Back to Journals » Infection and Drug Resistance » Volume 16
oprL Gene Sequencing, Resistance Patterns, Virulence Genes, Quorum Sensing and Antibiotic Resistance Genes of XDR Pseudomonas aeruginosa Isolated from Broiler Chickens
Authors Algammal AM , Eidaroos NH, Alfifi KJ , Alatawy M, Al-Harbi AI , Alanazi YF , Ghobashy MOI, khafagy AR, Esawy AM, El-Sadda SS, Hetta HF , El-Tarabili RM
Received 14 December 2022
Accepted for publication 8 February 2023
Published 13 February 2023 Volume 2023:16 Pages 853—867
DOI https://doi.org/10.2147/IDR.S401473
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Suresh Antony
Abdelazeem M Algammal,1 Nada H Eidaroos,1 Khyreyah J Alfifi,2 Marfat Alatawy,2 Alhanouf I Al-Harbi,3 Yasmene F Alanazi,4 Madeha OI Ghobashy,2,5 Ahmed R khafagy,6 Aboelkheir M Esawy,7 Soha S El-Sadda,7 Helal F Hetta,8 Reham M El-Tarabili1
1Department of Bacteriology, Immunology, and Mycology, Faculty of Veterinary Medicine, Suez Canal University, Ismailia, Egypt; 2Department of Biology, Faculty of Science, University of Tabuk, Tabuk, Saudi Arabia; 3Department of Medical Laboratory, College of Applied Medical Sciences, Taibah University, Yanbu, Saudi Arabia; 4Department of Biochemistry, Faculty of Science, University of Tabuk, Tabuk, Saudi Arabia; 5Microbiology Department, Faculty of Science, Ain Shams University, Cairo, Egypt; 6Department of Microbiology, Faculty of Veterinary Medicine, Ain Shams University, Cairo, Egypt; 7Animal Health Research Institute, Mansoura, Egypt; 8Department of Medical Microbiology and Immunology, Faculty of Medicine, Assiut University, Assiut, Egypt
Correspondence: Abdelazeem M Algammal, Department of Bacteriology, Immunology and Mycology, Faculty of Veterinary Medicine, Suez Canal University, Ismailia, 41522, Egypt, Email [email protected]
Background: Pseudomonas aeruginosa is incriminated in septicemia, significant economic losses in the poultry production sector, and severe respiratory infections in humans. This study aimed to investigate the occurrence, oprL sequencing, antimicrobial resistance patterns, virulence-determinant, Quorum sensing, and antibiotic resistance genes of P. aeruginosa retrieved from broiler chickens.
Methods: Two hundred samples were collected from 120 broiler chickens from broiler farms at Ismailia Governorate, Egypt. Consequently, the bacteriological examination was conducted and the obtained P. aeruginosa strains were tested for oprL gene sequencing, antibiogram, and PCR screening of virulence, Quorum sensing, and antibiotic resistance genes.
Results: The overall prevalence of P. aeruginosa in the examined birds was 28.3%. The oprL gene sequence analysis underlined that the tested strain expressed a notable genetic identity with various P. aeruginosa strains isolated from different geographical areas in the USA, India, China, Chile, and Ghana. PCR evidenced that the obtained P. aeruginosa strains, carrying virulence-related genes: oprL, toxA, aprA, phzM, and exoS in a prevalence of 100%, 100%, 42.5%, 33.3%, and 25.9%, respectively. Moreover, the recovered P. aeruginosa strains possessed the Quorum sensing genes: lasI, lasR, rhlI, and rhlR in a prevalence of 85.2%, 85.2%, 81.5%, and 81.5%, respectively. Furthermore, 40.7% of the isolated P. aeruginosa were XDR to seven antimicrobial classes, possessing sul 1, blaTEM, tetA, blaCTX-M, blaOXA-1, and aadA1 genes.
Conclusion: As we can tell, this is the first report emphasizing the evolution of XDR P. aeruginosa strains from broiler chicken in Egypt, which is supposed to be a serious threat to public health. The emerging XDR P. aeruginosa in poultry frequently harbored the oprL, toxA, and aprA virulence genes, the lasI, lasR, rhlI, and rhlR Quorum sensing genes, and the sul 1, blaTEM, tetA, blaCTXM, blaOXA-1, and aadA1 resistance genes.
Keywords: P. aeruginosa, MDR, XDR, oprL sequence analysis, Quorum sensing, virulence genes, resistance genes
Introduction
Pseudomonas species are ubiquitous microorganisms that are retrieved from various origins, such as poultry, drinking water, domestic and wild animals, human cases, and different food products.1,2 Pseudomonas infections in poultry are of the highest significance as the infection might spread speedily among the poultry flocks resulting in elevated mortalities in various ages.3 The genus Pseudomonas includes several species, but Pseudomonas aeruginosa (P. aeruginosa) is the most common specie incriminated in poultry infection all over the world. P. aeruginosa is an opportunistic microorganism able to infect various host tissues.4,5 P. aeruginosa infections in chickens mainly occur via skin wounds, contaminated vaccines, egg dipping, and contamination of needles used for injection. Moreover, the disease could be transmitted from infected flocks to susceptible ones in the same area due to poor hygienic conditions. Even though chickens of any age can be infected with P. aeruginosa, young chicks frequently are the most susceptible.5
P. aeruginosa are Gram-negative motile rods arranged mainly in a single manner or short chains. It is a strictly aerobic pathogen that rapidly grows on ordinary media and typically produces water-soluble fluorescein or pyocyanin pigments.6 P. aeruginosa is an opportunistic pathogen that is involved in high mortalities of young chicks as a result of yolk sac infections and omphalitis gained through hatchery.7 Furthermore, P. aeruginosa retains both cell-mediated and secreted virulence determinants. The cell-mediated virulence factors, such as lipopolysaccharide (LPS), flagella, and pili, play a vital role in motility, colonization of bacteria in the host tissues, and the invasion of bacterial active proteins into the target cells.8 Besides, the secreted virulence types enable microbial invasion and propagation, strengthen inflammatory conditions, initiate potent host-tissue damages, and increase the severity of infection. The most common secreted virulence determinants accompanying P. aeruginosa are exotoxin A and exotoxin S. Exotoxin A is accountable for the prevention of protein synthesis in the host cell, whereas exotoxin S is an extracellular protein that incriminated in the cell-apoptosis through the initiation of the GTPase and ribosyltransferases actions. Moreover, the pathogen secretes the biologically active phenazine compounds that play a vital role in bacterial virulence. The Quorum-Sensing phenomenon is a cell signaling mechanism present in certain bacterial species, enabling the bacterial cell response to extracellular signals. It is regulated by the las and rhl genes.9,10 Molecular techniques are essential for the rapid detection of P. aeruginosa by amplification of species-specific primers, especially oprL gene sequencing.11–13
Recently, multidrug resistance (MDR) has augmented worldwide that is deliberated public health threat. Several recent epidemiological investigations revealed the occurrence of XDR (extensively drug-resistant: resistant to ≥ 1 agent in all tested antimicrobial classes but ≤ 2 classes) and MDR (Multidrug-resistant: resistant to ≥ 1 agent in ≥ 3 antimicrobial classes) bacterial pathogens from different origins.14–17 P. aeruginosa frequently displayed various resistance patterns against several antibiotics. The antibiotic resistance in P. aeruginosa is accredited mainly to acquired and intrinsic resistance mechanisms to various antibiotics through low permeability of the outer membrane, antibiotic resistance genes, and active efflux pumps.18 In P. aeruginosa, the outer membrane proteins (oprL) play a significant role in antibiotics and antiseptics resistance.11 Moreover, the Extended β-lactamases (ESBLs), encoded by ESBLs genes, are responsible for the resistance of β-lactam antimicrobials (penicillin and cephalosporins). The blaCTX-M and blaTEM are the most common ESBLs genes related to P. aeruginosa.19,20 Therefore, the PCR screening of the most common antimicrobial resistance genes has to be accomplished to investigate the occurrence of MDR pathogens of public health importance.21
This work was conducted to demonstrate the occurrence, Sequence analysis of oprL gene, virulence, Quorum sensing, and resistance genes of XDR P. aeruginosa recovered from broiler chickens.
Methods
Animal Ethics
All protocols were conducted according to relevant USDA Animal Welfare guidelines followed for the welfare of the laboratory animals. Scientific Research Ethics Committee, Suez Canal University, Egypt, approved the handling of chickens and all the procedures (Approval no. 2022056).
Sampling
A total of 200 samples were congregated from 120 broiler chickens (2–4 weeks old) (40 apparently healthy birds: tracheal swabs (n=40), 40 Diseased birds: tracheal swabs (n=40), and 40 freshly dead birds: liver, lung, and heart (n=40 for each) from four commercial broilers farms (30 birds from each farm; 10 apparently healthy, 10 diseased birds, and 10 freshly dead birds, were examined) at Ismailia Province, Egypt (from April to June 2021). Diseased birds exhibited respiratory manifestations. Samples collection was carried out aseptically, and samples were immediately transmitted in the icebox to the laboratory as soon as possible.
Isolation and Identification of P. aeruginosa
The collected samples were inoculated in nutrient broth (Oxoid, UK) and incubated aerobically for 24 hrs at 37 °C. A loopful from the inoculated broth was streaked onto cetrimide agar and MacConkey agar (Oxoid, UK) and incubated at 37°C for 24 hrs under aerobic conditions. The recovered isolates were identified according to their morphological characteristics using Gram’s stain, culture characters, pigment production (fluorescent pigments), motility, and biochemical reactions using the following tests; oxidase, indole, H2S production, catalase, urease, methyl red, citrate utilization, gelatin hydrolysis, mannitol fermentation, and Voges-Proskauer tests as previously described by Mac Faddin.22 Likewise, the identification of P. aeruginosa was affirmed genetically using the species-specific set of primers aiming the oprL gene according to Xu.23
P. aeruginosa oprL Gene Sequencing
In this study, all the isolated P. aeruginosa strains disclosed coordination in their phenotypic traits (morphological, culture, and biochemical characteristics). Therefore, the PCR products of one randomly chosen P. aeruginosa strain were purified using the PureLink purification kit (Life Technologies, Renfrew, UK). Moreover, the attained sequences were stored in the GenBank (Accession no.: MW056321). Furthermore, numerous alignments were carried out on the recovered sequences. The phylogenetic tree was designed consistent with the neighbor-joining approach by the MEGA X software.24
Antibiogram of the Retrieved P. aeruginosa Isolates
The resistance patterns of the retrieved P. aeruginosa isolates were investigated using the disc diffusion test on Muller Hinton agar (Difco, USA). Eleven antimicrobial discs (Oxoid, UK) were implicated; streptomycin (S/10 μg), trimethoprim-sulfamethoxazole (SXT/1.25/23.75μg), ceftriaxone (CTX/30μg), norfloxacin (NOR/10μg), ampicillin (AMP/10μg), erythromycin (E/ 15μg), colistin sulfate (CT/10μg), amikacin (AK/30 μg), cefotaxime (CXT/30μg), tetracycline (TE/30 μg), and amoxicillin-clavulanic acid (AMC/30μg). The interpretation of the test results was accomplished in line with the instructions of CLSI, 2018.25 The P. aeruginosa ATCC 27853 was involved as a test control. Moreover, the tested P. aeruginosa strains were grouped into XDR and MDR in compliance with Magiorakos.26 Besides, the multiple antibiotic-resistance (MAR) indices were estimated according to Krumperman.27
Dissemination of Virulence, Quorum Sensing, and Resistance Genes in the Retrieved P. aeruginosa Isolates
PCR was used to monitor the virulence-related genes (oprL, toxA, aprA, exoS, and phzM), Quorum sensing genes (lasI, lasR, rhlI, and rhlR), and resistance genes (blaTEM, blaOXA, blaCTX-M, sul1, aadA1, and tetA) in the isolated P. aeruginosa. DNA was extracted using the PureLink DNA Extraction Kit (Life Technologies, Renfrew, UK / Cat. No. K182001). In each reaction, positive controls (Positive P. aeruginosa strains provided by the AHRI, Dokki, Egypt), as well as negative controls (DNA-free reaction) were applied. Moreover, the separation of PCR products was carried out with agar gel electrophoresis. Subsequently, the gel was photographed. The used primers (Life Technologies, Renfrew, UK) and PCR conditions are presented in Table 1.
 |
Table 1 Oligonucleotide Sequences and Conditions of PCR Assay |
Statistical Analyses
The analyses of the data frequencies were performed by the Chi-square test using SAS software (version 9.4, SAS Institute, Cary, NC, USA) (p-value < 0.05 specifies a significant difference). Furthermore, the correlations between the tested antibiotics and the resistance genes were estimated with the R-software (version 4.0.2; https://www.r-project.org/).
Results
Phenotypic Characteristics and the Occurrence of P. aeruginosa in the Examined Birds
The bacteriological assay proved that all recovered P. aeruginosa isolates (n=54) were Gram-negative, motile rods arranged singly or in short chains. On cetrimide agar, the colonies of retrieved P. aeruginosa isolates were large and irregular with a fruity odor and disseminate the characteristic fluorescent pigment (yellowish-green). Moreover, the recovered isolates displayed smooth, pale (non-lactose fermenter), and flat colonies on macConkey agar. Biochemically, the tested P. aeruginosa isolates were positive for oxidase, mannitol fermentation, gelatin hydrolysis, catalase, citrate utilization, and nitrate reduction tests. Furthermore, the retrieved P. aeruginosa isolates were negative for methyl red, H2S production, indole, urease, and Voges-Proskauer tests. Besides, all the recovered isolates were positive for the species-specific oprL gene.
The occurrence of P. aeruginosa in the inspected birds was 28.3% (34/120). Moreover, the percentage of P. aeruginosa in the inspected apparently healthy, diseased, and freshly dead chickens was 12.5% (5/40), 42.5% (17/40), and 30% (12/40), respectively (as described in Table 2). Statistically, there is a significant difference (p < 0.05) in the occurrence of P. aeruginosa in the examined apparently healthy, diseased, and freshly dead birds. With regard to the intensity of P. aeruginosa in the examined internal organs of the freshly dead birds, the predominant affected organs were the lung and liver (22.2% for each), followed by the heart (14.8%) (Table 3 and Figure 1).
 |
Table 2 Prevalence of P. aeruginosa Among the Examined Birds |
 |
Table 3 The Dissemination of P. aeruginosa Among Different Examined Samples of Chickens |
 |
Figure 1 The dissemination of P. aeruginosa among various examined samples. |
Sequence Analysis of the oprL Gene of P. aeruginosa
The oprL gene sequence analysis underlined that the tested strain (Accession No. MW056321) exposed high genetic identity (100%) with other P. aeruginosa strains isolated from different origins and geographical areas (Figure 2), for example, P. aeruginosa strain PA0750 of India (Accession no. CP034908), P. aeruginosa strain LIUYANG-C of China (Accession no. CP050053), P. aeruginosa PAC1 of USA (CP053706), P. aeruginosa strain delta 6_4 of USA (Accession no. CP047063), P. aeruginosa strain delta 6_6 of USA (Accession no. CP047065), P. aeruginosa strain Cas9_1 of USA (Accession no. CP047067), and P. aeruginosa strain PGN4 of USA (Accession no. CP032540). Besides, it proved 99.7% genetic identity with P. aeruginosa strain PA-1 of human origin in Chile (Accession no. CP097709) and P. aeruginosa strain PA0011 isolated from tissue biopsy in Ghana (Accession no. CP100761).
Antibiogram of the Retrieved P. aeruginosa Isolates
The tested P. aeruginosa isolates disclosed high resistance to different antimicrobial agents including; trimethoprim-sulfamethoxazole, penicillin, and tetracycline (100% for each), ceftriaxone and cefotaxime (92.6% for each), streptomycin and amikacin (90.7% for each), amoxicillin-clavulanic acid (88.8%), and erythromycin (77.7%). Furthermore, the tested isolates displayed a striking sensitivity to norfloxacin (81.5%) and colistin sulfate (74.07%) (Table 4 and Figure 3). Statistically, there is a significant difference (p < 0.05) in the sensitivity of P. aeruginosa strains to different antimicrobial agents. Likewise, notable positive correlations were verified between TE and AMP (r = 1); SXT, TE, and AMP (r = 1); AK, CXT, CTX, SXT, TE, and AMP (r = 1); E, AMC, and S (r = 1); S, AK, CXT, CTX, SXT, TE, and AMP (r = 1); AMC, S, AK, and CXT (r = 1); NOR and CT (r = 1) (Figure 4).
 |
Table 4 Antimicrobial Resistance Patterns of the Retrieved P. aeruginosa |
 |
Figure 3 The heat-map explicates the antibiogram of the retrieved P. aeruginosa strains from broiler chickens. |
 |
Figure 4 The heat-map simplifies the correlation coefficient (r) between the tested antimicrobial agents in this study. |
The Occurrence of Virulence, Quorum Sensing, and Resistance Genes in the Isolated P. aeruginosa Isolates
Using PCR revealed that the tested P. aeruginosa strains possessing the virulence-related genes: oprL, toxA, aprA, phzM, and exoS with a prevalence of 100%, 100%, 42.5%, 33.3%, and 25.9%, respectively. Moreover, the recovered P. aeruginosa strains possessed the Quorum sensing genes: lasI, lasR, rhlI, and rhlR in a prevalence of 85.2%, 85.2%, 81.5%, and 81.5%, respectively. With reference to the dissemination of the resistance genes, all the isolated P. aeruginosa strains (100%) held the blaTEM, sul1, and tetA resistance genes. Besides, recovered P. aeruginosa isolates harbored the antimicrobial-resistance genes; aadA1, blaCTX-M, and blaOXA in a prevalence of 90.7%, 88.8%, and 81.5%, respectively. The statistical analysis emphasized a significant difference (p < 0.05) in the dissemination of virulence genes in the obtained P. aeruginosa. On the contrary, a non-significant difference (p > 0.05) was recorded in the occurrence of Quorum sensing and resistance genes in the tested P. aeruginosa (Table 5 and Figure 5).
 |
Table 5 Distribution of Virulence, Quorum Sensing, and Antimicrobial Resistance Genes |
 |
Figure 5 The distribution of virulence, quorum sensing, and resistance genes in the recovered P. aeruginosa isolates. |
Resistance Patterns and the Distribution of Antimicrobial Resistance Genes Between the Isolated P. aeruginosa Strains from Birds
It was noticed that 40.7% (22/54) of the obtained P. aeruginosa isolates were XDR to 9 antimicrobial agents in seven antimicrobial classes and carrying sul1, blaTEM, tetA, blaCTX-M, blaOXA-1, and aadA1 genes. Moreover, 18.5% (10/54) of the tested P. aeruginosa strains revealed extensive-drug resistance to 9 antimicrobial agents in seven antimicrobial classes and carried sul1, blaTEM, tetA, blaCTXM, and aadA1 genes. Furthermore, 16.7% (9/54) of the obtained P. aeruginosa isolates were XDR to 10 antimicrobial agents in eight classes, possessing sul1, blaTEM, tetA, blaOXA-1, blaCTX-M, and aadA1 genes. Besides, 9.3% (5/54) of the obtained P. aeruginosa isolates were MDR to 6 antimicrobial agents in five classes, carrying sul1, blaTEM, tetA, blaOXA-1, and blaCTXM genes. Also, 7.4% (4/54) of the tested P. aeruginosa isolates were MDR 7 antimicrobial agents in six classes and possessing sul1, blaTEM, tetA, blaOXA-1, and aadA1 genes as illustrated in Table 6 and Figure 6. Likewise, our results emphasized that the MAR index values were > 0.2, suggesting that the P. aeruginosa strains recovered from birds derived from high-risk contamination. Additionally, our findings reported notable positive correlations between the blaTEM gene and AMP (r=1); blaTEM, CTX, and CXT (r=1); sul1 and SXT (r=1); tetA and TE (r=1); aadA1, AK, and S (r=1); blaCTX-M and CTX (r=1); blaCTX-M and AMP (r=1); blaCTX-M and CXT (r=0.99); blaTEM and AMC (r=0.99); blaCTX-M and AMC (r=0.98); blaOXA-1, AMP, and CTX (r=0.97); blaOXA-1 and CXT (r=0.96); blaOXA-1 and AMC (r=0.93) (Figure 7).
 |
Table 6 Resistance Patterns and Distribution of Resistance Genes Between P. aeruginosa Strains Isolated from Birds |
 |
Figure 6 Illustrates the occurrence and distribution of XDR and MDR resistance patterns among the isolated P. aeruginosa strains from the examined broiler chickens. |
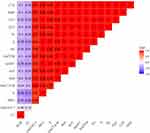 |
Figure 7 The heat-map elucidates the correlation coefficient (r) among the resistance genes of P. aeruginosa strains and different tested antimicrobial agents. |
Discussion
P. aeruginosa is one of the primary causes of septicemia in broiler chickens, triggering notable economic losses in the poultry production sector all over the world.7 Regarding the phenotypic features of P. aeruginosa, the bacteriological investigation emphasized that all the obtained isolates from birds disclosed the distinctive morphological, cultural, and biochemical features of P. aeruginosa. These outcomes are consistent with the findings verified by Abdelmoez.35
In this work, the overall prevalence of P. aeruginosa in the inspected birds was 28.3%. The highest prevalence was noted in the diseased chickens, followed by the freshly dead ones. Higher prevalence was recorded by Abd El-Tawab,36 who stated that the percentage of P. aeruginosa in the inspected broiler chickens was 34%. Moreover, a lower prevalence (20%) was reported by Shahat.4 P. aeruginosa represents a serious pathogen in poultry, causing severe respiratory infections and high mortality rates in broiler chicken flocks.37
Disparities in prevalence may be attributed to the hygienic conditions, the time of sampling, geographical variation, management practices, environmental stresses, and bird age and immunity.7 A high standard of hygienic measures is essential to prevent the spreading of P. aeruginosa infection between birds in poultry farms. Regular cleaning and disinfection of poultry farms frequently lead to better results for infection control. Moreover, limitations of antibiotic use in the poultry industry should be carried out.5,35
The molecular-based identification of P. aeruginosa is essential to overcome the limitations of conventional assays. Moreover, the amplification of species-specific primers, such as the oprL gene is beneficial to obtain rapid, reliable, and accurate identification of P. aeruginosa.38 In the present study, all retrieved isolates of P. aeruginosa from birds tested positive for the oprL gene. Likewise, the oprL sequence analyses emphasized that the tested P. aeruginosa strain displayed a notable genetic matching (100%) with several P. aeruginosa strains originated from various sources and areas. For example, P. aeruginosa strain LIUYANG-C of China (Accession no. CP050053), P. aeruginosa PAC1 of USA (CP053706), P. aeruginosa strain delta 6_4 of USA (Accession no. CP047063), P. aeruginosa strain delta 6_6 of USA (Accession no. CP047065), and P. aeruginosa strain PA0750 of India (Accession no. CP034908).39 These outcomes accentuate the epidemiological map and highlight the public health impact of P. aeruginosa.
Concerning the antimicrobial susceptibility testing, the retrieved P. aeruginosa strains were highly resistant to several antimicrobial classes including aminoglycosides, tetracycline, sulfonamides, penicillin, cephalosporins, macrolides, and β-Lactam-β-lactamase-inhibitor combination. These findings nearly agreed with those recorded by Kousar40 and Mohamed.41 On the other hand, norfloxacin and colistin-sulfate exhibited optimistic antimicrobial activity toward the tested P. aeruginosa strains recovered from broiler chicken. The sensitivity of P. aeruginosa to norfloxacin and colistin sulfate was informed previously by Sans-Serramitjana42 and Rafique.43 The remarkable resistance of P. aeruginosa to various antimicrobials is deliberated public health threat. The widespread use of antibiotics in the poultry production sector and the proficiency of P. aeruginosa to attain resistance genes from other superbugs are the fundamental reasons endorsing the occurrence of MDR and XDR strains. Moreover, the antimicrobial resistance in P. aeruginosa is attributed mainly to acquired and intrinsic resistance mechanisms through low permeability of the outer membrane as well as harboring resistance genes. Consequently, reliable application of susceptibility testing and investigation of the occurrence of XDR and MDR pathogens are indispensable for choosing the most effective antibiotics.44,45
Concerning the distribution of virulence genes, using PCR proved that the retrieved P. aeruginosa strains commonly have the oprL and toxA genes, followed by aprA, phzM, and exoS genes. Our findings are nearly in accordance with those recorded by Al-Dahmoshi,46 Bakheet and Torra,47 and Qian.48 The demonstration of virulence-related genes is crucial for the assessment of the potential pathogenicity of P. aeruginosa. The outer membrane lipoprotein (regulated by the oprL gene) is accountable for the intrinsic resistance of P. aeruginosa to antiseptics and antimicrobial agents.49,50 Moreover, Exotoxin A (regulated by the toxA gene) is a cytotoxic compound that is considered a key virulence determinant of P. aeruginosa. It is responsible for hindering protein biosynthesis in the host. Besides, exotoxin S is an extracellular protein that incriminated cell apoptosis through the initiation of the GTPase and ribosyltransferase actions. Likewise, the pathogen secretes the biologically active phenazine compounds (encoded by the phzM gene) that play a vital role in bacterial virulence. Alkaline protease (encoded by aprA gene) is a metalloprotease enzyme that splits different immune proteins such as TNF-α, IL-6, IFN-γ, and laminin, resulting in reduced immune response.51,52
In this study, the majority of the recovered P. aeruginosa strains had the lasI, lasR, rhlI, and rhlR Quorum sensing genes. Our results are consent with those stated by Sabharwal.28 Quorum-Sensing plays a substantial role in the expression of virulence-related genes, antimicrobial resistance, and biofilm formation in P. aeruginosa. Quorum-Sensing molecules are regulated by the las and rhl genes.53–55
Regarding the occurrence of resistance patterns in the recovered P. aeruginosa, the majorities of the tested P. aeruginosa were XDR to seven or eight antimicrobial classes, carrying the sul1, blaTEM, tetA, blaCTXM, blaOXA-1, and aadA1 genes. Besides, a high proportion of the retrieved P. aeruginosa disclosed MDR to five or six classes and harbored sul1, blaTEM, tetA, blaOXA-1, and blaCTXM genes. The tremendous increase in antimicrobial resistance is believed to be a major public health threat globally. The misapplication of antibiotics in poultry farms and health facilities and the transportation of resistance genes between bacteria are the primary predisposing determinants of multi-drug resistance.56,57 The Extended β-lactamases (ESBLs), encoded by ESBLs genes, are responsible for the resistance of β-lactam antimicrobials (penicillin and cephalosporins). The blaCTX-M, blaTEM, and blaOXA are the most common ESBLs genes in P. aeruginosa. The blaTEM gene is responsible for penicillin resistance. Moreover, the resistance to cephalosporins is ascribed to the blaCTX-M gene. Furthermore, the resistance to the β-Lactam-β-lactamase-inhibitor-combinations is endorsed by the synergism between blaOXA-1 and blaCTX-M resistance genes. Besides, P. aeruginosa is usually resistant to aminoglycosides, sulfonamides, and tetracycline due to the occurrence of aadA1, sul1, and tetA resistance genes, respectively.58–60 In the present study, there is a positive relationship between the occurrence of antimicrobial resistance and the distribution of antimicrobial resistance genes and virulence genes among the recovered P. aeruginosa strains from broiler chickens. On the other hand, Gajdács61 reported no correlation between virulence determinants, antimicrobial resistance, and biofilm production in the in-vitro-tested P. aeruginosa strains.
Conclusion
In brief, for all we know, this is the first report that underscored the evolution of XDR P. aeruginosa strains from broiler chicken in Egypt. The retrieved XDR P. aeruginosa strains commonly harbored the oprL, toxA, aprA, phzM, and exoS virulence-determinant genes and the lasI, lasR, rhlI, and rhlR Quorum sensing genes. The re-emerging P. aeruginosa strains in broiler chickens were XDR to many antimicrobial classes (cephalosporins, tetracycline, aminoglycosides, sulfonamides, penicillin, macrolides, and β-Lactam-β-lactamase-inhibitor combination) and usually carrying sul1, blaTEM, tetA, blaCTX-M, blaOXA-1, and aadA1 genes. Norfloxacin and colistin-sulfate displayed a potent in-vitro antimicrobial activity toward the emerging XDR P. aeruginosa strains. The synergistic application of traditional and molecular diagnostic assays is a precise epidemiological tool for the investigation of P. aeruginosa. Worryingly, the existence of XDR P. aeruginosa strains is reflected as a public health threat. The evolution of XDR P. aeruginosa strains consequently recommends the reliable conducting of sensitivity tests and the restricted use of antibiotics in poultry farms and the health sector.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure
The authors declare no conflicts of interest in this work.
References
1. Handley JA, Park SH, Kim SA, Ricke SC. Microbiome profiles of commercial broilers through evisceration and immersion chilling during poultry slaughter and the identification of potential indicator microorganisms. Front Microbiol. 2018;9:345. doi:10.3389/fmicb.2018.00345
2. Abdel-Tawab AA, Nasef SA, Ibrahim OA. Bacteriological and molecular studies on bacteria causing omphalitis in chicks with regard to disinfectant resistance. Glob Veterinaria. 2016;17(6):539–545.
3. Shukla S, Mishra P. Pseudomonas aeruginosa infection in broiler chicks in Jabalpur. Int J Ext Res. 2015;6:37–39.
4. Shahat HS, Mohamed H, Al-Azeem A, Mohammed W, Nasef SA. Molecular detection of some virulence genes in Pseudomonas aeruginosa isolated from chicken embryos and broilers with regard to disinfectant resistance. Int J Vet Sci. 2019;2(2):52–70. doi:10.21608/svu.2019.12365.1011
5. Saad ZA, Nasef SA, Elhariri M, Elhelw R, Ezzeldeen N. Resistance patterns associated with bacterial pathogens causing omphalitis in baby chicks. Biosci Res. 2017;14(4):845–851.
6. Reynolds D, Kollef M. The epidemiology and pathogenesis and treatment of Pseudomonas aeruginosa infections: an update. Drugs. 2021;81(18):2117–2131. doi:10.1007/s40265-021-01635-6
7. Eraky R, Abd El-Ghany W, Soliman K. Studies on Pseudomonas aeruginosa infection in hatcheries and chicken. J Hellenic Vet Med Soc. 2020;71(1):1953–1962. doi:10.12681/jhvms.22937
8. Mesquita CS, Soares-Castro P, Santos PM, Mendez-Vilas A. Pseudomonas aeruginosa: phenotypic flexibility and antimicrobial resistance. Sci Technol Educ. 2013;1:650–665.
9. Fazeli N, Momtaz H. Virulence gene profiles of multidrug-resistant Pseudomonas aeruginosa isolated from Iranian hospital infections. Iran Red Crescent Med J. 2014;16(10). doi:10.5812/ircmj.15722
10. Jurado-Martín I, Sainz-Mejías M, McClean S. Pseudomonas aeruginosa: an audacious pathogen with an adaptable arsenal of virulence factors. Int J Mol Sci. 2021;22(6):3128. doi:10.3390/ijms22063128
11. Nikbin V, Aslani MM, Sharafi Z, Hashemipour M, Shahcheraghi F, Ebrahimipour G. Molecular identification and detection of virulence genes among Pseudomonas aeruginosa isolated from different infectious origins. Iran J Microbiol. 2012;4(3):118.
12. Gong Q, Ruan M, Niu M, Qin C, Hou Y, Guo J. Immune efficacy of DNA vaccines based on oprL and oprF genes of Pseudomonas aeruginosa in chickens. Poult Sci. 2018;97(12):4219–4227. doi:10.3382/ps/pey307
13. Rocha AJ, Barsottini M, Rocha RR, Laurindo MV, Moraes FL, Rocha SL. Pseudomonas aeruginosa: virulence factors and antibiotic resistance genes. Braz Arch Biol Technol. 2019;62. doi:10.1590/1678-4324-2019180503
14. Algammal AM, Hashem HR, Al-Otaibi AS, et al. Emerging MDR-Mycobacterium avium subsp. avium in house-reared domestic birds as the first report in Egypt. BMC Microbiol. 2021;21(1):1–11. doi:10.1186/s12866-021-02287-y
15. Elbehiry A, Marzouk E, Aldubaib M, et al. Pseudomonas species prevalence, protein analysis, and antibiotic resistance: an evolving public health challenge. AMB Express. 2022;12(1):1–14. doi:10.1186/s13568-022-01390-1
16. Algammal AM, Abo Hashem ME, Alfifi KJ, et al. Sequence analysis, antibiogram profile, virulence and antibiotic resistance genes of XDR and MDR Gallibacterium anatis isolated from layer chickens in Egypt. Infect Drug Resist. 2022:4321–4334. doi:10.2147/IDR.S377797
17. Algammal AM, Ibrahim RA, Alfifi KJ, et al. A first report of molecular typing, virulence traits, and phenotypic and genotypic resistance patterns of newly emerging XDR and MDR Aeromonas veronii in Mugil seheli. Pathogens. 2022;11(11):1262. doi:10.3390/pathogens11111262
18. Langendonk RF, Neill DR, Fothergill JL. The building blocks of antimicrobial resistance in Pseudomonas aeruginosa: implications for current resistance-breaking therapies. Front Cell Infect Microbiol. 2021;11:665759. doi:10.3389/fcimb.2021.665759
19. Peymani A, Naserpour-Farivar T, Zare E, Azarhoosh K. Distribution of blaTEM, blaSHV, and blaCTX-M genes among ESBL-producing P. aeruginosa isolated from Qazvin and Tehran hospitals, Iran. J Prev Med Hyg. 2017;58(2):E155.
20. Heir E, Moen B, Åsli AW, Sunde M, Langsrud S. Antibiotic resistance and phylogeny of Pseudomonas spp. isolated over three decades from chicken meat in the Norwegian food chain. Microorganisms. 2021;9(2):207. doi:10.3390/microorganisms9020207
21. Meng L, Liu H, Lan T, et al. Antibiotic resistance patterns of Pseudomonas spp. isolated from raw milk revealed by whole genome sequencing. Front Microbiol. 2020;11:1005. doi:10.3389/fmicb.2020.01005
22. Mac Faddin JF. Media for Isolation-Cultivation-Identification-Maintenance of Medical Bacteria. Williams & Wilkins; 1985.
23. Xu J, Moore JE, Murphy PG, Millar BC, Elborn JS. Early detection of Pseudomonas aeruginosa–comparison of conventional versus molecular (PCR) detection directly from adult patients with cystic fibrosis (CF). Ann Clin Microbiol Antimicrob. 2004;3(1):1–5. doi:10.1186/1476-0711-3-21
24. Tamura K, Nei M, Kumar S. Prospects for inferring very large phylogenies by using the neighbor-joining method. Proc Natl Acad Sci. 2004;2004:11030–11035.
25. Clinical Laboratory Standards Institute. Performance standards for antimicrobial disk and dilution susceptibility tests for bacteria isolated from animals.
26. Magiorakos A-P, Srinivasan A, Carey RB, et al. Multidrug-resistant, extensively drug-resistant and pandrug-resistant bacteria: an international expert proposal for interim standard definitions for acquired resistance. Clin Microbiol Infect. 2012;18(3):268–281. doi:10.1111/j.1469-0691.2011.03570.x
27. Krumperman PH. Multiple antibiotic resistance indexing of Escherichia coli to identify high-risk sources of fecal contamination of foods. Appl Environ Microbiol. 1983;46(1):165–170. doi:10.1128/aem.46.1.165-170.1983
28. Sabharwal N, Dhall S, Chhibber S, Harjai K. Molecular detection of virulence genes as markers in Pseudomonas aeruginosa isolated from urinary tract infections. Int J Mol Epidemiol Genet. 2014;5(3):125.
29. Winstanley C, Kaye SB, Neal TJ, Chilton HJ, Miksch S, Hart CA. Genotypic and phenotypic characteristics of Pseudomonas aeruginosa isolates associated with ulcerative keratitis. J Med Microbiol. 2005;54(6):519–526. doi:10.1099/jmm.0.46005-0
30. Finnan S, Morrissey JP, O’Gara F, Boyd EF. Genome diversity of Pseudomonas aeruginosa isolates from cystic fibrosis patients and the hospital environment. J Clin Microbiol. 2004;42(12):5783–5792. doi:10.1128/JCM.42.12.5783-5792.2004
31. Colom K, Pérez J, Alonso R, Fernández-Aranguiz A, Lariño E, Cisterna R. Simple and reliable multiplex PCR assay for detection of blaTEM, blaSHV and blaOXA–1 genes in Enterobacteriaceae. FEMS Microbiol Lett. 2003;223(2):147–151. doi:10.1016/S0378-1097(03)00306-9
32. Archambault M, Petrov P, Hendriksen RS, et al. Molecular characterization and occurrence of extended-spectrum β-lactamase resistance genes among Salmonella enterica serovar Corvallis from Thailand, Bulgaria, and Denmark. Microb Drug Resist. 2006;12(3):192–198. doi:10.1089/mdr.2006.12.192
33. Randall L, Cooles S, Osborn M, Piddock L, Woodward MJ. Antibiotic resistance genes, integrons and multiple antibiotic resistance in thirty-five serotypes of Salmonella enterica isolated from humans and animals in the UK. J Antimicrob Chemother. 2004;53(2):208–216. doi:10.1093/jac/dkh070
34. Ibekwe AM, Murinda SE, Graves AK. Genetic diversity and antimicrobial resistance of Escherichia coli from human and animal sources uncovers multiple resistances from human sources. PLoS One. 2011;6(6):e20819. doi:10.1371/journal.pone.0020819
35. Abdelmoez N, Shawky M, Abdelhady H, Lebdah M, Salama S. Isolation and identification of some possible causative agents of swollen head syndrome (SHS) in broiler chickens in Egypt. Slov Vet Res. 2019;56:542.
36. Abd El-Tawab A, El-Hofy F, Khater D, Al-Adl M. Virulence, resistance genes detection and sequencing of gyrA gene of Pseudomonas aeruginosa isolated from chickens and human in Egypt. Nat Sci. 2018;16(2):32–39.
37. Abd El-Ghany WA. Pseudomonas aeruginosa infection of avian origin: zoonosis and one health implications. Vet World. 2021;14(8):2155. doi:10.14202/vetworld.2021.2155-2159
38. Algammal AM, Mabrok M, Sivaramasamy E, et al. Emerging MDR-Pseudomonas aeruginosa in fish commonly harbor oprL and toxA virulence genes and blaTEM, blaCTX-M, and tetA antibiotic-resistance genes. Sci Rep. 2020;10(1):1–12. doi:10.1038/s41598-020-72264-4
39. Kumar A, Chua K-L, Schweizer HP. Method for regulated expression of single-copy efflux pump genes in a surrogate Pseudomonas aeruginosa strain: identification of the BpeEF-OprC chloramphenicol and trimethoprim efflux pump of Burkholderia pseudomallei 1026b. Antimicrob Agents Chemother. 2006;50(10):3460–3463. doi:10.1128/AAC.00440-06
40. Kousar S, Rehman N, Javed A, et al. Intensive poultry farming practices influence antibiotic resistance profiles in Pseudomonas aeruginosa inhabiting nearby soils. Infect Drug Resist. 2021;14:4511. doi:10.2147/IDR.S324055
41. Mohamed HM, Alnasser SM, Abd-Elhafeez HH, Alotaibi M, Batiha GE-S, Younis W. Detection of β-lactamase resistance and biofilm genes in pseudomonas species isolated from chickens. Microorganisms. 2022;10(10):1975. doi:10.3390/microorganisms10101975
42. Sans-Serramitjana E, Fusté E, Martínez-Garriga B, et al. Killing effect of nanoencapsulated colistin sulfate on Pseudomonas aeruginosa from cystic fibrosis patients. J Cystic Fibrosis. 2016;15(5):611–618. doi:10.1016/j.jcf.2015.12.005
43. Rafique A, Andleeb S, Ghous T, Shahzad N, Shafique I. Antibacterial activity of traditional herbs and standard antibiotics against poultry associated Pseudomonas aeruginosa. J Pharm Sci Innov. 2012;1(5):12–16.
44. Makharita RR, El-Kholy I, Hetta HF, et al. Antibiogram and genetic characterization of carbapenem-resistant gram-negative pathogens incriminated in healthcare-associated infections. Infect Drug Resist. 2020;13:3991. doi:10.2147/IDR.S276975
45. Langaee TY, Gagnon L, Huletsky A. Inactivation of the ampD gene in Pseudomonas aeruginosa leads to moderate-basal-level and hyperinducible AmpC β-lactamase expression. Antimicrob Agents Chemother. 2000;44(3):583–589. doi:10.1128/AAC.44.3.583-589.2000
46. Al-Dahmoshi HO, Al-Khafaji NS, Jeyad AA, Shareef HK, Al-Jebori RF. Molecular detection of some virulence traits among Pseudomonas aeruginosa isolates, Hilla-Iraq. Biomed Pharmacol J. 2018;11(2):835–842. doi:10.13005/bpj/1439
47. Bakheet AA, Torra DE. Detection of Pseudomonas aeruginosa in dead chicken embryo with reference to pathological changes and virulence genes. Alexandria J Vet Sci. 2020;65(1):81. doi:10.5455/ajvs.101343
48. Qian Z, Hui P, Han L, et al. Serotypes and virulence genes of Pseudomonas aeruginosa isolated from mink and its pathogenicity in mink. Microb Pathog. 2020;139:103904. doi:10.1016/j.micpath.2019.103904
49. Douraghi M, Ghasemi F, Dallal MS, Rahbar M, Rahimiforoushani A. Molecular identification of Pseudomonas aeruginosa recovered from cystic fibrosis patients. J Prev Med Hyg. 2014;55(2):50.
50. Vanderwoude J, Fleming D, Azimi S, Trivedi U, Rumbaugh KP, Diggle SP. The evolution of virulence in Pseudomonas aeruginosa during chronic wound infection. Proc Royal Soc B. 2020;287(1937):20202272. doi:10.1098/rspb.2020.2272
51. Iiyama K, Takahashi E, Lee JM, et al. Alkaline protease contributes to pyocyanin production in Pseudomonas aeruginosa. FEMS Microbiol Lett. 2017;364(7). doi:10.1093/femsle/fnx051
52. Butterworth MB, Zhang L, Heidrich EM, Myerburg MM, Thibodeau PH. Activation of the epithelial sodium channel (ENaC) by the alkaline protease from Pseudomonas aeruginosa. J Biol Chem. 2012;287:32556–32565. doi:10.1074/jbc.M112.369520
53. Hartmann A, Schikora A. Quorum sensing of bacteria and trans-kingdom interactions of N-acyl homoserine lactones with eukaryotes. J Chem Ecol. 2012;38(6):704–713. doi:10.1007/s10886-012-0141-7
54. O’Loughlin CT, Miller LC, Siryaporn A, Drescher K, Semmelhack MF, Bassler BL. A quorum-sensing inhibitor blocks Pseudomonas aeruginosa virulence and biofilm formation. Proc Natl Acad Sci. 2013;110(44):17981–17986. doi:10.1073/pnas.1316981110
55. Khorvash F, Yazdani M, Shabani S, Soudi A. Pseudomonas aeruginosa-producing metallo-β-lactamases (VIM, IMP, SME, and AIM) in the clinical isolates of intensive care units, a university hospital in Isfahan, Iran. Adv Biomed Res. 2017;6:25.
56. Sanz-García F, Hernando-Amado S, Martínez JL. Mutation-driven evolution of Pseudomonas aeruginosa in the presence of either ceftazidime or ceftazidime-avibactam. Antimicrob Agents Chemother. 2018;62(10):e01379–01318. doi:10.1128/AAC.01379-18
57. Krishnan G, Sethumadhavan A, Muthusamy S, Mani M. Antibiotic resistant clinical isolates of Pseudomonas aeruginosa harbor LasA gene. Internet J Microbiol. 2019;16:125.
58. Cho J-K, Kim J-H, Kim J-M, Park C-K, Kim K-S. Antimicrobial resistance and distribution of resistance gene in Enterobacteriaceae and Pseudomonas aeruginosa isolated from dogs and cats. Korean J Vet Service. 2013;36(3):171–180. doi:10.7853/kjvs.2013.36.3.171
59. Botelho J, Grosso F, Peixe L. Antibiotic resistance in Pseudomonas aeruginosa–mechanisms, epidemiology and evolution. Drug Resist Updates. 2019;44:100640. doi:10.1016/j.drup.2019.07.002
60. Pang Z, Raudonis R, Glick BR, Lin T-J, Cheng Z. Antibiotic resistance in Pseudomonas aeruginosa: mechanisms and alternative therapeutic strategies. Biotechnol Adv. 2019;37(1):177–192. doi:10.1016/j.biotechadv.2018.11.013
61. Gajdács M, Baráth Z, Kárpáti K, et al. No correlation between biofilm formation, virulence factors, and antibiotic resistance in Pseudomonas aeruginosa: results from a laboratory-based in vitro study. Antibiotics. 2021;10:1134. doi:10.3390/antibiotics10091134
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

