Back to Journals » Neuropsychiatric Disease and Treatment » Volume 17
Optimal Method of Electrical Stimulation for the Treatment of Upper Limb Dysfunction After Stroke: A Systematic Review and Bayesian Network Meta-Analysis of Randomized Controlled Trials
Authors Tang Y, Wang L, He J, Xu Y, Huang S, Fang Y
Received 6 August 2021
Accepted for publication 1 September 2021
Published 15 September 2021 Volume 2021:17 Pages 2937—2954
DOI https://doi.org/10.2147/NDT.S332967
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Taro Kishi
Yuqi Tang,1,* Linjia Wang,2,* Jinxi He,3 Yipeng Xu,2 Shijie Huang,2 Yu Fang1
1Department of Neurology, Hospital of Chengdu University of Traditional Chinese Medicine, Chengdu, 610072, People’s Republic of China; 2School of Acu-Mox and Tuina, Chengdu University of Traditional Chinese Medicine, Chengdu, 610075, People’s Republic of China; 3Department of Pain, Sichuan Provincial Transportation Department Road Bureau Hospital, Chengdu, 611731, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Yu Fang Email [email protected]
Background: The obstacle of limb motor caused by stroke, especially the decline of motor function of upper limbs, can directly affect the activities of daily living of stroke patients with hemiplegia. Based on long-term clinical practice, the treatment effect of electrical stimulation methods for stroke limb dysfunction has been widely recognized and supported by authoritative guidelines and systematic reviews. However, which electrical stimulation method is the optimum in the treatment of stroke limb dysfunction is still a controversial issue.
Objective: In this paper, we adopted Network Meta-Analysis (NMA) to rank the priorities of various electrical stimulation methods, so as to select the optimal electrical stimulation method and discuss its rationality in guiding clinical practice.
Methods: We carried out a systematic review by searching a total of 6806 studies from 8 databases and 2 clinical trial registries, and finally screened out 34 studies for further investigation. Then, pairwise meta-analysis and Bayesian network meta-analysis were employed to evaluate the effectiveness and ranking of various interventions. The primary outcome measure was Fugl-Meyer Assessment Upper Extremity (FMA-UE), and the secondary outcome measures were Modified Barthel Index (MBI) and Modified Ashworth Scale (MAS). Finally, the risk of bias, publication bias and sensitivity of the Randomized Controlled Trials (RCTs) were evaluated.
Results: On the basis of comprehensive rehabilitation treatment (RT), the Functional Electrical Stimulation (FES) was superior than other electrical stimulation methods in improving both FMA-UE and MBI. Meanwhile, the results indicated that the Transcutaneous Electrical Acupoint Stimulation (TEAS) was the only electrical stimulation method that showed treatment advantages in reducing MAS.
Conclusion: The study showed that FES had the optimal overall rehabilitation effect on upper limb dysfunction of stroke patients based on the comprehensive RT, while the treatment effect of TEAS on upper limb spasticity after stroke was the most significant.
Keywords: network meta-analysis, electrical stimulation, stroke, upper limb dysfunction
Background
Stroke is a kind of cerebral blood circulation disorder which can cause motor dysfunction, and it has become one of the main causes of disability in the elderly.1 A global epidemiological prediction report on nervous system diseases shows that stroke has been accounted for a larger proportion of the global disease burden in recent years.2 The motor dysfunction of limbs caused by stroke, especially the decline of motor function of disabled upper limbs, can directly have an adverse effect on activities of daily living of stroke patients with hemiplegia. A number of studies show that 30–66% of the patients with hemiplegia after stroke still have no motor function in their upper limbs 6 months later.3 Various of treatment methods have been applied to the treatment of this kind of disease, but it is still very tricky to select an optimal treatment method when determining the treatment scheme for the recovery of upper limb function.4 The rehabilitation of upper limb dysfunction after stroke is still one of the difficulties in the treatment of stroke-related sequelae.
At present, in the Rehabilitation Treatment (RT) of stroke patients with hemiplegia, various rehabilitation techniques based on the concepts and theories of Bobath, Rood, Brunnstrom and Proprioceptive Neuromuscular Facilitation (PNF) are the primary rehabilitation training methods. Recently, an increasing number of novel treatments have been applied to the rehabilitation of stroke patients with upper limb motor dysfunction, including Virtual Reality (VR),5 Brain-machine Interface (BI),6 Mental Practice (MP),7 extracorporeal shock wave therapy,8 music therapy9,10 and so on. However, the effect of these novel treatments in clinical practice has not been fully verified. It is still a burning issue for clinicians to use practical and effective treatments based on traditional rehabilitation. The electrical stimulation has been widely regarded as an effective approach for the treatment of stroke limb dysfunction through long-term clinical practice.11
Diverse electrical stimulation methods have been applied in medical field. The frequently adopted methods for limb dysfunction after stroke are including: Transcutaneous Electrical Nerve Stimulation (TENS), Neuromuscular Electrical Stimulation (NMES), Functional Electrical Stimulation (FES), Transcranial Direct Current Stimulation (tDCS) and Transcutaneous Electrical Acupoint Stimulation (TEAS). TENS is transdermal output pulse current to effectively control the pain and stimulate the sensory, thus increasing muscle power and movement function and decreasing spasticity.12 TEAS is basically the combination of TENS and acupuncture points. FES is the electrical stimulation of motor neurons to stimulate muscle contraction and generate/increase joint torque. Some researchers define FES as electrical stimulation applied in voluntary movement.13 NMES typically adopts higher frequencies (20–50 Hz) and can be used to produce muscle tetany and contraction for “functional” purposes.14 tDCS works by applying a weak and constant direct current to the brain and is capable of enhancing or suppressing cortical excitability.15 During the past few years, many efforts have been dedicated to the systematic reviews and meta-analyses on the effect of the above electrical stimulation methods in the treatment of upper limb dysfunction after stroke. Vafadar et al16 studied the effect of FES on preventing or reducing shoulder subluxation in early stage after stroke. Eraifej et al13 investigated the effect of FES on improving activities of daily living and motor function after stroke. Elsner et al17 proposed that tDCS can improve the activities of daily living and cognitive function of stroke patients. Nevertheless, it can be found that most of the research concentrates on only one type of electrical stimulation method or isolated upper limb parts (such as hand, elbow, shoulder). There is a lack of comprehensive analysis and discussion on the treatment effect of various electrical stimulation methods in the related public literature. Generally, more than one rehabilitation treatments are adopted as the basic treatment method in the actual clinical treatment process.18,19 Therefore, it is extremely essential to study and select an optimal electrical stimulation method for upper limb motor dysfunction after stroke based on comprehensive RT. In this paper, we applied NMA to establish a network of RCTs by combining direct and indirect evidence in the network of RCTs to compare different treatment schemes. NMA, also known as multiple treatment meta-analysis or mixed treatment comparison, is an extension of paired meta-analysis, allowing more than two interventions to be compared in a single and coherent analysis of all relevant RCTs.20 Since NMA can contribute to evaluate the comparative effectiveness of different treatment methods frequently used in clinical practice, it has triggered a widespread attention among clinicians.21 Systematic assessment of RCTs has been generally considered to be the authoritative evidence to verify the effectiveness of interventions.22,23 Based on the proposed approach, we employed NMA to rank the priority of different electrical stimulation methods aiming at selecting an optimal electrical stimulation method and discussing its rationality in guiding clinical practice.
Methods
This study abided by the PRISMA-NMA guide.24 (Additional file 1) And a version of this study was registered on Open Science Framework (Registration DOI: 10.17605/OSF.IO/TAZ8D).
Search Strategy
In order to ensure the sufficient number of selected literature, the retrieval time was set from the establishment date of each database to July 20, 2021. The literature language was set within Chinese and English. The search databases included: China National Knowledge Infrastructure (CNKI), VIP Database for Chinese Technical Periodicals (VIP), WANFANG Database (WF), Chinese biomedical literature service system (SinoMed), PubMed, Web of Science (WOS), Embase, and Cochrane Library. The clinical trial registries included the International Standard Randomized Controlled Trial Number (ISRCTN) Register and the Chinese Clinical Trial Registry (ChiCTR). In addition, MeSH terms and free words used in this study included: (1) transcutaneous electrical nerve stimulation, transcutaneous electrical acupoint stimulation, neuromuscular electrical stimulation, functional electrical stimulation et al (2) stroke, cerebrovascular accident, brain vascular accident et al (3) randomized controlled trials, clinical trials et al. Taking PubMed database as an example, the complete data retrieval strategy is shown in Table 1.
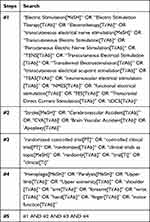 |
Table 1 Data Retrieval Strategy for PubMed Database |
Inclusion and Exclusion Criteria
Type of Study
All the included literature covered the journal articles of both Chinese and English RCTs based on all selected databases from the establishment date of the database to July 20, 2021. There was no restriction on the publication status of articles in this paper. The excluded types were conference articles, newspaper articles, and book abstracts in all databases. Meanwhile, master’s and doctoral theses were included as well. Moreover, negative results may be achieved in the case of that the included patients are far from enough. In this case, the intervention was actually effective. However, due to the small sample size, false negative results were produced.25 Therefore, for the purpose of ensuring the quality of the included literature and reducing the bias of this study, we excluded the literature with a total sample size of less than 30.
Type of Participant
The patients in the included literature were supposed to meet the relevant diagnostic criteria of ischemic or hemorrhagic stroke. This study had no restrictions on gender, age, course of disease and race of patients. The excluded types were hemiplegia or limb dysfunction caused by various other reasons, such as Spinal Cord Injury (SCI).
Type of Intervention
All the included literature was required to meet implementation standards of intervention set by us for both experimental and control group. The specified standards were as follows: (1) The experimental group should include one or more methods of TENS, FES, NMES, TEAS, tDCS combined with RT. Due to the complexity of the condition of stroke patients, the RT techniques used in RCTs are not be exactly same. We consulted the relevant guidelines26,27 in the preliminary work. The scope of RT was determined on the basis of the guidelines and combined with clinical practice. RT should cover various comprehensive RT methods, such as exercise therapy, occupational therapy, rehabilitation training, rehabilitation education, functional exercise, conventional drug treatment as well as routine nursing for stroke. (2) The control group should include one or more methods of TENS, FES, NMES, TEAS, tDCS combined with RT (same as the experimental group) or only RT; in the meantime, RT combined with Sham Stimulation (SS) should be also included in this group. The excluded interventions were following: (1) The research focus of the experimental group or the control group was a certain rehabilitation therapy, such as stretching training and mirror therapy combined with electrical stimulation, rather than comprehensive rehabilitation therapy. (2) The comparisons of different stimulation sites, stimulation frequency, stimulation methods and stimulation duration between the same or different kinds of electrical stimulation. Furthermore, if the intervention methods for the different groups in multi-arm studies were identical, but the intervention measures were different, we would combine the data of the two groups. In addition, there were no restrictions on the treatment dose, stimulation site, treatment duration and treatment frequency of electrical stimulation.
Type of Outcome Measure
In the present paper, the primary outcome measure was FMA-UE, and the secondary outcome measures were MBI and MAS. FMA-UE is a well-designed, feasible and effective clinical examination method, which has been widely used in motor function evaluation of stroke patients.28 MAS is a frequently-used clinical assessment tool for muscle tone increase, which is mainly used to assess spasticity caused by stroke.29 MBI was adopted to evaluate the activities of daily living, thus reflecting the improvement of limb dysfunction.30 It is worth mentioning that the baseline data is the first evaluation data before the first treatment, and the outcome measure data is the first evaluation data after the last treatment. In addition, studies that did not use any of the specified outcomes were excluded.
Study Screening Process
Firstly, we adopted the aforementioned search strategy to retrieve relevant studies from databases (CNKI, VIP, WF, SinoMed, PubMed, WOS, Embase and Cochrane Library) and the clinical trial registries (ISRCTN and ChiCTR), and then imported them into NoteExpress (V3.4.0) database. Subsequently, two professionally trained reviewers (LW and YT) would conduct a preliminary screening for all studies. Only the studies with complete titles and abstracts meeting the above inclusion criteria can be preliminarily screened out for further study. Besides, the studies with incomplete titles and abstracts were marked and would be re-screened in the following steps. Finally, the reviewers would review the preliminary selected studies thoroughly, and further screened out the studies completely meeting the inclusion criteria. In order to ensure the objectivity and reliability of the selected results, the two reviewers were required to select independently. If there were divergences between the two reviewers, the third professional reviewer (YF) would intervene in for further evaluation.
Data Processing and Analysis Process
The data extraction of the included studies was carried out by two professional reviewers (YX and SH) independently. The extracted data included title, reviewer’s name, publication time, country, stroke type and classification, course of disease, sample size and sample proportion, age, sex ratio, intervention method, treatment cycle and outcome measure (mean and standard deviation).
We used Review Manager (Revman V5.3) and Aggregate Data Drug Information System (ADDIS V1.16.8) to implement the meta-analysis. Pairwise meta-analysis was adopted for direct comparison. Where there was no significant heterogeneity (I2 < 50%), we used the fixed effect model for comprehensive analysis. Whereas if there was obvious heterogeneity between studies (I2 ≥50%), the random effect model would be adopted. A Markov chain Monte Carlo method was used to conduct NMA using the ADDIS. The NMA network plots of three outcome measures were generated in Stata software (V16.0 MP). For any possible situation, NMA was performed only when different interventions were connected into a network. Moreover, the node-splitting method was employed to divide the evidence of each comparison into direct evidence and indirect evidence. The surface under the cumulative ranking curve (SUCRA) was used to rank the advantages and disadvantages of different electrical stimulation methods.
Risk of Bias Assessment
In this study, the Cochrane Collaboration risk of bias tool was used to assess the bias risk of RCTs. The assessment items included: (1) Random sequence generation (selection bias); (2) Allocation concealment (selection bias); (3) Blinding of participants and personnel (performance bias); (4) Blinding of outcome assessment (detection bias); (5) Incomplete outcome data (attrition bias); (6) Selective reporting (reporting bias); (7) Other bias. The assessment results would be divided into high risk (H), low risk (L) and unclear risk (N). If all of the assessment items of one trial were low risk, or there were less than three unclear risk items, the trial would be finally judged as low risk. If two or more assessment items of one trial were high risk, the trial would be considered as high risk.31 Other trials would be classified as unclear risk.
Publication Bias Assessment
The publication bias was assessed by funnel plots generated by Stata software.
Sensitivity Analysis
We evaluated the robustness of each result by sensitivity analyses, excluding high-risk bias studies.
Results
Literature Research
In this study, a total of 6806 studies were retrieved from eight databases and two clinical trial registries. A total of 342 studies meeting the basic inclusion criteria were preliminarily selected after the titles and abstracts reviewed by two reviewers. Then, the preliminary selected studies would be thoroughly reviewed by the other two reviewers. Meanwhile, the third reviewer would be involved to re-evaluate the studies selected for further study. Finally, a total of 34 RCTs32–65 were included in this study. The specific study screening process is illustrated in Figure 1.
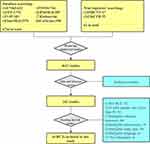 |
Figure 1 Flow chart of study selection. |
In this study, a total of 34 RCTs with 2383 patients, of which 46 patients dropped out during the trial were included. The details of all included studies are shown in Additional file 2. As the progress of stroke has a dramatic impact on the upper limb motor function, most studies described the baseline data of stroke types and course (duration of stroke). Only 7 studies32,33,35,36,48,52,54 accurately described the stroke stages, and 13 studies32,34,38,39,41,43–47,49,55,61 specified Brunnstrom stages. The types of stroke were classified as ischemic or hemorrhagic in all studies describing stroke types, whereas the course of stroke were not specified in four studies describing the stroke course.33,36,53,57 Except that the patient allocation ratio of the two studies59,63 was 1:1:1, the allocation ratio of the other studies was 1:1. One study33 only described the average age, while another study53 described the overall age and sex ratio of patients without distinguishing between the control group and the experimental group. Other trials had detailed age and sex baseline data. Additionally, the interventions, treatment cycles and outcome measures of all studies were complete.
Since there were no restrictions on the treatment dose, stimulation site, treatment duration and treatment frequency of electrical stimulation in inclusion criteria, in order to ensure the continuity and integrity of this study, the two reviewers independently sorted out the information of the above influencing factors during the study data entry stage. The details are shown in Additional file 3. In most studies, the frequency/intensity, site and duration of the same electrical stimulation were roughly the same, while the frequency/intensity of different electrical stimulation were quite different, which was related to the basic stimulation dose required by different electrical stimulation treatments. The treatment frequency was mainly once a day, and 5 days a week.
The results of bias risk assessment are illustrated in Figures 2 and 3, and the detailed assessment results are shown in Additional file 4. The results indicated that 5 trials were not classified into high-risk category by standard random allocation method, 4 trials of which56,58,61,64 were allocated according to the order of inclusion, and one of which37 was grouped according to the wishes of patients and their families. 5 trials44,51,55,62,63 were classified as high risk due to the inappropriate adopted blinding method. Moreover, 8 trials33,34,43,47,56,59,63,65 had other high-risk biases, including 4 trials in which the gender ratio of patients in the two groups was seriously unbalanced, 4 trials in which the proportion of stroke types was significantly different between the two groups, and one trial in which the course of disease was considerably different between the two groups. Among the 8 trials, one trial59 with a drop-out rate >20% at 8 weeks of follow-up was also included due to the complete outcome measure data after 4 weeks of treatment. In addition, it can be found that there was no high-risk bias trial related to 4 assessment items, including allocation concealment (selection bias), incomplete outcome data (attrition bias), selective reporting (reporting bias), blinding of outcome assessment (detection bias). In general, two trials56,63 were classified as high-risk overall bias, accounting for 5.9%.
 |
Figure 2 Risk of bias graph. |
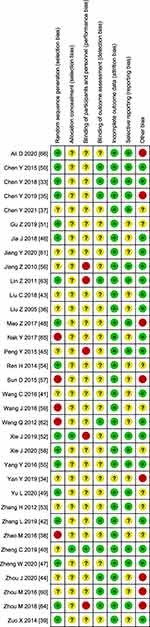 |
Figure 3 Risk of bias summary. |
Pairwise Meta-Analysis
In the present paper, the two intervention methods were compared comprehensively by pairwise meta-analysis. The results are shown in Additional file 5. The results of three outcome measures are summarized in Table 2, highlighting the intervention groups with meaningful comprehensive effects. When we used FMA-UE to evaluate the trial results, the treatment effect of FES, tDCS, TENS or TEAS combined with RT was superior than that of RT. When we adopted MBI to evaluate the trial results, the FES, tDCS or TEAS combined with RT outperformed RT. As for MAS, the treatment effect of TEAS combined with RT was better than that of RT. Meanwhile, compared with RT, the tDCS combined with RT had a more desirable treatment effect using FMA-UE and MBI to evaluate. In addition, in a trial adopting MAS for evaluation,38 the treatment effect of tDCS combined with RT surpassed that of RT combined with SS.
 |
Table 2 The Results of Three Outcome Measures |
Network Meta-Analysis
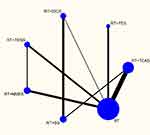
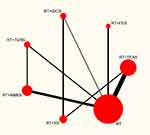
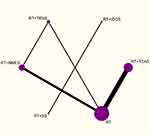
Figures 4–6 depict the network plots of different interventions for three outcome measures. The node size of each intervention represents the total number of patients included in the RCTs, and the thickness of the line between two nodes represents the total number of RCTs involving the two groups of interventions. FMA-UE and MBI were used for the evaluation of 7 interventions respectively, and MAS was used to evaluate 6 interventions. To be specific, FMA-UE was the outcome measure for 33 trials involving 1927 patient in total; MBI was the outcome measure for 27 trials with a total of 1648 patients; MAS was the outcome measure for 12 trials with 624 patients. As shown in Figures 4 and 5, the network structures among the interventions evaluated by FMA-UE and MBI were approximately the same. The primary difference is the number of patients with different interventions and RCTs, indicating that FMA-UE and MBI were common outcome measures for evaluating the rehabilitation of upper limb dysfunction after stroke. As illustrated in Figure 6, the trials without using FES were evaluated by MAS, and the number of trials and patients involved is less than that involved in FMA-UE and MBI. It is worth noting that the trials of tDCS combined with RT and SS combined with RT were not directly related to other intervention groups, and the number of trials and patients was also small. In general, except for RT, TEAS or NMES combined with RT had the largest number of trials and patients.
Since the interventions of one group of trials (RT+tDCS vs RT+SS) from the MAS network plot were independent of any interventions of other trials (shown in Figure 6), and this group of trials did not meet the requirements for subsequent NMA, it cannot be processed by Stata software. Thus, this group of trials was excluded for further analysis.
The effectiveness of NMA results depended on the internal consistency of the evidence network: the sources of direct evidence and various indirect evidence should be consistent.66 In this study, we adopted the segmented node method to test inconsistency in the NMA (Additional file 6). The results indicated that the direct or indirect comparisons of each segmented node had no statistical significance (p>0.05), demonstrating that there was no evidence of design inconsistency. We tested the convergence of the model, and the potential scale reduction factor was 1 (Additional file 7).
Figures 7 and 8 show the NMA results. It can be seen from the figures that in terms of improving FMA-UE score, FES, TEAS and TENS combined with RT were better than RT. In the meantime, the treatment effect of FES combined with RT was superior than that of NMES, TENS and SS combined with RT. In addition, the treatment effect of SS combined with RT was inferior than that of TEAS, TENS and tDCS combined with RT. When it comes to improving MBI score, FES, TEAS and tDCS combined with RT outperformed RT. On the basis of RT, the treatment effect of TEAS, tDCS and FES were better than that of SS, and the treatment effect of NMES was not as desirable as that of TEAS. The decrease of MAS score represents the relief of upper limb spasm. The treatment effect of TEAS combined with RT was more excellent than that of RT. Moreover, there was no significant treatment effect among the other interventions.
In this paper, we adopted a consistent model using ADDIS to rank various interventions in NMA, and the results were illustrated as ranking probability matrix (Figures 9–11). The ranking value corresponding to each intervention represented its probability. It can be seen from Figure 9 that FES combined RT was the most effective method to promote the FMA-UE score, followed by TEAS, tDCS, TENS and NMES. TEAS had the most significant effect on improving the MBI score (Figure 10). Additionally, TEAS had an optimal effect on reducing the MAS score (Figure 11). The SUCRA scores are specified in Additional file 8.
Sensitivity Analysis
Sensitivity analysis was carried out for all pairwise meta-analyses after excluding trials with high-risk bias. The results indicated that the treatment effect of NMES combined with RT was superior than that of RT (SMD 0.71, 95% CI (0.06, 1.73)).
Publication Bias
The funnel plots were used to evaluate the publication bias (Figures 12–14). As shown in these figures, the quantity distributions of trials involved in the three outcome measures were relatively symmetrical. Due to the limited sample size of RCTs, most trials were distributed in the lower middle part of the figures. As for all of the three outcome measures, there were a small number of trials outside the 95% confidence interval, indicating that there was potential heterogeneity in these trials. Furthermore, the missing angle of MBI (Figure 13) on the left side of the red vertical line (odds ratio=0) may be relevant to the unpublished trials with negative results.
Adverse Events
In the present paper, a total of 4 RCTs had adverse events with 12 patients having adverse reactions. Among these RCTs, two trials38,39 using tDCS combined with RT had 7 patients with skin discomfort, of which 2 patients had sleep disorders at the same time, of which 1 patient had dizziness under SS treatment. In one trial,51 one patient in the experimental group had severe shoulder pain under NMES combined with RT, and the other patient in the control group had recurrent cerebral infarction only under RT. In another trial,63 2 patients with poor physical conditions used non-steroidal anti-inflammatory drugs during the follow-up period, and had aggravated shoulder pain caused by improper care.
Discussion
Main Findings
We conducted a systematic review and Bayesian network meta-analysis on the relevant evidence of 34 RCTs (including 2383 patients). Various common electrical stimulation methods for the treatment of limb dysfunction after stroke were selected for the study in this paper, including FES, NMES, TEAS, TENS and tDCS. Among them, TEAS is within the scope of TENS. At present, TEAS has been widely used in the treatment of various diseases, and multiple relevant RCTs have been carried out.67 Therefore, we studied TEAS independently of TENS in this paper. Although the above 5 electrical stimulation methods have been extensively adopted in clinic, the strengths and weaknesses of these methods still need to be further investigated.
It can be deduced from the results that all of the selected electrical stimulation methods are effective in improving FMA-UE and MBI scores. Nevertheless, compared with other studies, some studies61–63 presented an opposite trend in MAS, which may be related to the course of the disease of the patient. Besides, unlike the points system of FMA-UE and MBI, MAS adopts the rating system to evaluate the condition of patients,68 implying that the variation trend of MAS may better reflect the change rule of the disease. In pairwise meta analysis and NMA, whether FMA-UE or MBI was used for evaluation, FES presented an optimal treatment effect. Some scholars have done relevant research and found the advantages of FES in the treatment of upper limb dysfunction after stroke.13 Recent studies69,70 have indicated that this may be related to the fact that FES can induce more plasticity changes and brain remodeling. Nevertheless, taking the uncontrollable factors of interventions into account, the RCTs included in this study did not consider some FES that were not widely used in clinic or need additional devices, such as brain computer interface, EMG biofeedback or new auxiliary electrical stimulation devices. We intended to systematically study on various new treatment schemes of FES in the future.
It has been proved that TEAS and tDCS were also effective treatment methods. Especially when reducing the MAS, TEAS was the only electrical stimulation method that showed treatment advantages, demonstrating that it had a significant effect on improving upper limb spasm after stroke. In the meantime, TENS did not show the same effect as TEAS, indicating that the combination of traditional Chinese medicine acupuncture points can improve the effect of electrical stimulation to a certain extent. Moreover, it should also be noted that only one trial of TENS evaluated by MAS was included, and this trial was classified as a high-risk bias due to the large difference between the experimental group and the control group, so the results may be biased. More high-quality RCTs need to be conducted in the future to further verify this result. Furthermore, NMES was not as effective as the other 4 electrical stimulation methods. It can be seen from the ranking probability matrix (Figures 9 and 10) that NMES ranked fifth in improving the scores of FMA-UE and MBI. As shown in the network plots (Figures 4–6), the number of RCTs involving NMES was second only to that involving TEAS, and so did the number of patients. In clinic, NMES has been extensively adopted for patients with limb function after stroke, but its treatment effect remains to be further investigated. Some studies have shown that NMES has limitations in recovering motor function, which may be related to the recruitment of motor units.71,72 Some researchers have claimed that the actual clinical treatment effect of NMES depends on the systematic treatment scheme.73
In addition, the treatment effect of either RT or SS was the worst. However, it is interesting to find out that the treatment effect of RT alone was better than that of RT combined with SS in the evaluation of FMA-UE and MAS. On the one hand, it may prove that the placebo effect of SS on patients is not strong enough. On the other hand, it may also be related to the publication bias.
Overall Quality of Evidence
In this study, the RCTs included in the study have been carefully selected. The results manifested that four RCTs had adverse events. To be specific, a total of 12 patients had adverse reactions, most of the adverse reactions were local skin discomfort, and only one patient had recurrent cerebral infarction during RT. Consequently, it was proved that the electrical stimulation methods were safe.
Unlike the drug test, it is quite hard to realize double blindness in the actual clinical practice. The blinding of participants or personnel is impractical for various electrical stimulation methods. For instance, it is difficult for personnel to be unaware of whether the electrical stimulator is on during the trials. After evaluating each trial through the Cochrane Collaboration risk of bias tool, we found that only three trials44,55,63 clearly did not use the blind method, one trial39 clearly used blind method, and other trials did not clarified the relevant information. Unblinded results may bias the final comprehensive effect evaluation. On average, unblinded evaluators of subjective binary outcomes generated substantially biased effect estimates in randomized clinical trials, exaggerating the odds ratio by 36%.74 As a result, the evaluation of the results of such trials should be treated carefully.
Based on the above reasons, we conducted the sensitivity analyses, and the results showed that most of the results were reliable. Moreover, publication bias also deserved attention. As for all of the three outcome measures, there were a small number of trials outside the 95% confidence interval, indicating that there was potential heterogeneity in these trials. Additionally, the missing angle of MBI (Figure 13) on the left side of the red vertical line (odds ratio=0) may be relevant to the unpublished trials with negative results.75,76 Furthermore, when it was not clear whether some trial data were normally distributed, we did not convert the quartile and the median or the minimum/maximum of the median into the mean and standard deviation, thus influencing the publication bias to a some degree. In this study, the research team carried out a comprehensive search of 8 databases and 2 clinical trial registries, and even searched for unpublished data. The asymmetric funnel plot (Figure 14) indicated that there may be small-study effects in the whole network.77 However, considering the comprehensive retrieval in the early stage of this study, we did not regard the asymmetry of the funnel plot as a specific evidence of publication bias.78
Strengths and Limitations
The strengths of this study are as follows. Firstly, this study was conducted according to PRISMA-NMA24 and PRISMA guidelines and checklist.79 In order to ensure the reliability of the final conclusion, the research team implemented a comprehensive search of 8 databases and 2 clinical trial registries, including RCTs only. Secondly, the Bayesian analysis approach adopted in this study was more accurate than the frequency-based methods. In addition, there were few relevant studies on the treatment effect comparison of different electrical stimulation methods for upper limb dysfunction after stroke, and this was the first academic study to investigate five different electrical stimulation methods and comprehensively compare their treatment effects on the basis of comprehensive RT. Furthermore, we made efforts to get the included trials closer to clinical practice by strictly restricting comprehensive RT methods, thus making the results have more guiding significance for clinicians.
Nevertheless, this study still has some limitations. First of all, most RCTs did not accurately describe the stages of stroke, and the duration of some trials varied significantly, which had a dramatic impact on the baseline data and outcome measure data. Besides, the dose settings (including frequency and current) of various electrical stimulation methods or the same electrical stimulation in different RCTs were quite different, and there was no standard for dose setting. Moreover, the specific stimulation site, treatment duration and treatment frequency of the same electrical stimulation method in different RCTs were also various. In this paper, we did not set any restrictions on the above influencing factors, but this may increase the risk of research bias. In the future, we will study the frequency/intensity of the same or different electrical stimulation methods in detail. Second, the language limitations of the literature, the differences of patient gender and acupoint selection of TEAS may lead to heterogeneity. Third, the two studies60,63 were three-arm tests. We divided them into three groups for comparison of different interventions, but each group had small sample size, which may reduce the reliability of the analysis results. Finally, the statistical results show that there were 50% of the studies received project funding, which may also affect the heterogeneity of the study. However, limited by the amount of research data, we did not take the impact of this factor into account.
Conclusions
This study provides plenty of effective and convincing evidence for electrical stimulation in the treatment of upper limb dysfunction after stroke. The results indicate that on the basis of comprehensive rehabilitation treatment, FES is superior than other electrical stimulation methods in improving the scores (FMA-UE and MBI) of stroke patients with upper limb dysfunction. Meanwhile, it is proved that TEAS is the only electrical stimulation method showing treatment strengths in reducing the MAS rating, indicating that TEAS has a significant effect on improving upper limb spasm after stroke. However, due to the limitations of the number of studies included and various details of RCTs, more high-quality RCTs are required to provide strong evidence to support the results.
Abbreviations
NMA, Network Meta-Analysis; FMA-UE, Fugl-Meyer Assessment for Upper Extremity; MBI, Modified Barthel Index; MAS, Modified Ashworth Scale; FES, Functional Electrical Stimulation; TENS, Transcutaneous Electrical Nerve Stimulation; TEAS, Transcutaneous Electrical Acupoint Stimulation; NMES, Neuromuscular Electrical Stimulation; tDCS, Transcranial Direct Current Stimulation; RT, Rehabilitation Treatment; PNF, Proprioceptive Neuromuscular Facilitation; VR, Virtual Reality; BI, Brain-machine Interface; MP, Mental Practice; RCT, Randomized Controlled Trial; CNKI, China National Knowledge Infrastructure; VIP, VIP Database for Chinese Technical Periodical; WF, WANFANG Database; SinoMed, Chinese biomedical literature service system; WOS, Web of Science; ISRCTN, International Standard Randomised Controlled Trial Number Register; ChiCTR, Chinese Clinical Trial Registry; Revman, Review Manager software; SMD, Standardized Mean Difference; SUCRA, Surface Under The Cumulative Ranking Curve; SCI, Spinal Cord Injury; SS, Sham Stimulation.
Data Sharing Statement
All data relevant to the study are included in the article or uploaded as supplementary information.
Acknowledgments
Thanks to Mengyu Chen for her professional writing assistance.
Author Contributions
YT designed the study and write the manuscript. LW implemented the thought with software, analyzed the data. YX and SH screened the literature and extracted the relevant data and evaluated the quality of RCTs. YT and JH dedicated in results analysis and manuscript revision. YF participated into analysis implementation and organized discussion of the results. All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval of the version to be published; and agree to be accountable for all aspects of the work.
Funding
There is no funding to report.
Disclosure
The authors declare that they have no competing interests.
References
1. Bai X, Guo Z, He L, Ren L, McClure MA, Mu Q. Different therapeutic effects of transcranial direct current stimulation on upper and lower limb recovery of stroke patients with motor dysfunction: a meta analysis. Neural Plast. 2019;2019:1372138.
2. Menken M, Munsat TL, Toole JF. The global burden of disease study: implications for neurology. Arch Neurol. 2000;57(3):418–420. doi:10.1001/archneur.57.3.418
3. Kwakkel G, Kollen BJ, van der Grond J, Prevo AJ. Probability of regaining dexterity in the flaccid upper limb: impact of severity of paresis and time since onset in acute stroke. Stroke. 2003;34(9):2181–2186. doi:10.1161/01.STR.0000087172.16305.CD
4. Bai X, Guo Z, He L, Ren L, McClure MA, Mu Q. Different therapeutic effects of transcranial direct current stimulation on upper and lower limb recovery of stroke patients with motor dysfunction: a meta-analysis. Neural Plast. 2019;2019:1372138.
5. Kim WS, Cho S, Ku J, et al. Clinical application of virtual reality for upper limb motor rehabilitation in stroke: review of technologies and clinical evidence. J Clin Med. 2020;9(10):3369. doi:10.3390/jcm9103369
6. Carvalho R, Dias N, Cerqueira JJ. Brain-machine interface of upper limb recovery in stroke patients rehabilitation: a systematic review. Physiother Res Int. 2019;24(2):e1764. doi:10.1002/pri.1764
7. Park SW, Kim JH, Yang YJ. Mental practice for upper limb rehabilitation after stroke: a systematic review and meta-analysis. Int J Rehabil Res. 2018;41(3):197–203. doi:10.1097/MRR.0000000000000298
8. Cabanas-Valdés R, Serra-Llobet P, Rodriguez-Rubio PR, López-de-celis C, Llauró-Fores M, Calvo-Sanz J. The effectiveness of extracorporeal shock wave therapy for improving upper limb spasticity and functionality in stroke patients: a systematic review and meta-analysis. Clin Rehabil. 2020;34(9):1141–1156. doi:10.1177/0269215520932196
9. Roche Bueno JC, Mincholé Lapuente E.Eficacia de la terapia musical en la recuperación funcional del miembro superior después de un ictus: una revisión sistemática y meta-análisis [Efficacy of Music Therapy in Functional Recovery of the Upper Limb After a Stroke: a Systematic Review and Meta-Analysis]. Rehabilitacion. 2019;53(3):181–188.
10. Syros A, Kotlia P, Fotakopoulos G. Preliminary findings from an Acupuncture and experiential/traditional music therapy during the standard care of rehabilitation exercise program for Recovery on post-stroke upper limb dysfunction. Int J Neurosci. 2021;18:1–11. doi:10.1080/00207454.2020.1860972
11. Sharififar S, Shuster JJ, Bishop MD. Adding electrical stimulation during standard rehabilitation after stroke to improve motor function. A systematic review and meta-analysis. Ann Phys Rehabil Med. 2018;61(5):339–344. doi:10.1016/j.rehab.2018.06.005
12. Lin S, Sun Q, Wang H, Xie G. Influence of transcutaneous electrical nerve stimulation on spasticity, balance, and walking speed in stroke patients: a systematic review and meta-analysis. J Rehabil Med. 2018;50(1):3–7. doi:10.2340/16501977-2266
13. Eraifej J, Clark W, France B, Desando S, Moore D. Effectiveness of upper limb functional electrical stimulation after stroke for the improvement of activities of daily living and motor function: a systematic review and meta-analysis. Syst Rev. 2017;6(1):40.
14. Doucet BM, Lam A, Griffin L. Neuromuscular electrical stimulation for skeletal muscle function. Yale J Biol Med. 2012;85(2):201–215.
15. Elsner B, Kwakkel G, Kugler J, Mehrholz J. Transcranial direct current stimulation (tDCS) for improving capacity in activities and arm function after stroke: a network meta-analysis of randomised controlled trials. J Neuroeng Rehabil. 2017;14(1):95. doi:10.1186/s12984-017-0301-7
16. Vafadar AK, Côté JN, Archambault PS. Effectiveness of functional electrical stimulation in improving clinical outcomes in the upper arm following stroke: a systematic review and meta-analysis. Biomed Res Int. 2015;2015:729768. doi:10.1155/2015/729768
17. Elsner B, Kugler J, Pohl M, Mehrholz J. Transcranial direct current stimulation (tDCS) for improving activities of daily living, and physical and cognitive functioning, in people after stroke. Cochrane Database Syst Rev. 2016;3(3):CD009645.
18. Wu Q, Yue Z, Ge Y, et al. Brain functional networks study of subacute stroke patients with upper limb dysfunction after comprehensive rehabilitation including BCI training. Front Neurol. 2020;27(10):1419. doi:10.3389/fneur.2019.01419
19. Hebert D, Lindsay MP, McIntyre A, et al. Canadian stroke best practice recommendations: stroke rehabilitation practice guidelines, update 2015. Int J Stroke. 2016;11(4):459–484. doi:10.1177/1747493016643553
20. Dias S, Caldwell DM. Network meta-analysis explained. Arch Dis Child Fetal Neonatal Ed. 2019;104(1):F8–F12. doi:10.1136/archdischild-2018-315224
21. Rouse B, Chaimani A, Li T. Network meta-analysis: an introduction for clinicians. Intern Emerg Med. 2017;12(1):103–111. doi:10.1007/s11739-016-1583-7
22. Brighton B, Bhandari M, Tornetta P
23. Evans D. Hierarchy of evidence: a framework for ranking evidence evaluating healthcare interventions. J Clin Nurs. 2003;12(1):77–84. doi:10.1046/j.1365-2702.2003.00662.x
24. Hutton B, Salanti G, Caldwell DM, et al. The PRISMA extension statement for reporting of systematic reviews incorporating network meta-analyses of health care interventions: checklist and explanations. Ann Intern Med. 2015;162(11):777–784. doi:10.7326/M14-2385
25. Pocock SJ, Stone GW. The primary outcome fails - what next? N Engl J Med. 2016;375(9):861–870. doi:10.1056/NEJMra1510064
26. Winstein CJ, Stein J, Arena R, et al.; American Heart Association Stroke Council, Council on Cardiovascular and Stroke Nursing, Council on Clinical Cardiology, and Council on Quality of Care and Outcomes Research. Guidelines for Adult Stroke Rehabilitation and Recovery: a Guideline for Healthcare Professionals From the American Heart Association/American Stroke Association. Stroke. 2016;47(6):e98–e169.
27. Gittler M, Davis AM. Guidelines for adult stroke rehabilitation and recovery. JAMA. 2018;319(8):820–821. doi:10.1001/jama.2017.22036
28. Gladstone DJ, Danells CJ, Black SE. The fugl-meyer assessment of motor recovery after stroke: a critical review of its measurement properties. Neurorehabil Neural Repair. 2002;16(3):232–240. doi:10.1177/154596802401105171
29. Harb A, Kishner S. Modified ashworth scale. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2021: 1-2.
30. Ohura T, Hase K, Nakajima Y, Nakayama T. Validity and reliability of a performance evaluation tool based on the modified Barthel Index for stroke patients. BMC Med Res Methodol. 2017;17(1):131. doi:10.1186/s12874-017-0409-2
31. Wang L, Yin Z, Zhang Y, et al. Optimal acupuncture methods for nonspecific low back pain: a systematic review and bayesian network meta-analysis of randomized controlled trials. J Pain Res. 2021;20(14):1097–1112. doi:10.2147/JPR.S310385
32. Chen Y, Wang HW, Xiang YZ, et al. Effects of low frequency transcutaneous electric acupoint stimulation on upper limbs and hands function for chronic stroke patients. Chinese J Rehabil Theory Pract. 2018;24(12):1371–1375.
33. Yan YL, Ding XM, Lu YJ. Low frequency transcutaneous electrical acupoint stimulation for stroke sequelae stage hand And upper limb dysfunction. Cardiovasc Dis J Integrated Trad Chinese Western Med. 2019;7(12):87–88.
34. Chen Y, Wang HW, Xiang YZ, et al. Short-term effectiveness of community-based low frequency transcutaneous electric acupoint stimulation on upper limb and hand function rehabilitation in stroke patients. Chinese J Rehabil. 2019;34(12):669–672.
35. Liu ZL, Song L, Guan S, et al. Effect of functional electrical stimulation on recovery of upper limb motor function in stroke patients with hemiplegia. Chinese J Rehabil. 2005;20(1):52.
36. Chen YL, Yuan XM, Li YH, et al. Therapeutic effects of functional electrical stimulation combined with comprehensive rehabilitation therapy on patients with shoulder subluxation after stroke. Acta Medicinae Sinica. 2021;34(02):75–78. doi:10.19296/j.cnki.1008-2409.2021-02-021
37. Zhao M. Clinical study of functional electrical stimulation in treatment of upper limb dyskinesia after cerebral storke. World Latest Med Inf. 2016;16(46):67–68.
38. Zuo XQ. Effect of Transcranial Direct Current Stimulation on Motor Function of Upper Limb and Aphasia in Stroke Patients. Hebei Medical University; 2014.
39. Zheng CJ, Xia GW, Duan C. Effect of transcranial direct current stimulation on upper limb and hand function in stroke: a double-blind randomized controlled trial. Chinese J Rehabil. 2019;34(12):623–626.
40. Wang CX, Yang FX, Zou WG. Effect of transcranial direct current stimulation combined with routine rehabilitation in the rehabilitation of upper limb motor function and aphasia in stroke hemiplegia. Chinese J Pract Nerv Dis. 2016;19(4):48–49.
41. Zhang LQ. Mechanism of Transcranial Direct Current Stimulation in the Clinical Application of Upper Limb Post-Stroke Spasticity. Kunming Medical University; 2019.
42. Liu CM. Rehabilitation effect of transcutaneous electrical nerve stimulation therapy on sensory disorders after cerebral apoplexy. Shenzhen J Integrated Trad Chinese Western Med. 2018;28(4):175–177.
43. Zhou JY, Gu LH, Chen QG, et al. Clinical application of percutaneous acupoint electrical stimulation on upper limbs and hand dysfunction after cerebral apoplexy. Chinese J Trauma Disability Med. 2020;28(10):17–19.
44. Peng Y, Zhang J, Su CC, et al. Effects of transcutaneous acupoint electrical stimulation on upper limb function and motor evoked potential in stroke patients. Chinese J Rehabil Med. 2015;30(6):547–550,571.
45. Jia J, Zhong JB, Liu J. Clinical study of TEAS coordinated with basic rehabilitation training on hand dysfunction after stroke. J Clin Acupunct Moxibustion. 2018;34(05):22–25.
46. Zheng WY, Mao ZF. Application of transcutaneous acupoint electrical stimulation in rehabilitation of elderly patients with post-stroke hand dysfunction. China Mod Doctor. 2020;58(20):92–95.
47. Mao ZF, Wang XL, Yang RC, et al. effect of transcutaneous electrical acupoint stimulation in patients with hand dysfunction after stroke. Mod Pract Med. 2017;29(12):1565–1566.
48. Yu LH, Jiang M, Mao ZF. Application of percutaneous acupoint electrical stimulation in the rehabil-itation of hand dysfunction after stroke. China Mod Doctor. 2020;58(1):93–96.
49. Chen Y, Tian Y, Xie BJ. Effects of transcutaneous electrical acupoint stimulation treatment in stroke survivors with upper dysfunction. Chinese J Rehabil Med. 2015;30(5):454–457.
50. Gu ZW, Lei B, Deng WZ. Effect of transcutaneous electrical acupoint stimulation in patients with hand dysfunction after stroke in the community. Chinese J Clin Rational Drug Use. 2019;12(29):112–113.
51. Xie JJ, Li JX, Sun Q. A randomized controlled trial of transcutaneous electrical acupoint stimulation for upper extremity dysfunction after stroke. J Basic Chinese Med. 2019;25(07):969–971.
52. Zhang HL. Effect oF transcutaneous electrical nerve stimulation on acupuncture points in acute stroke patients with upper extremity motor function. J Emerg Tradi Chinese Med. 2012;21(9):1475–1476.
53. Ren H, He YH. Long-term recovery of upper limb function after functional electrical stimulation in stroke patients with hemiplegia. Chinese Foreign Med Res. 2014;12:129–131.
54. Yang YZ. Application of neuromuscular electrical stimulation technology in the improvement of the quality of life of 40 cases of patients with cerebral apoplexy hemiplegia. Shanghai Med Pharm J. 2016;37(21):40–43.
55. Jiang Z, He J, Cai SF, et al. Effects of neuromuscular electrical stimulation therapy on function of upper limbs of stroke patients. J Fujian Univ Trad Chinese Med. 2010;20(5):12–14.
56. Sun DJ, Chen M. Clinical observation of electrical acupoint stimulation on recovery of upper limb function after cerebral apoplexy. Hainan Med J. 2015;2:292–293.
57. Xie JJ, Li JX, Sun Q. Clinical observation of transcutaneous electrical acupoint stimulation at motor points in treating hemiplegia upper limb proprioceptive disturbance in patients with ischemic stroke. J Guangzhou Univ Trad Chinese Med. 2020;37(02):274–279.
58. Wang JH. Comprehensive rehabilitation training in combination with transcutaneous electrical nerve stimulation for patients with omalgia after stroke-induced hemiplegia. Int Med Health Guidance News. 2016;22(10):1401–1403. doi:10.3760/cma.j.issn.1007-1245.2016.10.021
59. Zhou MM. A Study on Efficacy of Neuromuscular Electrical Stimulation and Transcutaneous Nerve Stimulation in Hemiplegic Shoulder Pain. BengBu Medical College; 2016.
60. Jiang Y. Therapeutic Effect of Transcranial Direct Current Stimulation on Hand Dysfunction After Stroke. Inner Mongolia Medical University; 2020.
61. Wang QS. Research the Effect of Nerve Muscle Electrical Stimulation on Muscle Tension and Muscle Strength of Upper Limb with Stroke Patients. Jilin University; 2012.
62. Lin Z, Yan T. Long-term effectiveness of neuromuscular electrical stimulation for promoting motor recovery of the upper extremity after stroke. J Rehabil Med. 2011;43(6):506–510. doi:10.2340/16501977-0807
63. Zhou M, Li F, Lu W, Wu J, Pei S. Efficiency of neuromuscular electrical stimulation and transcutaneous nerve stimulation on hemiplegic shoulder pain: a randomized controlled trial. Arch Phys Med Rehabil. 2018;99(9):1730–1739. doi:10.1016/j.apmr.2018.04.020
64. Nakipoğlu Yuzer GF, Köse Dönmez B, Özgirgin N. A randomized controlled study: effectiveness of functional electrical stimulation on wrist and finger flexor spasticity in hemiplegia. J Stroke Cerebrovasc Dis. 2017;26(7):1467–1471. doi:10.1016/j.jstrokecerebrovasdis.2017.03.011
65. Alisar DC, Ozen S, Sozay S. Effects of bihemispheric transcranial direct current stimulation on upper extremity function in stroke patients: a randomized double-blind sham-controlled study. J Stroke Cerebrovasc Dis. 2020;29(1):104454. doi:10.1016/j.jstrokecerebrovasdis.2019.104454
66. Shim S, Yoon BH, Shin IS, Bae JM. Network meta-analysis: application and practice using Stata. Epidemiol Health. 2017;27(39):e2017047. doi:10.4178/epih.e2017047
67. Chen J, Tu Q, Miao S, Zhou Z, Hu S. Transcutaneous electrical acupoint stimulation for preventing postoperative nausea and vomiting after general anesthesia: a meta-analysis of randomized controlled trials. Int J Surg. 2020;73:57–64. doi:10.1016/j.ijsu.2019.10.036
68. Ansari NN, Naghdi S, Arab TK, Jalaie S. The interrater and intrarater reliability of the Modified Ashworth Scale in the assessment of muscle spasticity: limb and muscle group effect. NeuroRehabilitation. 2008;23(3):231–237. doi:10.3233/NRE-2008-23304
69. Zheng X, Chen D, Yan T, et al. A randomized clinical trial of a functional electrical stimulation mimic to gait promotes motor recovery and brain remodeling in acute stroke. Behav Neurol. 2018;18(2018):8923520.
70. Hara Y. Brain plasticity and rehabilitation in stroke patients. J Nippon Med Sch. 2015;82(1):4–13. doi:10.1272/jnms.82.4
71. Bickel CS, Gregory CM, Dean JC. Motor unit recruitment during neuromuscular electrical stimulation: a critical appraisal. Eur J Appl Physiol. 2011;111(10):2399–2407. doi:10.1007/s00421-011-2128-4
72. Maffiuletti NA. Physiological and methodological considerations for the use of neuromuscular electrical stimulation. Eur J Appl Physiol. 2010;110(2):223–234. doi:10.1007/s00421-010-1502-y
73. Maffiuletti NA, Gondin J, Place N, Stevens-Lapsley J, Vivodtzev I, Minetto MA. Clinical use of neuromuscular electrical stimulation for neuromuscular rehabilitation: what are we overlooking? Arch Phys Med Rehabil. 2018;99(4):806–812. doi:10.1016/j.apmr.2017.10.028
74. Hróbjartsson A, Thomsen AS, Emanuelsson F, et al. Observer bias in randomised clinical trials with binary outcomes: systematic review of trials with both blinded and non-blinded outcome assessors. BMJ. 2012;27(344):e1119. doi:10.1136/bmj.e1119
75. Mlinarić A, Horvat M, Šupak Smolčić V. Dealing with the positive publication bias: why you should really publish your negative results. Biochem Med (Zagreb). 2017;27(3):030201. doi:10.11613/BM.2017.030201
76. Lin L, Chu H. Quantifying publication bias in meta-analysis. Biometrics. 2018;74(3):785–794. doi:10.1111/biom.12817
77. Shi C, Dumville JC, Cullum N. Support surfaces for pressure ulcer prevention: a network meta-analysis. PLoS One. 2018;13(2):e0192707. doi:10.1371/journal.pone.0192707
78. Salanti G, Del Giovane C, Chaimani A, Caldwell DM, Higgins JP. Evaluating the quality of evidence from a network meta-analysis. PLoS One. 2014;9(7):e99682. doi:10.1371/journal.pone.0099682
79. Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ. 2009;21(339):b2700. doi:10.1136/bmj.b2700
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.