Back to Journals » Cancer Management and Research » Volume 15
Optimal Delivery of Follow-Up Care Following Treatment for Adults Treated for Ewing Sarcoma
Authors Digklia A, Dolcan A , Kucharczyk MA, Jones RL, Napolitano A
Received 5 December 2022
Accepted for publication 23 May 2023
Published 17 June 2023 Volume 2023:15 Pages 537—545
DOI https://doi.org/10.2147/CMAR.S362693
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Seema Singh
Antonia Digklia,1 Ana Dolcan,1 Monika A Kucharczyk,2 Robin L Jones,3 Andrea Napolitano3
1Department of Oncology, Lausanne University Hospital, University of Lausanne, Lausanne, 1011, Switzerland; 2Clinical Practice Research Datalink, MHRA, London, E14 4PU, UK; 3Sarcoma Unit, The Royal Marsden NHS Foundation Trust and Institute of Cancer Research, London, UK
Correspondence: Antonia Digklia, Email [email protected]
Abstract: Ewing sarcoma (ES) is a rare, highly malignant sarcoma. It usually presents in the second decade of life; however, patients can be diagnosed as early as newborns and as late as in their seventies. ES is most frequently found in the long bones of the extremities and the pelvis. In older patients, ES can also arise in the soft tissues. Currently, there is no standard schedule for surveillance of adult patients with ES after their initial treatment for localised disease, not only for the early detection of recurrence but also for long-term side effects. Follow-up is based on group recommendations using extrapolated data obtained primarily from studies with paediatric patients. The main objective of this review is to summarise the data available on treatment-associated complications in long-term survivors. Furthermore, we provide a set of recommendations for optimising the follow-up of adults ES survivors, as well as for managing the sequelae that result from intensive multimodal treatment.
Keywords: Ewing, sarcoma, follow-up, toxicities, survivor, review
Introduction
Ewing Sarcoma (ES) is a rare subtype of sarcoma. Only 30% of affected patients are older than 20 years of age and their treatment is currently based on data from younger patients, since the majority of clinical trials only recruited patients younger than 18 years of age.
The diagnosis of ES is largely based on morphology and immunohistochemistry (small round cell tumours, CD99+, FLI+). Molecular confirmation by fluorescence in-situ hybridization (FISH) or next-generation sequencing for ES breakpoint region 1 (EWSR1) rearrangements has currently become very common, since 90% of ES carry it. This translocation fuses the EWSR1 gene on chromosome 22 to the friend of leukemia virus integration site 1 gene (FLI1) on chromosome 11 t(11;22)(q24;q12). The resulting EWSR1-FLI1 fusion product functions as an oncoprotein.1
Prior to the 1970s, ES tumours were treated with surgery, radiation therapy (RT) or a combination of both. Nearly all patients eventually developed primary or distant relapse. Clinical trials performed in the 1970s established the efficacy of different combinations of chemotherapy agents in ES, including the combination of vincristine and cyclophosphamide, or the combination of vincristine, actinomycin-D, cyclophosphamide and doxorubicin.
Furthermore, patients with ES benefit the most by advances in multimodal approach developed over the last 40 years. This multimodal approach includes perioperative chemotherapy, surgery and/or radiotherapy followed by post-operative chemotherapy, leading to remarkable improvement in survival and greater likelihood of limb sparing surgery with fewer toxicities.1 Currently, based on the Phase III AEWS0031 and Euro Ewing 2012 trials, adult patients are treated with induction chemotherapy with VDC/IE (alternating cycles of vincristine + doxorubicin + cyclophosphamide, and ifosfamide + etoposide) with surgical resection or radiation therapy or both for the primary site. The role of consolidation high dose chemotherapy with autologous hematopoietic cell transplantation remains controversial.2
Similar to the paediatric population, relapse is often systemic (71–73%), and only 11–15% of patients develop local recurrence, leading to five-year post-local relapse survival rates of 15–25%. The prognosis of patients with metastatic disease is dismal, with the exception of limited lung disease.3,4 Review of the European Intergroup Cooperative Ewing Sarcoma Studies showed that five year event-free survival (EFS) for patients with isolated lung metastases was 34%, for those with bone/bone marrow metastases it was 28%, and for patients with combined lung and bone/bone marrow metastases it was 14%. Currently, patients with recurrent ES in the lungs, are treated with radical multimodal therapy which can provide long-term survival. Furthermore, most ES relapses occur within the first 2 years.5
Early detection of disease relapse is expected to give patients a chance for longer survival. On the other hand, little is known about late effects of the therapy and their optimal management in long-term survivors. Furthermore, many aspects of the disease require further study, and there is no consensus between national and international guidelines for standard practice of follow-up of ES patients.2 In this context, physicians base their approach on their individual experience and there are significant differences among various oncology practices. Hence, several aspects of long-term survivors need to be examined.
Therefore, in this review we discuss both the existing standards and remaining questions in optimal follow-up (FU) of adult survivors of ES to further improve clinical practice and quality of life (QoL) of the patients. For the purposes of this article, an ES survivor is an adult patient who has completed a specific treatment with no detectable residual cancer.
Patients affected by specific syndromes, such as Li Fraumeni (mutations in TP53), Fanconi Anemia Complementation Group M (FANCM) or deletions of the CDKN2A locus, are excluded from this guide due to the special clinical characteristics and specific preventive aspects of these disorders.6,7
Follow-Up
As discussed above, 40% of curatively treated ES patients will present with a relapse. Most metastases develop in the lungs and bones. Currently, the objective of follow-up programs is the early detection of relapse to allow, whenever technically possible and after multidisciplinary evaluation, surgical resection of the lesions, most commonly in the case of limited lung disease. In this context, the identification of presence of tumour in the hilar and mediastinal lymph nodes, and invasion of the mediastinum and pleura is of utmost importance.
According to published guidelines, regular follow-up after therapy of primary ES includes computed tomography (CT) chest (to detect lung metastases in asymptomatic patients) every 3 months for the first two years, and every 6 months for the first 5 years. After the completion of the 5-year surveillance, a CT chest is recommended once or twice per year up to 10 years after completion of treatment. Long term surveillance beyond 10 years should be performed based on clinical indications.
Optimal follow-up for local recurrence should include a careful physical examination of the region around the primary site resection, combined with a CT or magnetic resonance imaging (MRI). However, no consensus has been reached whether MRI is superior in the follow-up of ES patients and many centres still use CT.2 Following a tight follow-up schedule is of higher importance in patients who are likely to benefit by and tolerate further treatments.
On the other hand, given the risks and potential consequences of late complications, there is a strong need for appropriate systematic long-term follow-up for ES survivors to optimise clinical outcomes including secondary cancers, reduced fertility, and wound complications. Unfortunately, most of the current recommendations do not address these aspects and our comments are not based on prospective evidence, but only supported by retrospective reports.2
Cardiotoxicity
Doxorubicin is known to lead to cardiotoxicity. It is dose-dependent and doses over 450 mg/m2 increase significant the likelihood of toxicity occurrence.8 A history of cardiovascular disease such as hypertension, hyperlipidaemia, or atherosclerosis, diabetes, age (>65 years), gender (female), is associated with increased risk of doxorubicin-associated cardiotoxicity. Tobacco use, poor nutrition, or physical inactivity appear to also increase the risk of cardiotoxicity. The exact mechanisms by which doxorubicin-associated cardiotoxicity develops remain elusive. Several mechanisms have been proposed including decreased antioxidant effects, decreased mitochondrial function, increased lipid peroxidation, and increased inflammatory response. Recent data showing that doxorubicin significantly upregulates the expression of death receptors (DRs) (TNFR1, Fas, DR4 and DR5) in cardiomyocytes at both protein and mRNA levels, suggest an additional mechanism of cardiotoxicity.9
Although, preventive treatments are being delivered to reduce and prevent cardiotoxicity development no standard approach has been established.10 Co-administration of dexrazoxane, an iron chelator, that reduces oxidative stress, for preventing doxorubicin-associated cardiotoxicity is approved by the FDA. It has been shown to prevent left ventricular dysfunction and heart failure in children with osteosarcoma (OS) receiving doxorubicin, especially in girls. No data is available specifically for ES patients.11 In adults, in the interim analysis of the ANNOUNCE phase III trial, pre-treatment with dexrazoxane did not appear to reduce progression-free survival (PFS) in patients with soft-tissue sarcomas (STS) treated with doxorubicin. Furthermore, Van Tine et al in a Phase II single-arm noninferiority trial testing the upfront use of dexrazoxane with doxorubicin on PFS and cardiac function in STS showed reported an increase in PFS from a historical 4.6 to 8.4 months. The 3 patients who were removed from the study due to cardiotoxicity were exposed on >600 mg/m2 doxorubicin. No other patient developed persistent cardiac dysfunction with left ventricular ejection fraction (LVEF) remaining below 50%.12,13
Regarding ES, the phase II clinical trial (NCT00038142) aimed to determine whether dose intensive vincristine, doxorubicin, cyclophosphamide and dexrazoxane (VACdxr) with or without ImmTherTM can increase the 2-year disease-free survival seen with standard VAC therapy for high-risk ES. In this trial, 46 patients were randomized in a 12 year period and the study closed early with low enrolment.
Currently, patients are defined as having experienced cardiotoxicity during the treatment if they have had a decrease in LVEF of >10% to <50% or had heart failure (HF)-related hospitalization. Upshaw et al, in a recent longitudinal cohort study of 362 patients with breast cancer, highlighted the doxorubicin-induced diastolic dysfunction.14
In a recent prospective evaluation of cardiotoxicity development among patients with sarcoma treated with anthracyclines the incidence of cancer therapeutics-related cardiac dysfunction was as high as 14%.15 Interestingly, incidence of less than 5% was reported in the position paper on cancer treatments and cardiovascular toxicity released by the European Society of Cardiology (ESC).16,17
Although asymptomatic cardiotoxicity, cardiomyopathy, and arrhythmias have been identified years and even decades after the conclusion of chemotherapy, there are no long-term detailed cardiac safety data due to the poor prognosis of ES patients, so limited information is available, and no special recommendations are available.18
Currently, LVEF function should be analysed by transthoracic ultrasound (TTE), at least prior to treatment initiation and after 6 cycles of doxorubicin, with additional interim evaluations for patients at high-risk of cardiotoxicity. All patients demonstrating a LVEF reduction or with concerns for cardiotoxicity should be referred to a cardiologist. Treatment with beta-blockers and renin-angiotensin-aldosterone system (RAAS) inhibitors can be suggested if there are any concerns for chemotherapy-induced cardiotoxicity. To date, anthracycline-associated cardiotoxicity is considered early-onset of chronic progressive cardiomyopathy that tends to present during, or within 5–10 years following therapy. The risk is further increased if the heart is in the radiotherapy treatment volume.17
Furthermore, whole lung irradiation (WLI) that was employed in the management of lung metastases in patients with ES was found to have a cumulative impact on late cardiac failure in childhood cancer survivors. Currently, cardiac sparing (CS) whole lung irradiation (WLI) using IMRT has led to an improvement of cardiac impact19,20 However, the evidence for this approach is limited and the results of the Euro-Ewing-Intergroup EE99 study particularly for younger patients up to the age of 49 are awaited (NCT00020566).
In this context, we propose to follow the 2022 ESC guidelines for sarcoma survivors who received a high total cumulative anthracycline dose (doxorubicin>450 mg/m2) and to obtain an assessment at 12 months after the end of treatment with clinical examination, TTE, electrocardiogram and natriuretic peptides (NP) measurements. If it is normal, new assessment is proposed every five years as well as non-invasive screening for carotid disease.17
Neurotoxicity Due to Treatment of ES
A number of studies indicate that neurotoxicity can occur following chemotherapy for ES. Ifosfamide is a well-known agent used in ES treatment that can lead to encephalopathy in up to 30% of patients. While this is often transient and reversible it may cause persistent neurological dysfunction or death. Malnutrition characterized by low albumin and simultaneous use of CYP3A4 inhibitors such as aprepitant are identified risk factors.21,22 Preventive treatment with methylene blue, thiamin, and glucose 5% infusions can reduced the risk of developing ifosfamide-induced encephalopathy.23 In a series of 97 patients with malignant solid tumours including ES, self-limiting neurotoxicity was usually associated with pre-treatment with cisplatin.24 In another study involving 28 patients with sarcomas, neurotoxicity was significantly reduced by decreasing the dose of antiemetics and narcotics.
Vincristine can also lead to a form of neuropathic dysmotility, which usually subsides after the treatment is completed.25 This has also been reported to manifest as limb weakness and areflexia.
In general, neurotoxicity induced by chemotherapeutics in adult ES patients is low and resolves either with preventive measures or by the end of treatment. In this context, no particular follow-up is proposed. For symptomatic patients, although several therapeutical approaches have been tested (ie physical therapy, acupuncture, anti-inflammatory therapies, pregabalin, cannabinoids, etc), no standard of care has been established.26 Currently, duloxetine is the only drug to show decrease of chemotherapy-induced peripheral neuropathy.27 Due to the complexity of the problem, these patients should be followed by pain medicine specialists. Still, further effort is needed to better understand mechanisms in order to develop drugs that can protect the nervous system and reduce the symptoms.
Neurological Complications in Patients with Ewing Sarcoma Due to Disease Localization
Disease-induced neurological deficits arise primarily in ES that originates in the spine or the cranium since ES metastatic to the brain is a rare event and few cases have been reported.28,29 Boussios et al summarized 69 ES cases with initial presentation of cord or radicular compression of spinal cord, arising from primary or metastatic ES.30 About 30% of these patients were paraplegic, while 46% presented with imminent paraplegia and 25% with cauda equina syndrome. On the other hand, primary or metastatic intracranial ES can present with non-specific symptoms like headache, seizures or changes in behaviour.31 In one of the most encompassing reviews that included 125 patients with ES with vertebral origin, 53 had a detailed clinical information. Of these, 91% presented with pain and 40% with neurologic deficits.32 Usually, symptoms subside after surgical removal of the tumour or chemotherapy. In the case of spinal involvement, decompression can be achieved with chemotherapy while laminectomy is not always necessary.33 There are reports suggesting that chemotherapy should precede surgical removal even if there are major neurologic symptoms. Neurological sequelae (32%), spinal curvature deformation (35%), spinal reduction mobility (40%) and spinal pain (25%) were observed in ES spine survivor patients in a French cohort.34
Chemotherapy-Associated Infertility
Chemotherapy-associated infertility is known to be a major concern for both males and females. The risk of infertility is well-studied in many standard treatment regimens for breast cancer, but in patients with ES remains uncertain. This is a serious problem for younger patients who have not considered childbearing, nor can they comprehend the impact of cancer-directed therapy decisions. Moreover, in females, the intensive chemotherapy regimen employed, which includes cyclophosphamide and doxorubicin, may not only diminish fertility but lead to amenorrhea and early/premature menopause via ovarian atrophy, stromal fibrosis and vascular toxicity, with a significant impact on quality of life. Those patients who have the potential for childbearing and receive an ES diagnosis, should be referred to a team specialised in fertility and reproduction prior to the initiation of perioperative chemotherapy. In this case, methods to preserve fertility (eg, oocyte cryopreservation for women and sperm freezing for men) should be considered. This approach may delay therapy for 1–2 months. In the case of patients with breast cancer, ovarian function suppression with gonadotropin-releasing hormone agonists which leads to reduction of the vulnerability of maturing ovarian follicles to cytotoxic chemotherapy is used, but currently no such study has shown a benefit to ES survivors. Consequently, it is not recommended as a standard fertility preservation technique for patients with ES, although it may be discussed on an individual basis with patients, especially in those cases wherein other procedures would cause an excessive delay in initiating the treatment.
For male patients who want to verify their potential fertility, semen sample analysis should be performed, preferably at minimum 2 and up to 5 years after the conclusion of chemotherapy. Clinicians should be aware also of the probability of sexual dysfunction (ie, decreased libido), so dosing of total testosterone and LH levels should be proposed in symptomatic male survivors. In pre-menopausal women (<50 years), FSH, LH, and 17β-estradiol should be performed if amenorrhea or significant alterations in menstrual cycles persist for at least 6 months.
Emotional Effects and Psychosocial Wellbeing
In general, emotional effects associated with cancer range from anxiety to frustration, feeling out of control and anger. On the positive side, patients have frequently reported the ability to change priorities and adopt a new perspective on life. There are only a limited number of studies focusing on the psychological sequelae of sarcomas and even fewer have focused on ES.35 Most studies have focused on quality of life (QoL) by measuring of patient-reported outcome (PRO) using well-known questionnaires such as the SF-36.35
Recently, studies have included the body image questionnaire (MBSRQ) and the Rosenberg Self-Esteem (RSE-scale). For example, a study including 58 patients who had been treated for ES and 56 healthy individuals as a control group showed that while patients with ES reported statistically significant lower quality of life and body image, there was no difference with respect to self-esteem.36 These results confirmed what had already been reported in smaller series of patients.37,38 A large study that included 618 long-term survivors of ES showed that while the patients reported somewhat diminished physical activity and were prone to more functional problems, compared to the control group, their mental health wellbeing appeared at the same level as the controls.39
In a study, in which long-term survivors of paediatric sarcoma had been evaluated, the authors reported that about 12% of them experienced symptoms similar to those of post-traumatic stress disorder.40 Males appeared to be more severely affected than females. In a separate study in which only a few patients had ES and older patients were included, the authors stratified them based on age, sex and education. In this cohort, older and primarily retired patients appeared to lose contact with their social environment, and, in contrast to the previous study, females were more likely to feel emotionally burdened than male patients. The availability of psychological services, which highly educated patients were more likely to use, appeared to improve the psychological wellbeing. These highly educated patients had a more positive attitude than patients that had a low level of education.41 In most studies, the availability of psycho-oncological services to patients who have been treated for ES appears to be beneficial.
Recently, it has been reported that adult survivors of childhood ES score worse in emotional regulation and task efficiency, and present with more neurocognitive difficulties compared to their siblings.42
In this context, additional studies investigating emotional stability is survivors and efficacy of strategies such as comprehensive cancer survivorship counselling for these patients are indicated to determine if they can improve long-term outcomes.
Nephrotoxicity
Developing treatment-related nephrotoxicity after ES therapy is a possibility especially due to the use of ifosfamide. Farry et al reported significant long-term renal toxicity in a large cohort study with adult patients treated with ifosfamide (median age 43.5 years old, up to 53% of 154 5-year survivors with chronic kidney disease (CKD) stage ≥3). Patient age and concomitant exposure to carboplatin significantly affected the eGFR (estimated glomerular filtration rate).43 In a recent study with patients >40 years treated in low- and middle-income countries, 6.5% of patients developed renal toxicity.44
On the other hand, platinum agents including cisplatin and carboplatin may also cause acute and chronic glomerular and tubular toxicity. Carboplatin-associated nephrotoxicity is usually less severe than cisplatin-associated toxicity. In a large study 29% of 533 1-year survivors and 33% of 397 5-year survivors treated with cisplatin developed stage 3 CKD.45 In contrast to ifosfamide associated CKD, platinum-associated tubular damage leads to magnesuria and subsequently to chronic hypomagnesaemia and secondary hypocalcaemia. Rarely, kidney dysfunction may be due to platinum-induced thrombotic microangiopathy.
Recently, Ensergueix et al reported 34 adult patients with ifosfamide-associated nephrotoxicity in a retrospective multicentre French trial in which 41% had also received cisplatin.46 The most common forms of ifosfamide-associated nephrotoxicity were proximal tubular dysfunction and acute kidney injury. eGFR decreased progressively in 16 of 34 patients, 10 patients developed stage 5 CKD, 6 required haemodialysis and 6 died. Notably, kidney biopsy in 3/14 patients suggested mitochondrial dysfunction as a possible mechanism of ifosfamide-associated nephrotoxicity.43
N-acetylcysteine seems to have a renal protective effect in vitro and in vivo in rats exposed to ifosfamide.47 Hanly et al reported acute renal failure reversibility by intravenous administration of N-acetylcysteine in children.48,49 However, no controlled study has yet validated the prophylactic use of N-acetylcysteine in this indication, as well as its curative use.
Although our knowledge of nephrotoxicity occurrence is increasing, greater understanding of the pathogenesis will help us how to better prevent it, especially in the adult population. Prospective studies are therefore needed to evaluate this incidence, but also to determine the risk factors and establish prevention strategies, by using nephroprotective reagents, as has been described above with N-acetylcysteine. In this context, we propose annual blood-urea nitrogen, creatinine and electrolyte laboratory test with blood pressure monitoring for cancer survivors.
Challenges
In recent years, there have been increasing efforts to optimize practices and mitigate challenges in providing follow-up care to survivors of childhood-onset cancers, particularly in the transition between paediatric and adult care. Neuromuscular dysfunction, increased incidence of cancer or disabling chronic health conditions, and reduced fertility are well-known observations underlying the need of establishing survivorship clinics.50,51 In general, the risk of chronic conditions including musculoskeletal and cardiac complications is elevated, and the cumulative incidence of successive cancer 25 years after ES diagnosis is 15%.52,53 ES survivors, appeared to possess reduced walking efficiency, mobility, strength and endurance. In patients with osteosarcoma, accumulation of ≥4 grade 3–4 chronic conditions has been associated with reduced ability of executive function and attention.54,55 Similar results were observed in a recent study, in which bone sarcoma survivors reported much higher difficulties with task efficiency and emotional regulation that was associated with reduced employment attainment.42
Due to the growing curative and life-extending cancer treatments leading to growing cancer survivor populations, identification of adequate survivorship care is crucial. In addition to helping preserve quality of life and increase survival rate by preventing long-term complications, follow-up care may increase survivors’ ability to return to work or resume important social functions. However, little is known about the long-term impact of our treatments on survivors of adult-onset cancers including ES. In a recent study describing follow-up care for breast and colorectal cancer survivors in 27 countries from six continents with varying levels of resources, less than half had a national plan addressing survivorship care.56 Lack of reimbursement for follow-up care was often the reason especially in low-income countries. In contrast, a recent pilot study indicated that there are substandard levels of adherence to individualized healthcare recommendations in long-term survivors of childhood cancer. In this context, further studies are needed to evaluate the factors, incentives and methods that lead to increased survivor adherence to healthcare recommendations. Currently, the scientific focus is on patients under active treatment and little research has been invested on survivors. More funding and support is required to establish survivorship clinics that can cover the holistic needs of this population.
Conclusion
These last years it is becoming more and more evident that cancer rehabilitation and survivorship care need to include different approaches. A better understanding of these needs and behaviours could help identifying specific gaps and inform follow-up interventions in the future. Unfortunately, evidence in this area for ES survivors remains scarce. Individualized written survivorship care plans that include treatment information and recommendations for long-term monitoring may be a solution for now. On the other hand, we acknowledge that setting-up recommendations of meeting the needs of ES survivors, is not easy since it needs a wide range of different specialties to be considered.
Our review includes only some components that may contribute to the process of establishing a bundle of basic and robust interventions for ES survivors (Figure 1). However, there is an unmet need to better understand the ES patient’s requirements and support for living beyond cancer. Further research is required to adequately meet the needs of this growing group of people.
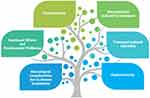 |
Figure 1 Challenges for survivorship clinics. |
Disclosure
Dr Ana Dolcan reports personal fees from PharmaMar and Novartis, outside the submitted work. The authors report no other conflicts of interest in this work.
References
1. Zollner SK, Amatruda JF, Bauer S, et al. Ewing sarcoma-diagnosis, treatment, clinical challenges and future perspectives. J Clin Med. 2021;10(8):1685. doi:10.3390/jcm10081685
2. Strauss SJ, Frezza AM, Abecassis N, et al. Bone sarcomas: ESMO-EURACAN-GENTURIS-ERN PaedCan clinical practice guideline for diagnosis, treatment and follow-up. Ann Oncol. 2021;32(12):1520–1536. doi:10.1016/j.annonc.2021.08.1995
3. Ahmed SK, Robinson SI, Okuno SH, et al. Adult Ewing sarcoma: survival and local control outcomes in 102 patients with localized disease. Sarcoma. 2013;2013:681425. doi:10.1155/2013/681425
4. Pretz JL, Barysauskas CM, George S, et al. Localized adult Ewing sarcoma: favorable outcomes with alternating vincristine, doxorubicin, cyclophosphamide, and ifosfamide, etoposide (VDC/IE)-based multimodality therapy. Oncologist. 2017;22(10):1265–1270. doi:10.1634/theoncologist.2016-0463
5. Cotterill SJ, Ahrens S, Paulussen M, et al. Prognostic factors in Ewing’s tumor of bone: analysis of 975 patients from the European Intergroup Cooperative Ewing’s Sarcoma Study Group. J Clin Oncol. 2000;18(17):3108–3114. doi:10.1200/JCO.2000.18.17.3108
6. Randall RL, Lessnick SL, Jones KB, et al. Is there a predisposition gene for Ewing’s sarcoma? J Oncol. 2010;2010:397632. doi:10.1155/2010/397632
7. Brohl AS, Patidar R, Turner CE, et al. Frequent inactivating germline mutations in DNA repair genes in patients with Ewing sarcoma. Genet Med. 2017;19(8):955–958. doi:10.1038/gim.2016.206
8. Reinbolt RE, Patel R, Pan X, et al. Risk factors for anthracycline-associated cardiotoxicity. Support Care Cancer. 2016;24(5):2173–2180. doi:10.1007/s00520-015-3008-y
9. Zhao L, Zhang B. Doxorubicin induces cardiotoxicity through upregulation of death receptors mediated apoptosis in cardiomyocytes. Sci Rep. 2017;7:44735. doi:10.1038/srep44735
10. Stansfeld A, Radia U, Goggin C, et al. Pharmacological strategies to reduce anthracycline-associated cardiotoxicity in cancer patients. Expert Opin Pharmacother. 2022;23(14):1641–1650. doi:10.1080/14656566.2022.2124107
11. Narayan HK, Putt ME, Kosaraju N, et al. Dexrazoxane preferentially mitigates doxorubicin cardiotoxicity in female children with sarcoma. Open Heart. 2019;6(1):e001025. doi:10.1136/openhrt-2019-001025
12. Van Tine BA, Hirbe AC, Oppelt P, et al. Interim analysis of the phase II study: noninferiority study of doxorubicin with upfront dexrazoxane plus olaratumab for advanced or metastatic soft-tissue sarcoma. Clin Cancer Res. 2021;27(14):3854–3860. doi:10.1158/1078-0432.CCR-20-4621
13. Jones RL, Wagner AJ, Kawai A, et al. Prospective evaluation of doxorubicin cardiotoxicity in patients with advanced soft-tissue sarcoma treated in the ANNOUNCE phase III randomized trial. Clin Cancer Res. 2021;27(14):3861–3866. doi:10.1158/1078-0432.CCR-20-4592
14. Upshaw JN, Finkelman B, Hubbard RA, et al. Comprehensive assessment of changes in left ventricular diastolic function with contemporary breast cancer therapy. JACC Cardiovasc Imaging. 2020;13(1):198–210. doi:10.1016/j.jcmg.2019.07.018
15. Shamai S, Rozenbaum Z, Merimsky O, et al. Cardio-toxicity among patients with sarcoma: a cardio-oncology registry. BMC Cancer. 2020;20(1):609. doi:10.1186/s12885-020-07104-9
16. Zamorano JL, Lancellotti P, Rodriguez Muñoz D, et al. 2016 ESC Position Paper on cancer treatments and cardiovascular toxicity developed under the auspices of the ESC Committee for Practice Guidelines: the Task Force for cancer treatments and cardiovascular toxicity of the European Society of Cardiology (ESC). Eur Heart J. 2016;37(36):2768–2801. doi:10.1093/eurheartj/ehw211
17. Lyon AR, López-Fernández T, Couch LS, et al. 2022 ESC guidelines on cardio-oncology developed in collaboration with the European Hematology Association (EHA), the European Society for Therapeutic Radiology and Oncology (ESTRO) and the International Cardio-Oncology Society (IC-OS). Eur Heart J. 2022;43(41):4229–4361. doi:10.1093/eurheartj/ehac244
18. Jones RL, Swanton C, Ewer MS. Anthracycline cardiotoxicity. Expert Opin Drug Saf. 2006;5(6):791–809. doi:10.1517/14740338.5.6.791
19. Longhi A, Ferrari S, Tamburini A, et al. Late effects of chemotherapy and radiotherapy in osteosarcoma and Ewing sarcoma patients: the Italian Sarcoma Group Experience (1983–2006). Cancer. 2012;118(20):5050–5059. doi:10.1002/cncr.27493
20. Kalapurakal JA, Gopalakrishnan M, Walterhouse DO, et al. Cardiac-sparing whole lung IMRT in patients with pediatric tumors and lung metastasis: final report of a prospective multicenter clinical trial. Int J Radiat Oncol Biol Phys. 2019;103(1):28–37. doi:10.1016/j.ijrobp.2018.08.034
21. Sweiss KI, Beri R, Shord SS. Encephalopathy after high-dose Ifosfamide: a retrospective cohort study and review of the literature. Drug Saf. 2008;31(11):989–996. doi:10.2165/00002018-200831110-00003
22. Howell JE, Szabatura AH, Hatfield Seung A, et al. Characterization of the occurrence of ifosfamide-induced neurotoxicity with concomitant aprepitant. J Oncol Pharm Pract. 2008;14(3):157–162. doi:10.1177/1078155208093930
23. Kasper B, Harter C, Meissner J, et al. Prophylactic treatment of known ifosfamide-induced encephalopathy for chemotherapy with high-dose ifosfamide? Support Care Cancer. 2004;12(3):205–207. doi:10.1007/s00520-003-0573-2
24. Schwartzman E, Scopinaro M, Angueyra N. Phase II study of ifosfamide as a single drug for relapsed paediatric patients. Cancer Chemother Pharmacol. 1989;24(Suppl 1):S11–2. doi:10.1007/BF00253230
25. Drach J, Huang H, Samoilova O, et al. Efficacy and safety of frontline rituximab, cyclophosphamide, doxorubicin and prednisone plus bortezomib (VR-CAP) or vincristine (R-CHOP) in a subset of newly diagnosed mantle cell lymphoma patients medically eligible for transplantation in the randomized, Phase 3 LYM-3002 study. Leuk Lymphoma. 2018;59(4):896–903. doi:10.1080/10428194.2017.1365855
26. Avallone A, Bimonte S, Cardone C, et al. Pathophysiology and therapeutic perspectives for chemotherapy-induced peripheral neuropathy. Anticancer Res. 2022;42(10):4667–4678. doi:10.21873/anticanres.15971
27. Smith EM, Pang H, Cirrincione C, et al. Effect of duloxetine on pain, function, and quality of life among patients with chemotherapy-induced painful peripheral neuropathy: a randomized clinical trial. JAMA. 2013;309(13):1359–1367. doi:10.1001/jama.2013.2813
28. Ben Nsir A, Boughamoura M, Maatouk M, et al. Dural metastasis of Ewing’s sarcoma. Surg Neurol Int. 2013;4:96. doi:10.4103/2152-7806.115487
29. Rana K, Wadhwa V, Bhargava EK, et al. Ewing’s sarcoma multifocal metastases to temporal and occipital bone: a rare presentation. J Clin Diagn Res. 2015;9(6):MD04–5. doi:10.7860/JCDR/2015/13254.6071
30. Boussios S, Hayward C, Cooke D, et al. Spinal Ewing sarcoma debuting with cord compression: have we discovered the thread of Ariadne? Anticancer Res. 2018;38(10):5589–5597. doi:10.21873/anticanres.12893
31. Haveman LM, Ranft A, van den Berg H, et al. Primary and metastatic intracranial Ewing sarcoma at diagnosis: retrospective international study and systematic review. Cancers. 2020;12(6):1675. doi:10.3390/cancers12061675
32. Ilaslan H, Sundaram M, Unni KK, et al. Primary Ewing’s sarcoma of the vertebral column. Skeletal Radiol. 2004;33(9):506–513. doi:10.1007/s00256-004-0810-x
33. Mirzaei L, Kaal SEJ, Schreuder HWB, et al. The neurological compromised spine due to Ewing sarcoma. What first: surgery or chemotherapy? Therapy, survival, and neurological outcome of 15 cases with primary Ewing sarcoma of the vertebral column. Neurosurgery. 2015;77(5):718–24; discussion 724–5. doi:10.1227/NEU.0000000000000903
34. Vogin G, Helfre S, Glorion C, et al. Local control and sequelae in localised Ewing tumours of the spine: a French retrospective study. Eur J Cancer. 2013;49(6):1314–1323. doi:10.1016/j.ejca.2012.12.005
35. Storey L, Fern LA, Martins A, et al. A critical review of the impact of sarcoma on psychosocial wellbeing. Sarcoma. 2019;2019:9730867. doi:10.1155/2019/9730867
36. Holzer LA, Huyer N, Friesenbichler J, et al. Body image, self-esteem, and quality of life in patients with primary malignant bone tumors. Arch Orthop Trauma Surg. 2020;140(1):1–10. doi:10.1007/s00402-019-03205-8
37. van Riel CA, Meijer-van den Bergh EEM, Kemps HLM, et al. Self-perception and quality of life in adolescents during treatment for a primary malignant bone tumour. Eur J Oncol Nurs. 2014;18(3):267–272. doi:10.1016/j.ejon.2014.01.005
38. Lopez-Guerra JL, Marquez-Vega C, Praena-Fernandez JM, et al. Health related quality of life and late side effects of long-term survivors of Ewing’s sarcoma of bone. J BUON. 2011;16(3):528–536.
39. Ranft A, Seidel C, Hoffmann C, et al. Quality of survivorship in a rare disease: clinicofunctional outcome and physical activity in an observational cohort study of 618 long-term survivors of Ewing sarcoma. J Clin Oncol. 2017;35(15):1704–1712. doi:10.1200/JCO.2016.70.6226
40. Wiener L, Battles H, Bernstein D, et al. Persistent psychological distress in long-term survivors of pediatric sarcoma: the experience at a single institution. Psychooncology. 2006;15(10):898–910. doi:10.1002/pon.1024
41. Sachsenmaier SM, Ipach I, Kluba T. Quality of life, physical and mental status and contentment of patients with localized soft tissue or bone sarcoma: a questionnaire analysis. Orthop Rev (Pavia). 2015;7(2):5920.
42. Kadan-Lottick NS, Zheng DJ, Wang M, et al. Patient-reported neurocognitive function in adult survivors of childhood and adolescent osteosarcoma and Ewing sarcoma. J Cancer Surviv. 2022. doi:10.1007/s11764-021-01154-z
43. Farry JK, Flombaum CD, Latcha S. Long term renal toxicity of ifosfamide in adult patients--5 year data. Eur J Cancer. 2012;48(9):1326–1331. doi:10.1016/j.ejca.2012.03.009
44. Panda G, Chandrasekharan A, Das S, et al. Outcomes of Ewing sarcoma in adults over 40 years of age from a low-middle income country. Ecancermedicalscience. 2022;16:1361. doi:10.3332/ecancer.2022.1361
45. Latcha S, Jaimes EA, Patil S, et al. Long-term renal outcomes after cisplatin treatment. Clin J Am Soc Nephrol. 2016;11(7):1173–1179. doi:10.2215/CJN.08070715
46. Ensergueix G, Karras A. Néphrotoxicité de l’ifosfamide [Ifosphamide nephrotoxicity]. Nephrol Ther. 2018;14(Suppl 1):S125–S131. French. doi:10.1016/j.nephro.2018.02.008
47. Chen N, Aleksa K, Woodland C, et al. The effect of N-acetylcysteine on ifosfamide-induced nephrotoxicity: in vitro studies in renal tubular cells. Transl Res. 2007;150(1):51–57. doi:10.1016/j.trsl.2007.02.001
48. Hanly L, Rieder MJ, Huang S-HS, et al. N-acetylcysteine rescue protocol for nephrotoxicity in children caused by ifosfamide. J Popul Ther Clin Pharmacol. 2013;20(2):e132–45.
49. Hanly LN, Chen N, Aleksa K, et al. N-acetylcysteine as a novel prophylactic treatment for ifosfamide-induced nephrotoxicity in children: translational pharmacokinetics. J Clin Pharmacol. 2012;52(1):55–64. doi:10.1177/0091270010391790
50. Henderson TO, Moskowitz CS, Chou JF, et al. Breast cancer risk in childhood cancer survivors without a history of chest radiotherapy: a report from the childhood cancer survivor study. J Clin Oncol. 2016;34(9):910–918. doi:10.1200/JCO.2015.62.3314
51. Ginsberg JP, Goodman P, Leisenring W, et al. Long-term survivors of childhood Ewing sarcoma: report from the childhood cancer survivor study. J Natl Cancer Inst. 2010;102(16):1272–1283. doi:10.1093/jnci/djq278
52. Marina NM, Liu Q, Donaldson SS, et al. Longitudinal follow-up of adult survivors of Ewing sarcoma: a report from the Childhood Cancer Survivor Study. Cancer. 2017;123(13):2551–2560. doi:10.1002/cncr.30627
53. Friedman DN, Chastain K, Chou JF, et al. Morbidity and mortality after treatment of Ewing sarcoma: a single-institution experience. Pediatr Blood Cancer. 2017;64(11):e26562. doi:10.1002/pbc.26562
54. Rodwin RL, Chen Y, Yasui Y, et al. Longitudinal evaluation of neuromuscular dysfunction in long-term survivors of childhood cancer: a report from the childhood cancer survivor study. Cancer Epidemiol Biomarkers Prev. 2021;30(8):1536–1545. doi:10.1158/1055-9965.EPI-21-0154
55. Edelmann MN, Daryani VM, Bishop MW, et al. Neurocognitive and patient-reported outcomes in adult survivors of childhood osteosarcoma. JAMA Oncol. 2016;2(2):201–208. doi:10.1001/jamaoncol.2015.4398
56. Mollica MA, Mayer DK, Oeffinger KC, et al. Follow-up care for breast and colorectal cancer across the globe: survey findings from 27 countries. JCO Glob Oncol. 2020;6:1394–1411. doi:10.1200/GO.20.00180
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
