Back to Journals » Cancer Management and Research » Volume 14
Optical Coherence Tomography Benefits the Diagnosis and Follow-Up of Primary Central Nervous System Lymphoma with Intraocular Involvement
Authors Zhou X, Tian S , Zhou X, Shi H, Li Y, Xiao J, Chen K, Chen B, Xu G, Wang Q
Received 17 December 2021
Accepted for publication 24 February 2022
Published 5 March 2022 Volume 2022:14 Pages 1007—1018
DOI https://doi.org/10.2147/CMAR.S353142
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Seema Singh
Xianjin Zhou,1– 3 Sha Tian,3 Xian Zhou,3 Huimin Shi,3 Yi Li,3 Jianjiang Xiao,3 Kun Chen,4 Bobin Chen,5 Gezhi Xu,1,2 Qingping Wang3
1Eye Institute, Eye and ENT Hospital, College of Medicine, Fudan University, Shanghai, People’s Republic of China; 2Shanghai Key Laboratory of Visual Impairment and Restoration, Science and Technology Commission of Shanghai Municipality, Shanghai, People’s Republic of China; 3Department of Ophthalmology, Huashan Hospital, Fudan University, Shanghai, People’s Republic of China; 4Department of Laboratory Medicine, Huashan Hospital, Fudan University, Shanghai, People’s Republic of China; 5Department of Hematology, Huashan Hospital, Fudan University, Shanghai, People’s Republic of China
Correspondence: Qingping Wang, Department of Ophthalmology, Huashan Hospital, Fudan University, No. 12 Middle Urumqi Road, Shanghai, 200040, People’s Republic of China, Email [email protected] Gezhi Xu, Department of Ophthalmology, Eye & ENT Hospital, Fudan University, No. 83 Fenyang Road, Shanghai, 200031, People’s Republic of China, Email [email protected]
Purpose: To describe the characteristic manifestations of vitreoretinal lymphoma (VRL) with optical coherence tomography (OCT) and monitor their outcomes after treatmEnt.
Patients and Methods: Patients with primary central nervous system lymphoma (PCNSL) and intraocular involvement were assigned to the VRL group. OCT manifestations were analyzed and changes in abnormalities were recorded after intravitreal methotrexate injections. OCT manifestations of PCNSL patients without intraocular involvement were analyzed as well (non-VRL group).
Results: There were 48 eyes with high-quality OCT records in the VRL group, of which 19 had abnormal manifestations. The most frequent abnormality was outer retina (OR) fuzzy borders (14 of 19, 73.7%). Other abnormalities included: focal subretinal deposits (8 of 19, 42.1%), hyperreflective subretinal dots (2 of 19, 10.5%), pigment epithelium detachment (PED) (5 of 19, 26.3%), preretinal deposits (5 of 19, 26.3%), epiretinal membrane (3 of 19, 15.8%), cystoid macular edema (3 of 19, 15.8%), subretinal fluid (3 of 19, 15.8%), outer retina atrophy (2 of 19, 10.5%), unilateral optic papilledema (2 of 19, 10.5%), retinal thickening (1 of 19, 5.3%), and subretinal fibrosis (1 of 19, 5.3%). Nine eyes with retinal abnormalities were receiving regular intravitreal methotrexate. The retinal structure of seven eyes (only outer retina involved) returned to almost normal on OCT images. The remaining two eyes (with severe retinal vasculitis) showed little improvement after treatment.
Conclusion: OCT is helpful for the diagnosis of PCNSL with intraocular involvement and long-term follow-up of the disease.
Summary Statement: The characteristic manifestations of vitreoretinal lymphoma (VRL) with optical coherence tomography (OCT) were described and their outcomes after treatment were monitored. These findings suggested that OCT is helpful for the diagnosis of PCNSL with intraocular involvement and long-term follow-up of the disease.
Keywords: intravitreal injection, methotrexate, optical coherence tomography, primary central nervous system lymphoma, vitreoretinal lymphoma
Introduction
Primary central nervous system lymphoma (PCNSL) is an extranodal non-Hodgkin’s lymphoma that originates exclusively from the brain, leptomeninges, spinal cord, or eyes.1 Diffuse large B-cell lymphoma (DLBCL), the most common pathological type, accounts for approximately 90% of PCNSL. Rare cases of PCNSL correspond to Burkitt, low-grade, or T-cell lymphoma.2 PCNSL is a relatively rare disease that constitutes 4–6% of all extranodal lymphomas and less than 3% of all primary tumors of the CNS.3 Although technologies and strategies for treating PCNSL have improved in recent years, the prognosis of this disease remains poor, with a 5-year overall survival rate of about 30% for immunocompetent patients.4
Vitreoretinal lymphoma (VRL) is a subtype of PCNSL that predominantly affects the vitreous and/or retina. In a few cases, VRL may also involve the anterior segment and optic nerve head. VRL can be either primary or secondary as intraocular involvement of PCNSL. Of primary VRL patients, 60% to 80% have intracranial manifestations during the course of the disease.5 However, intraocular involvement of PCNSL occurs or ultimately develops in only 15–25% of patients with PCNSL. Accurate assessment of intraocular involvement of PCNSL is essential. It has been reported that PCNSL patients with intraocular involvement have a high risk of disease relapse and poor progression free survival (PFS).6
Diagnosis of vitreoretinal lymphoma has been difficult as it manifests as a masquerade syndrome sharing clinical features with noninfectious uveitis. The symptoms are non-specific, including blurred vision (40–50%) and floaters (20–25%).7 Most individuals with VRL exhibit no abnormal findings of the anterior segment. Lymphomatous cells in the vitreous is the major manifestation, which can be characterized as homogeneous collections of medium-amplitude mobile echoes in the anterior and middle segments of the vitreous by B-scan ultrasound.8 The infiltration of lymphomatous cells to the retina can be visualized on funduscopic examination as multiple creamy subretinal lesions and can develop into “leopard spots” pigmentation.9 Vasculitis may also be observed although infrequently.
Vitreous cytopathology has been the gold standard for the diagnosis of VRL with the existence of atypical lymphoid cells. Nevertheless, the positive rate is still not very high (about 50%10) due to the scarcity and fragility of the lymphoma cells and their reactiveness to steroid therapy.11 IL-10 is thought to be produced by malignant B-cell lymphomas and related to normal inflammatory states. Elevated IL-10 concentrations or IL-10/IL-6 >1 in the aqueous humor or vitreous fluid is indicative of B-cell lymphoma.12 Monoclonality of immunoglobulin heavy chain (IgH) and T-cell receptor (TCR) gene rearrangements have also been widely used to detect B and T cell lymphomas, respectively.10 Myeloid differentiation factor 88 (MYD88) and CD79B are two genes that are related to B-cell VRL. The mutations of these two genes were found to be positive in the vitreous fluid of a large proportion of VRL patients and this may become a helpful diagnostic tool in the future.13,14
Optical coherence tomography (OCT) is a powerful tool that can be used to assess the microstructure of the retina due to its high spatial resolution. It has been applied extensively to evaluate the condition of the fundus. Compared to the methods listed above, OCT is a non-invasive examination and can be performed repeatedly. Accordingly, OCT is a useful method for the assessment and follow-up of VRL. Although there are some existing data about the OCT manifestations of VRL, most of these studies have focused on primary VRL15,16 and the numbers of cases are limited.17,18 Thus, we present a relatively large series of OCT images from PCNSL patients, and we determined the characteristic manifestations of intraocular involvement with OCT and monitored the changes during follow-up.
Patients and Methods
Patient Eligibility
Consecutive patients confirmed as having PCNSL (DLBCL) with intraocular involvement between January 2016 and December 2019 were enrolled in the VRL group at Huashan Hospital North affiliated to Fudan University, Shanghai, China. Consecutive PCNSL (DLBCL) patients without intraocular involvement between January 2019 and December 2019 were enrolled in the non-VRL group. Medical records of the patients in the two groups were reviewed. This study was performed in accordance with the guidelines of the Declaration of Helsinki and was granted approval by the Ethics Committee of Huashan Hospital, Fudan University. All patients were informed about the purpose of the study and written informed consent was obtained. Written consent was acquired from every patient included in this study.
Treatment Methods
Non-ocular lesions were treated according to the advice of hematologists, including systemic high-dose-methotrexate-based (HD-MTX based) chemotherapy, whole brain radiotherapy, intracranial chemotherapy, and autologous stem-cell transplantation. VRL was treated with regular intravitreal injections of methotrexate (MTX). The implementation of intravitreal MTX was divided into three phases: induction, consolidation, and maintenance. MTX was intravitreally injected at a dosage of 400 μg/0.1 mL twice a week for the first 4 weeks (induction), weekly for the following 8 weeks (consolidation), and then monthly for the final 9 months (maintenance).19 If keratopathy was observed, injections were suspended to wait for restoration of the corneal epithelium.
Ophthalmic Assessment
When first admitted to our Hospital, all PCNSL patients received an ophthalmologic evaluation. Routine ophthalmic examinations were performed once a month in the non-VRL group. In the VRL group, ophthalmic examinations were administered before every intravitreal injection and 1 month after the last injection. Examinations included: slit lamp examination, pupil-dilated fundus photography, B-scan ultrasonography, and spectral domain-OCT (Spectralis, Heidelberg, Germany). OCT images were acquired using a 30° volume scan pattern with an 8.8×8.8 mm scanning area positioned at the center of the fovea.
Diagnosis
The diagnosis of PCNSL was confirmed with an intracranial biopsy. Then, the cases were pathologically classified into two groups: germinal center B-cell-like (GCB) and activated B-cell–like (ABC) using Hans’ criteria.20 In regard to suspected VRL, a vitrectomy was performed. About 0.8 mL of undiluted vitreous fluid was taken with a 600 cpm cutting rate, and then 5 mL of vitreous fluid diluted with balanced salt solution was acquired. The vitreous samples were sent to the cytopathology laboratory within 30 minutes for smear preparation, Wright’s stain, cell number counting, and immunohistochemistry (CD3, CD20, PAX-5, BCL-2, and BCL-6). Interleukins (IL-10, IL-6, and the IL10/IL6 ratio) were detected with a cytometric bead array method. If cytopathology revealed the presence of naïve atypical lymphocytes in the vitreous fluid, the diagnosis of VRL was confirmed. If cytopathology showed no obvious abnormalities, then the aqueous humor and/or vitreous fluid IL-10/IL-6 ratio were taken into consideration. If the aqueous humor and/or vitreous fluid IL-10/IL-6 ratio >1 and retinal lesions (subretinal creamy yellowish lesions or vasculitis) were observed, a diagnosis of VRL was presumed. Otherwise, the diagnosis was non-VRL.
Statistical Analysis
SPSS 25.0 (SPSS, Inc., Chicago, IL, USA) was used to perform the statistical analysis. Continuous variables were presented as the mean ± standard deviation (with a normal distribution) or median/ interquartile distance (without a normal distribution), and categorical variables were presented as numbers and proportions. A Student’s t-test was used to compare the average differences between the two groups. Categorical data were analyzed using a chi-squared test or chi-squared test with continuity correction (when the theoretical frequency was <5). A two-tailed p-value <0.05 was considered statistically significant.
Results
Patient Characteristics
A total of 120 (64 males, 56 females) PCNSL patients without intraocular involvement were enrolled in the non-VRL group, and 45 (25 males, 20 females) patients with intraocular involvement were enrolled in the VRL group. The mean age at diagnosis of PCNSL in the VRL and non-VRL groups was 56.9±10.5 and 56.8±11.2 years, respectively. A list of the main clinical characteristics of the patients in the two groups is summarized in Table 1. Of the 45 patients in the VRL group, 23 (51.1%) patients were diagnosed with concomitant intraocular involvement at the first ophthalmic evaluation. The remaining 22 patients developed vitreoretinal lymphoma during follow-up after a median of 12 months (range 1–54 months).
 |
Table 1 Demographic Information and Tumor Characteristics in the Two Groups |
OCT Manifestations
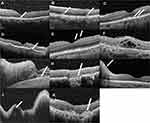
Of the 45 patients in the VRL group, the OCT images of 12 patients were either incomplete or had low quality. Finally, the OCT images of 33 patients (48 eyes) were analyzed. Abnormalities were found on the images of 19 (39.6%) eyes before the diagnosis of vitreoretinal lymphoma. The most frequent abnormality was outer retina (OR) fuzzy borders (14 of 19, 73.7%). Other abnormalities included: focal subretinal deposits (8 of 19, 42.1%), hyperreflective subretinal dots (2 of 19, 10.5%), pigment epithelium detachment (PED) (5 of 19, 26.3%), preretinal deposits (5 of 19, 26.3%), epiretinal membrane (3 of 19, 15.8%), cystoid macular edema (3 of 19, 15.8%), subretinal fluid (3 of 19, 15.8%), outer retina atrophy (2 of 19, 10.5%), unilateral optic papilledema (2 of 19, 10.5%), retinal thickening (1 of 19, 5.3%), and subretinal fibrosis (1 of 19, 5.3%). The details of the abnormalities observed on OCT images are shown in Figure 1.
Of the 120 patients in the non-VRL group, the OCT images of 12 patients were either incomplete or with low quality. Finally, the OCT images of 108 patients (216 eyes) were analyzed. Abnormalities were found on the images of 26 (12.0%) eyes. These images were reviewed by two independent experienced ophthalmologists to identify the relationship between the abnormalities and the intracranial lymphoma lesions. The majority of the abnormalities were not related to lymphoma, including focal RPE degeneration (nine eyes), epiretinal membrane (five eyes), vitreomacular traction (four eyes), solitary PED (four eyes), and focal elevated ellipsoid zone (one eye). The abnormality that was considered to be associated with lymphoma was hyperreflective subretinal dots, which was observed on the OCT images of two cases.
OCT manifestations of the patients in both groups are summarized in Table 2. OCT revealed specific abnormalities of VRL. Most of the abnormalities seen in the VRL group were not observed in the non-VRL group.
 |
Table 2 Optical Coherence Tomography Abnormalities in the Two Groups |
Follow-Up
After pars plana vitrectomy, the preretinal deposits all disappeared. The cystoid macular edema of the left eye of one patient spontaneously resolved before intravitreal injections. Of the 33 patients (48 eyes) with a complete OCT record in the VRL group, 21 patients (31 eyes) completed the induction phase of intravitreal chemotherapy (eight injections), 19 patients (28 eyes) completed the consolidation phase (16 injections), and nine patients (13 eyes) completed the maintenance phase (25 injections). No obvious side effects from intravitreal methotrexate were observed on OCT images except for transient cystoid macular edema in two cases and both spontaneously resolved one month later.
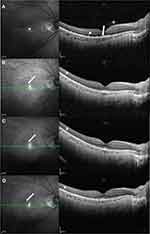
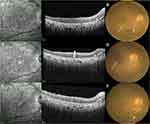
Seven patients (nine eyes) who had abnormalities (preretinal deposits and transient macular edema excluded) on OCT images before the first injection completed the whole treatment regimen and were analyzed for treatment outcomes (Table 3). OCT effectively revealed the treatment progress. Abnormalities, including OR fuzzy borders, PED, and focal subretinal deposits, fully disappeared in five eyes (eyes 1–4, 6). Three eyes (eyes 3, 4, 6) showed clearance of abnormalities after one, six, and seven injections, respectively, and developed hyperreflective subretinal dots. These subretinal dots were connected with the RPE and localized between the RPE and the ellipsoid zone. On near-infrared images, there were numerous scattered hyperreflective dots with a clear border and they remained stable during follow-up (Figure 2). Two eyes (eyes 1, 2) did not develop subretinal dots after disappearance of the abnormalities (after four and eight injections, respectively). The subretinal dots observed before intravitreal MTX of another two eyes (eyes 5, 7) also remained stable during follow-up. The OCT images and fundus photographs of the seven eyes above manifested only outer retina involvement. The remaining two eyes (eyes 8, 9) showed improvement during treatment. However, the structure of the retina was not restored to normal (Figure 3). The OCT images and fundus photographs of the two eyes revealed both inner and outer retina involvement, including retinal vasculitis, epiretinal membrane, cystoid macular edema, focal subretinal deposits, OR fuzzy borders, and PED.
 |
Table 3 Follow-Up of Abnormalities During Treatment |
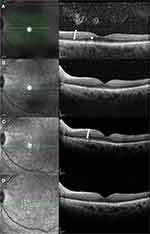
During follow-up, two patients had intraocular recurrence. The left eye of case I (eye 1) had vitreous opacity, OR fuzzy borders, and PED before intravitreal MTX, and these abnormalities disappeared after eight injections. After 21 injections (8 months), the vitreous opacity recurred without retina lesions on OCT images. The patient received the entire induction-consolidation-maintenance regimen again and did not develop a second relapse for 26 months. The left eye of case II (eye 3) had vitreous opacity, OR fuzzy borders, and focal subretinal deposits before intravitreal MTX, and these abnormalities disappeared after seven injections. After 19 injections (6 months), OR fuzzy borders appeared again on the OCT images and the intravitreal MTX were administered from the consolidation phase (weekly injections) again. The OR fuzzy borders rapidly resolved after four injections and the patient did not develop a second relapse for ten months (Figure 4).
Discussion
In this study, we describe the characteristic OCT manifestations of PCNSL with intraocular involvement and present the changes in these abnormalities after intravitreal injections. Our results suggest OCT is a useful tool for the evaluation and monitoring of vitreoretinal lymphoma.
Abnormalities were found on the OCT images of 19 eyes (39.6%) in this report. The percentage was similar to that of a study by Saito et al (37.2%).15 However, several studies have demonstrated a higher proportion, from 68%21 to 93.8%.16 This result is likely due to the different inclusion criteria for patients. Our study focused on the intraocular involvement of PCNSL, while the other studies paid more attention to primary vitreoretinal lymphoma. On one hand, VRL is difficult to diagnose considering its resemblance to noninfectious uveitis and VRL patients with retina infiltration are more likely to be correctly diagnosed. On the other hand, the mean duration between ocular symptoms and diagnosis of PVRL is 6 months and there is more time for the development of retinal infiltration. In addition, in this study all patients received a large macular scan so the abnormalities outside of the macular area were not recorded by OCT.
The signs viewed on OCT images are hypothesized to be due to the infiltration of lymphoma cells. The most frequently observed abnormalities on OCT images are OR fuzzy borders (14 of 19), focal subretinal deposits (8 of 19), and PED (5 of 19). Although described with different terms in other studies, it is consistent that the outer retina is more easily involved than the inner retina.16,21–23 The three types of abnormalities may refer to the infiltration of lymphoma cells into different layers of the retina. OR fuzzy borders are assumed to be a diffuse infiltration within the RPE and photoreceptor outer segment layer. Focal subretinal deposits represent focal infiltration superior to the RPE. PED manifests as infiltration between the RPE and Bruch’s membrane. The close relationship between the RPE layer and lymphoma involvement is probably due to the interactions of the cell molecules between RPE cells and B lymphoma cells. Chan et al24 conducted a human tissue study investigating the expression of cell biomarkers from three freshly enucleated eyes with PVRL and a normal autopsied eye. High expression levels of CXC chemokine receptor 4 (CXCR4) and CXCR5, combined with abundant transcripts, were found to be limited to the lymphoma cells. In contrast, CXC chemokine ligand-12 (CXCL12) and CXC chemokine ligand-13 (CXCL13) were found in the RPE. However, no chemokine expression was detected in the RPE cells in the normal control eyes.
Hyperreflective subretinal dots were a sign that arose after the disappearance of lymphoma infiltration and subsequently remained stable in three cases of the VRL group. To our knowledge, only Tan et al25 reported this phenomenon. These persistent hyperreflective dots may be the debris of lymphoma cells, degenerated RPE cells, or degenerated photoreceptor outer segments that were non-absorbable by the RPE layer. Interestingly, the sign was also observed in the first ophthalmic evaluation, in both the VRL and non-VRL groups, indicating the previous existence and regression of vitreoretinal lymphoma before intraocular treatment. This is possibly due to the systemic corticosteroids after intracranial biopsy. Another possible explanation is that vitreoretinal lymphoma is able to regress spontaneously, although only several cases have been reported.25–27
Inner retinal infiltration was not observed in our study. Some other studies reported different types of inner retinal infiltration, such as diffuse intraretinal infiltration,23,28 focal intraretinal deposits,15 and vertical hyperreflective lesions.29 However, the inner retina was not completely intact and was involved by means of retinal vasculitis in two cases. Furthermore, eyes with only outer retina infiltration could restore the retinal structure to almost normal after the induction phase, while the eyes with severe retinal vasculitis could not. This suggests that severe vasculitis is a predictor for a poor prognosis for structure restoration.
Methotrexate is the most widely used chemotherapeutic drug for the treatment of vitreoretinal lymphoma. No obvious adverse effects were observed during the follow-up on OCT images except for transient macular edema in two cases, suggesting the safety of an intravitreal MTX injection. Pigmentary maculopathy was reported as a complication due to the blood-brain barrier disruption treatment for intracranial lesions19 but was not observed in our study. In fact, the most frequent side effect reported for MTX is keratopathy, as the use of the antimetabolite agent with a short injection interval inhibited growth of the corneal epithelium.30
Other less frequent abnormalities observed in our study included cystoid macular edema, subretinal fluid, outer retina atrophy, unilateral optic papilledema, retinal thickening, and subretinal fibrosis. These signs are not characteristic of lymphoma. Nonetheless, they demonstrate the diversity of the clinical manifestations of vitreoretinal lymphoma. Saito et al15 reported 13 types of OCT abnormalities, which was even more than in our study. In any case, vitreoretinal lymphoma has a variety of manifestations on OCT images and should be considered a possible diagnosis in the context of one of these signs.
OCT is helpful in assessing intraocular involvement with PCNSL. When considering primary vitreoretinal lymphoma, abnormalities observed by OCT could not directly lead to the diagnosis, due to confounding by other possible explanations like uveitis. However, in the context of already existing PCNSL, OCT exhibits considerably more value. Most of the abnormalities seen in the patients with intraocular involvement are usually absent in the patients without intraocular involvement. Furthermore, as a non-invasive examination method with high resolution, OCT is an effective tool to evaluate the treatment effect and monitor the relapse as it is able to reveal minor changes during the course of the disease. Thus, OCT is advantageous for better decision-making and for reducing the morbidity and mortality of PCNSL patients.
Our study has several limitations. As a retrospective study, the OCT abnormalities located outside the macular area could not be analyzed. Second, IgH gene rearrangement was not studied because of technical problems. The diagnosis of vitreoretinal lymphoma could have been more accurate. Finally, the follow-up time in our study was relatively short. A longer time is required to further study the long-term performance of vitreoretinal lymphoma.
Conclusion
Our study demonstrated the characteristic OCT manifestations of vitreoretinal lymphoma and thus indicated that OCT is a useful tool for the diagnosis of PCNSL with intraocular involvement. Retinal structures can return to almost normal after the induction phase of intravitreal methotrexate treatment in a large proportion of patients. As a repeatable non-invasive examination, OCT can be utilized to assess the treatment response and monitor long-term development during the entire course of the disease. Therefore, OCT is beneficial for medical decision-making and contributes to the health of PCNSL patients.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
This work was supported by the Shanghai Xuhui district health and family planning commission key disease joint project (XHLHGG201807) and the Shanghai clinical three-year action plan-major clinical research project (SHDC2020CR2041B).
Disclosure
The authors report no conflicts of interest in this work.
References
1. Farrall AL, Smith JR. Eye involvement in primary central nervous system lymphoma. Surv Ophthalmol. 2020;65(5):548–561. doi:10.1016/j.survophthal.2020.02.001
2. Grommes C, DeAngelis LM. Primary CNS lymphoma. J Clin Oncol. 2017;35(21):2410–2418. doi:10.1200/JCO.2017.72.7602
3. Hoang-Xuan K, Bessell E, Bromberg J, et al. Diagnosis and treatment of primary CNS lymphoma in immunocompetent patients: guidelines from the European Association for Neuro-Oncology. Lancet Oncol. 2015;16(7):e322–e332. doi:10.1016/S1470-2045(15)00076-5
4. Yuan XG, Huang YR, Yu T, et al. Primary central nervous system lymphoma in China: a single-center retrospective analysis of 167 cases. Ann Hematol. 2020;99(1):93–104. doi:10.1007/s00277-019-03821-9
5. Coupland SE, Heimann H, Bechrakis NE. Primary intraocular lymphoma: a review of the clinical, histopathological and molecular biological features. Graefes Arch Clin Exp Ophthalmol. 2004;242(11):901–913. doi:10.1007/s00417-004-0973-0
6. Zhuang L, Lai J, Chen K, et al. Intraocular involvement is associated with a high risk of disease relapse in primary central nervous system lymphoma. Oncol Rep. 2019;41(1):397–404. doi:10.3892/or.2018.6781
7. Takhar JS, Doan TA, Gonzales JA. Primary vitreoretinal lymphoma: empowering our clinical suspicion. Curr Opin Ophthalmol. 2019;30(6):491–499. doi:10.1097/ICU.0000000000000620
8. Lai J, Chen K, Shi HM, et al. B-scan ultrasound and cytology of the vitreous in primary central nervous system lymphoma with vitreoretinal involvement. Int J Ophthalmol. 2019;12(6):1001–1007. doi:10.18240/ijo.2019.06.20
9. Fend F, Ferreri AJ, Coupland SE. How we diagnose and treat vitreoretinal lymphoma. Br J Haematol. 2016;173(5):680–692. doi:10.1111/bjh.14025
10. Kimura K, Usui Y, Goto H; Japanese Intraocular Lymphoma Study G. Clinical features and diagnostic significance of the intraocular fluid of 217 patients with intraocular lymphoma. Jpn J Ophthalmol. 2012;56(4):383–389. doi:10.1007/s10384-012-0150-7
11. Ito T, Takeda A, Fujiwara K, et al. Risk factors for failure of vitrectomy cell block technique in cytological diagnosis of vitreoretinal lymphoma. Graefes Arch Clin Exp Ophthalmol. 2019;257(5):1029–1036. doi:10.1007/s00417-019-04266-6
12. Pochat-Cotilloux C, Bienvenu J, Nguyen A-M, et al. Use of a threshold of interleukin-10 and IL-10/IL-6 ratio in ocular samples for the screening of vitreoretinal lymphoma. Retina. 2018;38(4):773–781. doi:10.1097/IAE.0000000000001922
13. Shi H, Zhou X, Chen B, et al. Clinical relevance of the high prevalence of MYD88 L265P mutated vitreoretinal lymphoma identified by droplet digital polymerase chain reaction. Ocul Immunol Inflamm. 2021;29:448–455.
14. Yonese I, Takase H, Yoshimori M, et al. CD79B mutations in primary vitreoretinal lymphoma: diagnostic and prognostic potential. Eur J Haematol. 2019;102(2):191–196. doi:10.1111/ejh.13191
15. Saito T, Ohguro N, Iwahashi C, Hashida N. Optical coherence tomography manifestations of primary vitreoretinal lymphoma. Graefes Arch Clin Exp Ophthalmol. 2016;254(12):2319–2326. doi:10.1007/s00417-016-3395-x
16. Barry RJ, Tasiopoulou A, Murray PI, et al. Characteristic optical coherence tomography findings in patients with primary vitreoretinal lymphoma: a novel aid to early diagnosis. Br J Ophthalmol. 2018;102(10):1362–1366. doi:10.1136/bjophthalmol-2017-311612
17. Jang HS, Sepah YJ, Sophie R, et al. Longitudinal spectral domain optical coherence tomography changes in eyes with intraocular lymphoma. J Ophthalmic Inflamm Infect. 2013;3(1):59. doi:10.1186/1869-5760-3-59
18. Egawa M, Mitamura Y, Hayashi Y, Naito T. Spectral-domain optical coherence tomographic and fundus autofluorescence findings in eyes with primary intraocular lymphoma. Clin Ophthalmol. 2014;8:335–341. doi:10.2147/OPTH.S58114
19. Frenkel S, Hendler K, Siegal T, Shalom E, Pe’er J. Intravitreal methotrexate for treating vitreoretinal lymphoma: 10 years of experience. Br J Ophthalmol. 2008;92(3):383–388. doi:10.1136/bjo.2007.127928
20. Hans CP, Weisenburger DD, Greiner TC, et al. Confirmation of the molecular classification of diffuse large B-cell lymphoma by immunohistochemistry using a tissue microarray. Blood. 2004;103(1):275–282. doi:10.1182/blood-2003-05-1545
21. Lavine JA, Singh AD, Sharma S, Baynes K, Lowder CY, Srivastava SK. Ultra-widefield multimodal imaging of primary vitreoretinal lymphoma. Retina. 2019;39(10):1861–1871. doi:10.1097/IAE.0000000000002260
22. Keino H, Okada AA, Watanabe T, et al. Spectral-domain optical coherence tomography patterns in intraocular lymphoma. Ocul Immunol Inflamm. 2016;24(3):268–273. doi:10.3109/09273948.2014.1002568
23. Yang X, Dalvin LA, Mazloumi M, et al. Spectral domain optical coherence tomography features of vitreoretinal lymphoma in 55 eyes. Retina. 2021;41:249–258.
24. Chan CC, Shen D, Hackett JJ, Buggage RR, Tuaillon N. Expression of chemokine receptors, CXCR4 and CXCR5, and chemokines, BLC and SDF-1, in the eyes of patients with primary intraocular lymphoma. Ophthalmology. 2003;110(2):421–426. doi:10.1016/S0161-6420(02)01737-2
25. Tan SZ, Steeples LR, Chhabra R, Jones NP. An unusual case report of primary vitreoretinal lymphoma. BMC Ophthalmol. 2018;18(Suppl 1):223. doi:10.1186/s12886-018-0860-9
26. Kase S, Namba K, Jin XH, Kubota KC, Ishida S. Spontaneous regression of intraocular lymphoma. Ophthalmology. 2012;119(5):1083–1084. doi:10.1016/j.ophtha.2011.12.011
27. Mantopoulos D, Cebulla CM. Multimodal imaging of spontaneously shifting primary vitreoretinal lymphoma. Ocul Oncol Pathol. 2015;1(4):237–240. doi:10.1159/000374121
28. Zhao H, Wang X, Mao Y, Peng X. Longitudinal observation of OCT imaging is a valuable tool to monitor primary vitreoretinal lymphoma treated with intravitreal injections of methotrexate. BMC Ophthalmol. 2020;20(1):10. doi:10.1186/s12886-019-1300-1
29. Deák GG, Goldstein DA, Zhou M, Fawzi AA, Jampol LM. Vertical hyperreflective lesions on optical coherence tomography in vitreoretinal lymphoma. JAMA Ophthalmol. 2019;137(2):194–198. doi:10.1001/jamaophthalmol.2018.5835
30. Zhou X, Zhou X, Shi H, et al. Reduced frequency of Intravitreal methotrexate injection lowers the risk of keratopathy in vitreoretinal lymphoma patients. BMC Ophthalmol. 2020;20(1):189. doi:10.1186/s12886-020-01464-3
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.