of patients experienced rash and 4.5% experienced pruritus and there was no evidence of anaemia or neutropenia or other clinically significant AEs. In the largest trial of LDV+SOF in treatment-experienced patients (ION-240), 3.7% experienced rash and 0.5% became anaemic, with no other significant AE signal detected. As the differential risk of AEs between regimens was small and the attributable expenditure incurred negligible in comparison to other cost elements,41 the cost of AEs was not included in the final model. An on-treatment utility decrement was, however, included for all patients.
The health state transition probabilities in the post-treatment phase are subject to some uncertainty. Although the majority of the values used were derived from a wide range of published models, many of these are based on populations being treated many years ago, and are not specific to current disease patterns in Italy. While Italian sources were identified for patient baseline characteristics and treatment costs, in some cases the patient groups from which they were derived do not necessarily reflect the exact population modeled in this analysis. Utilities were derived from a US population, although this dataset has been widely used in the past for published economic models of HCV treatment from an Italian perspective.
Finally, the model assumed that on the completion of treatment, no further antiviral therapy would be instituted for those patients who failed to achieve SVR. Although this is probably a legitimate assumption given the current state of understanding, the lifetime outputs of the model may well be susceptible to future change.
Conclusion
Oral interferon-free antiviral therapy regimens have been shown to offer substantial benefits over existing treatments, especially for those patients with more advanced disease who have failed to respond adequately to first line treatment. In a proportion of patients, however, resistance to NS5A inhibitors may result in suboptimal results. In this analysis carried out from the perspective of the Italian National Health Service, we have demonstrated that pretreatment testing for NS5A resistance may be a cost-effective strategy that allows therapy to be tailored appropriately to the individual patient. This strategy maximizes the clinical benefit of treatment while minimizing the risk of further emergence of resistant HCV strains.
Disclosure
At the time this analysis was carried out, Kirsten Y Westerhout, Walter Bouwmeester, Marjanne A Piena, and Maarten Treur were employed by Pharmerit BV, who received payment from Janssen EMEA to carry out the economic analysis described in this paper. Inge Duchesne, Marta Pisini, and Beatrice Gueron were employed by Janssen EMEA and Francesco Damele was employed by Janssen-Cilag SpA (Italy), sponsors of this analysis. Jonathan Belsey was employed by JB Medical Ltd, who received payment from Janssen EMEA to write the paper.
The authors report no other conflicts of interest in this work.
References
Tong MJ, el-Farra NS, Reikes AR, Co RL. Clinical outcomes after transfusion associated hepatitis C. N Engl J Med. 1995;332(22):1463–1466. | ||
Tremolada F, Casarin C, Alberti A, Drago C, Tagger A, Ribero ML, Realdi G. Long-term follow-up of non-A, non-B (type C) post-transfusion hepatitis. J Hepatol. 1992;16(3):273–281. | ||
Thein HH, Yi Q, Dore GJ, Krahn MD. Estimation of stage-specific fibrosis progression rates in chronic hepatitis C virus infection: a meta-analysis and meta-regression. Hepatology. 2008;48(2):418–431. | ||
El-Serag HB, Rudolph KL. Hepatocellular carcinoma: epidemiology and molecular carcinogenesis. Gastroenterology. 2007;132(7):2557–2576. | ||
Chou R, Hartung D, Rahman B, Wasson N, Cottrell EB, Fu R. Comparative effectiveness of antiviral treatment for hepatitis C virus infection in adults: a systematic review. Ann Intern Med. 2013;158(2):114–123. | ||
Fried MW, Shiffman ML, Reddy KR, et al. Peginterferon alfa-2a plus ribavirin for chronic hepatitis C virus infection. N Engl J Med. 2002;347(13):975–982. | ||
Pawlotsky JM. NS5A inhibitors in the treatment of hepatitis C. J Hepatol. 2013;59(2):375–382. | ||
Sarrazin C, Dvory-Sobol H, Svarovskaia E, et al. Baseline and Post-Baseline Resistance Analyses of Phase 2/3 Studies of Ledipasvir/Sofosbuvir ± RBV. AASLD Liver Meeting; 2014; Boston, MA, USA. | ||
Sarrazin C, Dvory-Sobol H, Svarovskaia E, et al. The prevalence and the effect of HCV NS5A Resistance-Associated Variants in Patients With Compensated Cirrhosis Treated With Ledipasvir/Sofosbuvir ± RBV. 50th Annual Meeting EASL, 2015; Vienna, Austria. | ||
Marcellusi A, Viti R, Capone A, Mennini F. The economic burden of HCV-induced diseases in Italy. A probabilistic cost of illness model. Eur Rev Med Pharmacol Sci. 2015;19(9):1610–1620. | ||
Lavitz E, Flamm S, Yang J et al. Retreatment of patients who failed 8 or 12 weeks of ledipasvir/sofosbuvir based regimens with ledipasvir/sofosbuvir for 24 weeks. 50th Annual Meeting of the European Association for the Study of the Liver; April 22–26, 2015; Vienna, Austria. | ||
Kwo P, Gitlin N, Nahass R, et al. Simeprevir plus sofosbuvir (12 and 8 weeks) in hepatitis C virus genotype 1-infected patients without cirrhosis: OPTIMIST-1, a phase 3, randomized study. Hepatology. 2016;64(2):370–380. | ||
Lawitz E, Sulkowski M, Ghalib R, et al. Simeprevir plus sofosbuvir, with or without ribavirin, to treat chronic infection with hepatitis C virus genotype 1 in non-responders to pegylated interferon and ribavirin and treatment-naive patients: the COSMOS randomised study. Lancet. 2014;384(9956):1756–1765. | ||
Lawitz E, Matusow G, DeJesus E, et al. Simeprevir plus sofosbuvir in patients with chronic hepatitis C virus genotype 1 infection and cirrhosis: A phase 3 study (OPTIMIST-2). Hepatology. 2016;64(2):360–369. | ||
Westerhout K, Treur M, Mehnert A, Pascoe K, Ladha I, Belsey J. A cost utility analysis of simeprevir used with peginterferon + ribavirin in the management of genotype 1 hepatitis C virus infection, from the perspective of the UK National Health Service. J Med Econ. 2015;18(10):838–849. | ||
Colombo M, Fernández I, Abdurakhmanov D, et al. Safety and on-treatment efficacy of telaprevir: the early access programme for patients with advanced hepatitis C. Gut. 2014;63(7):1150–1158. | ||
Colombo M, Piccioto A, Andreone P. Treatment of hepatitis C genotype 1 patients with severe fibrosis or compensated cirrhosis: the Italian telaprevir early access program. 46th A.I.S.F. Annual Meeting 2013/Digestive and Liver Disease 45S (2013), S1–S48. | ||
Townsend R, McEwan P, Kim R, Yuan Y. Structural frameworks and key model parameters in cost-effectiveness analyses for current and future treatments of chronic hepatitis C. Value Health. 2011;14(8):1068–1077. | ||
Fernandez-Rodriguez CM, Alonso S, Martinez SM, et al. Peginterferon plus ribavirin and sustained virological response in HCV-related cirrhosis: outcomes and factors predicting response. Am J Gastroenterol. 2010;105(10):2164–2172. | ||
Wright M, Grieve R, Roberts J, Main J, Thomas HC. Health benefits of antiviral therapy for mild chronic hepatitis C: randomised controlled trial and economic evaluation. Health Technol Assess. 2006;10(21):1–113. | ||
Siebert U, Sroczynski G, Rossol S, et al. Cost effectiveness of peginterferon alpha-2b plus ribavirin versus interferon alpha-2b plus ribavirin for initial treatment of chronic hepatitis C. Gut. 2003;52(3):425–432. | ||
Italian National Institute of Statistics. Life tables 2014. Available from: http://dati.istat.it/?lang=en&SubSessionId=a172e556-d13e-44c4-bfef-5ebf67942031&themetreeid=-200. Accessed November 30, 2016. | ||
Younossi Z, Boparai M, McCormick M, Price LL, Guyatt G. Assessment of utilities and health-related quality of life in patients with chronic liver disease. Am J Gastroenterol. 2001;96(2):579–583. | ||
Sullivan S, Jensen D, Bernstein D, et al. Cost-effectiveness of combination peginterferon alpha-2a and ribavirin compared with interferon alpha-2b and ribavirin in patients with chronic hepatitis C. Am J Gastroenterol. 2004;99(8):1490–1496. | ||
Kim WR, Poterucha JJ, Hermans JE, Therneau TM, Dickson ER, Evans RW, Gross JB Jr. Cost-effectiveness of 6 and 12 months of interferon-alpha therapy for chronic hepatitis C. Ann Intern Med. 1997;127(10):866–874. | ||
Bennett WG, Inoue Y, Beck JR, Wong JB, Pauker SG, Davis GL. Estimates of the cost-effectiveness of a single course of interferon-alpha 2b in patients with histologically mild chronic hepatitis C. Ann Intern Med. 1997;127(10):855–865. | ||
Sullivan SD, Craxi A, Alberti A, et al. Cost effectiveness of peginterferon alfa-2a plus ribavirin versus interferon alfa-2b plus ribavirin as initial therapy for treatment-naive chronic hepatitis C. Pharmacoeconomics. 2004;22(4):257–265. | ||
Camma C, Petta S, Enea M, et al. Cost-effectiveness of boceprevir or telaprevir for untreated patients with genotype 1 chronic hepatitis C. Hepatology. 2012;56(3):850–860. | ||
Camma C, Petta S, Cabibbo G, et al. Cost-effectiveness of boceprevir or telaprevir for previously treated patients with genotype 1 chronic hepatitis C. J Hepatol. 2013;59(4):658–666. | ||
Petta S, Cabibbo G, Enea M, et al. Personalized cost-effectiveness of boceprevir-based triple therapy for untreated patients with genotype 1 chronic hepatitis C. Dig Liver Dis. 2014;46(10):936–942. | ||
Petta S, Cabibbo G, Enea M, et al. Cost-effectiveness of sofosbuvir-based triple therapy for untreated patients with genotype 1 chronic hepatitis C. Hepatology. 2014;59(5):1692–1705. | ||
Younossi Z, Singer M, Mir H, Henry L, Hunt S. Impact of interferon free regimens on clinical and cost outcomes for chronic hepatitis C genotype 1 patients. J Hepatol. 2014;60(3):530–537. | ||
Associazione Italiana per lo Studio del Fegato. Documento di indirizzo dell’Associaziona Italiana per lo Studio del Fegato per l’usi razionale di antivirali diretti di seconda generazione nelle categorie di pazienti affetti da epatitie C cronical ammesse alla rimborsabilita in Italia. Available from: http://www.webaisf.org/pubblicazioni/documento-aisf-hcv-2015.aspx. Accessed November 30, 2016. | ||
Italian National Agency for Regional Healthcare Services (AGENAS). Available from: www.agenas.it/. Accessed April, 2013. | ||
Shepherd J, Jones J, Hartwell D, Davidson P, Price A, Waugh N. Interferon alpha (pegylated and non-pegylated) and ribavirin for the treatment of mild chronic hepatitis C: a systematic review and economic evaluation. Health Technol Assess. 2007;11(11):1–205. | ||
Hartwell D, Jones J, Baxter L, Shepherd J. Peginterferon alfa and ribavirin for chronic hepatitis C in patients eligible for shortened treatment, re-treatment or in HCV/HIV co-infection: a systematic review and economic evaluation. Health Technol Assess. 2011;15(17):1–210. | ||
National Institute for Health and Clinical Excellence (NICE). Final Appraisal Determination – Telaprevir for the Treatment of Genotype 1 Chronic Hepatitis C; 2012. Available from: https://www.nice.org.uk/guidance/ta252/documents/hepatitis-c-genotype-1-telaprevir-final-appraisal-determination-document2. Accessed November 30, 2016. | ||
Gemeinsamer Bundesausschuss (G-BA). MSD SHARP & DOHME GmbH. Dossier zur Nutzenbewertung. Modul 4. Boceprevir (VICTRELIS); 2011. Available from: https://www.g-ba.de/informationen/nutzenbewertung/8/. Accessed November 30, 2016. | ||
Drummond M, Jefferson T. Guidelines for authors and peer reviewers of economic submissions to the BMJ. The BMJ economic evaluation working party. BMJ. 1996;313(7052):275–283. | ||
Afdhal N, Reddy K, Nelson D, et al. Ledipasvir and sofosbuvir for previously treated HCV genotype 1 infection. N Engl J Med. 2014;370(16):1483–1493. | ||
Thorlund K, Druyts E, El Khoury AC, Mills EJ. Budget impact analysis of boceprevir and telaprevir for the treatment of hepatitis C genotype 1 infection. Clin Outcomes Res. 2012;4:349–359. |
 © 2017 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2017 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.