Back to Journals » Clinical Ophthalmology » Volume 17
Ophthalmological Manifestations of Axenfeld-Rieger Syndrome: Current Perspectives
Authors Michels K, Bohnsack BL
Received 13 January 2023
Accepted for publication 23 February 2023
Published 10 March 2023 Volume 2023:17 Pages 819—828
DOI https://doi.org/10.2147/OPTH.S379853
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Scott Fraser
Kristi Michels,1 Brenda L Bohnsack2
1Department of Ophthalmology, Northwestern University Feinberg School of Medicine, Chicago, IL, USA; 2Division of Ophthalmology, Ann & Robert H. Lurie Children’s Hospital of Chicago, Chicago, IL, USA
Correspondence: Brenda L Bohnsack, Email [email protected]
Abstract: Axenfeld-Rieger syndrome (ARS) is a rare congenital disease that is primarily characterized by ocular anterior segment anomalies but is also associated with craniofacial, dental, cardiac, and neurologic abnormalities. Over half of cases are linked with autosomal dominant mutations in either FOXC1 or PITX2, which reflects the molecular role of these genes in regulating neural crest cell contributions to the eye, face, and heart. Within the eye, ARS is classically defined as the combination of posterior embryotoxon with iris bridging strands (Axenfeld anomaly) and iris hypoplasia causing corectopia and pseudopolycoria (Rieger anomaly). Glaucoma due to iridogoniodysgenesis is the main source of morbidity and is typically diagnosed during infancy or childhood in over half of affected individuals. Angle bypass surgery, such as glaucoma drainage devices and trabeculectomies, is often needed to obtain intraocular pressure control. A multi-disciplinary approach including glaucoma specialists and pediatric ophthalmologists produces optimal outcomes as vision is dependent on many factors including glaucoma, refractive error, amblyopia and strabismus. Further, since ophthalmologists often make the diagnosis, it is important to refer patients with ARS to other specialists including dentistry, cardiology, and neurology.
Keywords: Axenfeld-Rieger syndrome, Axenfeld anomaly, Rieger anomaly, glaucoma, posterior embryotoxon, iris hypoplasia
Introduction
Axenfeld Rieger syndrome (ARS) is a clinically and genetically heterogenous group of conditions characterized by anterior segment dysgenesis of the eye and varying degrees of systemic congenital abnormalities.1–4 The disease was first recognized by Axenfeld in 1920 who reported a patient with anterior displacement of Schwalbe’s line (posterior embryotoxon) in combination with corectopia.5 In 1934, Rieger described two patients with “mesodermal dysgenesis” consisting of iris hypoplasia, pseudopolycoria, and posterior embryotoxon.6 Due to phenotypic similarities, the cases described by Axenfeld and Rieger were considered as part of the same group of disorders known today as ARS.
ARS occurs in 1 of 100,000–200,000 live births.4 Further, there is no gender or racial predilection and has been reported in ethnic groups in Europe, Africa, North and South America, Middle East, and Asia.4 Mutations in the PITX2 and FOXC1 genes, which are crucial for normal embryologic development of the anterior segment and other organs affected in ARS, account for 40–60% of cases.7–9 Vision loss is most commonly due to glaucoma, which affects more than 50% of individuals with ARS.10 Thus, although rare, it is important for eye specialists to recognize ARS in order to monitor for glaucoma and potentially arrange care with other specialists.
ARS Genetics and Pathogenesis
ARS is inherited in an autosomal dominant pattern with eye and systemic findings showing complete and incomplete penetrance, respectively.3,7,8,11 Family-based studies and linkage analysis identified that mutations in two genes, Paired-like Homeodomain 2 (PITX2) and Forehead Box C1 (FOXC1), are causative in approximately half of ARS cases.9,12–16 The PITX2 gene is located on 4q25, while the FOXC1 gene is localized to 6p25, and both genes encode for transcription factors predominantly expressed during embryogenesis.12,17 Disease causing mutations in PITX2 and FOXC1 are classified as type I and type III ARS. Type II ARS has been localized to 13q14, and a separate isolated case was associated with 16q24 deletion; however, the specific genes involved have yet to be identified.3,7,18,19 Further, anterior segment dysgenesis, and in some cases specifically referenced as ARS, has also been associated with mutations in CYP1B1, COL4A1, PAX6, FOXE3, CPAMD8, and PXDN.20–23 In addition, genetic testing utilizing targeted gene panels for anterior segment dysgenesis fails to identify gene mutations in many ARS patients indicating that much knowledge is still to be gained regarding the genetics of this disease.
As PITX2 and FOXC1 mutations are most common, the phenotypes associated with these genes are best described.3,15,24–31 Reports in the literature have suggested that FOXC1 mutations are more likely to cause isolated ocular findings such that craniofacial and dental anomalies are rare.3,32 This is in contrast to PITX2 mutations, which in addition to anterior segment dysgenesis have been reported to be commonly associated with craniofacial abnormalities.3,32–34 Further, a more recent study showed that FOXC1 mutations had more corneal involvement and higher incidence of glaucoma in contrast to PITX2 mutations which showed more iris abnormalities.35 However, personal experience with numerous multi-generation families with ARS and either FOXC1 or PITX2 mutations challenges this division of gene function. Further, there is phenotypic variation in eye and systemic findings amongst affected family members that carry the same genetic mutation.25,33,36
Nevertheless, the association between ARS and mutations in PITX2 and FOXC1 yields important insight into disease pathogenesis. Mouse, chick and zebrafish animal models have demonstrated that Pitx2 and Foxc1 are expressed in neural crest cells during embryogenesis.32,37–45 The neural crest is a transient stem cell population that originates at the edge of the neural tube, migrates throughout the embryo, and gives rise to a diverse set of tissues.46–48 Pitx2 and Foxc1 are expressed in the cranial and cardiac neural crest subpopulations. The cranial neural crest arises from the edge of the mesencephalon and rhombencephalon and migrates into the craniofacial region to populate the 1st and 2nd pharyngeal arches, frontonasal process, and periocular mesenchyme.49–51 In the mid- and lower face region, the cranial neural crest cells from the 1st and 2nd pharyngeal arches give rise to the odontoblasts and cementoblasts required for tooth formation, connective tissue, and the maxillary and mandibular bones.51–57 The cranial neural crest cells within the periocular mesenchyme migrate into the anterior segment of the eye via the ocular fissure and between the surface ectoderm-derived corneal epithelium and neural epithelial-derived optic cup to contribute to the corneal stroma and endothelium, iris stroma and muscles, ciliary body stroma, trabecular meshwork and aqueous outflow tracts, and sclera.49,51,55 The cardiac neural crest cells originate at the level of the third somite and migrate through the 3rd, 4th, and 5th pharyngeal arches on their way to the cardiac outflow region where they regulate tract septation and aortic arch formation.58,59
Genetic manipulation of Pitx2 and Foxc1 expression in animal models shows that absence of these genes halts cranial neural crest cell migration from the edge of the neural tube, which induces cell apoptosis.42,60 Thus, few neural crest cells reach the pharyngeal arches and periocular mesenchyme resulting in absence of jaw and mid-face bone formation and disruption of cornea, iris, and iridocorneal angle development. Further, Pitx2 and Foxc1 expressed in the periocular mesenchyme regulate closure of the optic fissure on the inferonasal edge of the optic cup such that loss of these genes in neural crest cells also causes microphthalmia and colobomas.41,42,60 Complete knockout or knockdown of Pitx2 or Foxc1 in mice and zebrafish results in embryonic lethality due to cardiac malformations; however, heterozygous Pitx2 mice show anterior segment anomalies similar to human ARS.43
As ARS is inherited in an autosomal dominant fashion, gene dosage likely plays a role in disease pathogenesis.61 In the heterozygous state, early craniofacial and cardiac neural crest development may proceed thereby preventing embryonic lethality; however, the phenotypes may reflect later roles of these genes. Pitx2 and Foxc1 continue to be expressed in neural crest-derived cells in the developing anterior segment. These neural crest cells form a continuous layer that separates the trabecular meshwork from the anterior chamber, preventing aqueous humor drainage.1,20 This cell layer eventually retracts to expose the trabecular meshwork and allow for aqueous outflow. Inhibition of this retraction along with contraction of the layer is hypothesized to result in iridogoniodysgenesis. While there is interaction between the Pitx2 and Foxc1 proteins within neural crest cells, specific downstream targets are not well defined.61 It is likely that additional genes associated with ARS that have yet to be identified interact within the PITX2 and FOXC1 pathways in neural crest cells and will yield further insight into disease pathogenesis.
Ocular Manifestations
ARS presents with characteristic anterior segment findings that are typically bilateral but can be asymmetric or, rarely, unilateral (Supplemental Table 1).4,15,16,25–27,31,35,62 By convention, ARS is the combination of posterior embryotoxon with iris bridging strands (Axenfeld anomaly) and iris hypoplasia (Rieger anomaly) (Figure 1A–D).2–4,8,24 Posterior embryotoxon is classically described as premature termination of Descemet’s membrane (Schwalbe’s line); however, a more recent histological study has shown that the line itself is a peripheral corneal stroma nub that is present due to an attenuated Descemet’s membrane.63 Clinically, posterior embryotoxon varies in presentation from a discontinuous subtle line in the peripheral cornea that runs parallel to the limbus to a prominent continuous white line (Figure 1E, arrowheads). Findings are more evident on gonioscopy, especially if iris strands that traverse the angle structures are present.3,64 These bridging strands may be thin or thick, but unlike peripheral anterior synechiae do not typically cause angle closure or restrict aqueous outflow.3 Although posterior embryotoxon is found in the majority of patients with ARS, it is not necessary for the diagnosis. Additionally, 15% of the normal population may have some degree of posterior embryotoxon, but it is less pronounced and not associated with iris bridging strands.64 Rieger anomaly refers to iris hypoplasia and can be associated with corectopia and pseudopolycoria (Figure 1A–D).2–4,8,24 Absence or malformation of the iris stroma and muscles (sphincter and dilator) in conjunction with iris bridging strands leads to pupil distortion and iris tears. The corectopia and pseudopolycoria are rarely visually significant but may cause cosmetic concerns. However, not all patients with ARS have corectopia or pseudopolycoria, and the hypoplasia may manifest as a gray, featureless (lack of crypts, furrows, and rings) iris (Figure 1F).35
In addition to these classic findings, other ocular structures can show abnormalities. Congenital and early-onset cataracts are common; however, lensectomy can be challenging due to corneal abnormalities, poor pupil dilation and iris floppiness.65,66 In rare cases, congenital cataracts in ARS can be associated with persistent fetal vasculature and microphthalmia, which further complicates surgical removal.17,24 Corneal involvement can be more extensive as eyes can also show Peters anomaly and sclerocornea.16,24,31,35,62,67–69 These congenital corneal abnormalities especially when coupled with glaucoma (discussed in next section) can predispose for corneal edema and scarring due to decompensation.35,62 Although traditionally considered an anterior segment dysgenesis, optic nerve and retinal abnormalities have also been reported in ARS.70 Optic nerve colobomas, hypoplasia, and dysplasia (Figure 2A), foveal hypoplasia (Figure 2B) and atrophy, and chorioretinal colobomas have all been reported in patients with ARS.16,25,71,72 The importance of recognizing these other abnormalities as part of the disease spectrum is emphasized by phenotypic variations causing delayed diagnosis in individuals belonging to families with genetically confirmed ARS.
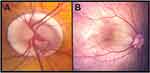 |
Figure 2 Posterior segment findings in ARS. Two eyes of patients with ARS show optic nerve dysplasia with significant peripapillary chorioretinal atrophy (A) and foveal hypoplasia (B). |
Glaucoma in ARS
Glaucoma secondary to the anterior segment dysgenesis is the main source of morbidity in ARS and affects more than 50% of patients.10,35 Although it is tempting to attribute elevated intraocular pressure (IOP) to the iris strands that bridge the iridocorneal angle, the pathogenesis of glaucoma is more likely due to congenital malformation of the iridocorneal angle structures as severing of the iris bridging strands is ineffective in obtaining pressure control.10
Elevated IOP may be present at birth or not manifest until adulthood; however, most individuals with glaucoma secondary to ARS will be diagnosed during childhood. Further, glaucoma is typically bilateral, but may asymmetrically affect one eye greater than the other.10 Thus, individuals with ARS need to be routinely and carefully monitored for signs and symptoms of glaucoma. In addition to increased IOP and optic nerve cupping, children under 5–7 years of age may also show the classic signs and symptoms usually associated with primary congenital glaucoma (PCG). These include buphthalmos with increased corneal diameter and axial length, Haab's striae (breaks in Descemet’s membrane), and corneal edema, which results in photophobia, blepharospasm, and epiphora.73,74 Especially in toddlers where IOP readings obtained in clinic may not be accurate due to poor cooperation, attention should be paid to changes in visual function, ocular preference, strabismus measurements, corneal clarity, cycloplegic refraction, and optic nerve appearance.
Glaucoma management typically starts with standard topical ocular anti-hypertensive medications.10 The exception can be neonates presenting at birth with buphthalmos and corneal edema who, unless there is a known family history of ARS, are often initially misdiagnosed with PCG. Careful slit-lamp examination and gonioscopy, if not obscured by corneal edema, can help distinguish between classic ARS and PCG. It is important to note that iridogoniodysgenesis, which should be classified as a variant of ARS due to its genetic association with PITX2 and FOXC1 mutations, shares more clinical similarity with PCG as posterior embryotoxon, iris bridging strands, corectopia and pseudopolycoria are often absent.75–78 Examination for craniofacial abnormalities (discussed below) as well as careful assessment of past medical history, review of systems, and family history often helps to differentiate between these two entities. This is relevant as unlike PCG, angle surgery (goniotomy and trabeculotomy) does not usually yield long-term IOP control in iridogoniodysgenesis or ARS.10
Glaucoma in ARS is often refractory to medications such that two-thirds of affected patients require at least one IOP-lowering surgery. As mentioned above, goniotomy and trabeculotomy are less effective in obtaining and maintaining IOP control.10 This suggests that the restriction of aqueous humor outflow does not solely reside within the trabecular meshwork, but also involves downstream and alternative tracts. However, this has not been confirmed histologically or via aqueous humor outflow imaging. As a result, the majority of patients with glaucoma secondary to ARS require angle-bypass surgery, typically trabeculectomy with anti-fibrotics or placement of a glaucoma drainage device (GDD) to achieve long-term IOP control.10 Trabeculectomy surgery in children and young adults requires adjunctive anti-fibrotics, usually mitomycin C, to prevent bleb scarring. While trabeculectomies avoid the use of hardware, this surgery presents post-operative challenges such as over- or under-filtration and bleb leaks that can be difficult to manage in infants and children. In addition, trabeculectomies carry a life-long risk of bleb-related infections that if not diagnosed and managed appropriately can result in vision loss due to endophthalmitis.79–81 Careful patient selection based on the ability to monitor and manipulate the bleb in clinic as well as patient and parent understanding of the risks associated with trabeculectomies is critical. Thus, despite the fact that trabeculectomies are generally able to achieve lower eye pressures, GDDs have become the mainstay of angle-bypass surgery in children.82–90
GDDs are divided into two main categories, valved and non-valved. Valved GDDs, such as the Ahmed FP7 and FP8, afford immediate IOP lowering effect and less risk of hypotony. While the post-operative management is more straightforward, early outflow of aqueous humor, which is hypothesized to carry pro-inflammatory cytokines, can lead to bleb encapsulation and the hypertensive phase by 4 to 8 weeks after surgery.91–94 This hypertensive phase is a poor prognostic indicator for overall success and survival of the Ahmed GDD. Non-valved GDDs, such as Baerveldt and Molteno implants, require either a dissolvable ligature suture or implantation in two steps as the resistance to aqueous outflow is dependent on the formation of a capsule around the GDD plate.88 The pressure lowering effect should be delayed for at least 3 weeks, but hypotony can still be a challenge.88,95 However, the absence of early aqueous humor outflow in non-valved implants is thought to limit plate encapsulation and ultimately increase success and survival time compared to valved GDDs.
Implantation of GDDs in ARS presents unique challenges. The iris bridging strands, especially when thick, can bleed and prevent ideal placement of the tube. In addition, the intraocular portion of the tube should be placed as far from the cornea as possible to decrease the risk of exacerbating the endothelial layer. However, this needs to be balanced with iris floppiness that can lead to tube obstruction. In cases of severe anterior segment dysgenesis with shallow anterior chambers, the lens may need to be removed such that the tube can be placed in the pars plana.96–98
Ciliary body ablation, either transscleral or endoscopic, can help control IOP, but is most effective after aqueous outflow has been established with a GDD.10,88,99 In severe anterior segment dysgenesis, transscleral cycloablation can seem like an attractive option given the challenges and potential complications of intraocular surgery in these complex eyes. However, the window between glaucoma and hypotony is slim if there is no adequate outflow. Multiple sessions of ciliary body ablation often yield minimal effect on IOP, and ultimately angle-bypass surgery may be required.88 However, the outflow may then be greater than aqueous production resulting in hypotony and phthisis.
When managing glaucoma secondary to ARS, it is important to develop a well thought out strategy since most eyes will require more than one IOP-lowering surgery.10 GDDs tend to be favored in children but may need to be combined with cycloablation for optimal pressure control.82–90 Trabeculectomy with anti-fibrotics is also highly effective but is typically reserved for adolescents and adults. Further, in children, there is a great need for maximizing surgical options for the future given the life-long need for treatment.
Visual Outcomes in ARS
Few studies have assessed visual outcomes in ARS due to the rarity of the disease. In one of the largest case series of ARS patients, the average best corrected visual acuity was approximately 20/60 in thirty-two affected individuals, but vision ranged from 20/20 to light perception.10 Visual outcomes in ARS are dependent on numerous factors. While glaucomatous optic neuropathy first affects the visual field and at late stages central visual acuity, other consequences of elevated IOP in children with ARS can impact visual outcomes.73,74 Haab's striae directly impair vision if present in the central visual axis but also indirectly affect vision by inducing high amounts of irregular astigmatism. Further, increased axial length associated with buphthalmos can lead to significant myopia.100,101 However, in the previously referenced study, there was no significant difference in average best corrected visual acuity between eyes with ARS that did or did not have glaucoma.10 Although more rare, involvement of other ocular structures including cataracts, Peters Anomaly, sclerocornea, optic nerve and retinal colobomas worsens visual prognosis.10,99 Since ARS is a congenital disease and glaucoma is typically diagnosed during childhood, it is also critical to address refractive error, amblyopia, and strabismus.101,102 Both high myopia and astigmatism can cause bilateral amblyopia if the refractive error is left uncorrected. Furthermore, asymmetry of refraction (anisometropia) or ocular findings affecting the cornea, lens, retinal or optic nerve can lead to unilateral amblyopia. This can be further exacerbated by strabismus, which due to asymmetry of vision and lack of fusion, is common.100 As a result, correction of refractive error with glasses or contact lenses, part-time occlusion and strabismus surgery may be needed to optimize visual outcomes. Thus, coordinated management between glaucoma specialists and pediatric ophthalmology is critical in patients with ARS.
Systemic Manifestations in ARS
While the ocular findings in ARS draw the most attention, there are systemic manifestations that are important to recognize as they may help solidify the clinical diagnosis and potentially require other subspecialty care (Supplemental Table 1).15,17–19,21,24,25,27,31,35,62 Due to the common origin of craniofacial and ocular neural crest cells, there is a characteristic craniofacial appearance associated with ARS which consists of maxillary hypoplasia with mid-face flattening, mandibular hypoplasia, hypertelorism, micrognathia, cleft palate, and telecanthus.3,4,17–19,25,30,31,33,34,62,103 Dental anomalies due to decreased odontoblast and cementoblasts are very common in ARS and are classically microdontia (small teeth) and oligodontia (too few teeth).2,15,19,21,24,25,27,31,35,62,68,104 Further, tooth enamel is often abnormal leading to a high rate of dental caries.62,105 As a result of these teeth abnormalities, children with ARS need evaluation and close monitoring by pediatric dentistry.106 Although less common than the craniofacial and dental abnormalities, congenital heart defects such as aortic and mitral valve stenosis and hypoplasia of the cardiovascular outflow tracts have been found in almost one-quarter of ARS patients.15,19,35,62,67,68,107–109 Additional systemic findings, including redundant periumbilical skin, hypospadias, anal stenosis, hearing loss, skeletal anomalies, and growth retardation have all been reported with ARS.10,17–19,25,27,31,33–35,62,68,110–113 Further, neurologic involvement including white matter hyperintensities, hydrocephalus, Dandy Walker malformations, and arachnoid cysts with developmental delays and learning disabilities have all been described in individuals with ARS.17,19,25,31,35,62,106,114 More recently, FOXC1 and PITX2 mutations have been associated with small cerebral vessel abnormalities that increase stroke risk such that all individuals diagnosed with ARS should undergo brain imaging.42 With the rarity of ARS, there is often under-recognition of the constellation of systemic and ocular manifestations. Greater knowledge of this disease amongst specialists including ophthalmologists, dentists, and cardiologists is needed.
Conclusions
As ARS is often diagnosed by ophthalmologists, it is important to recognize both the eye and systemic manifestations and coordinate appropriate care with other specialties.62 Glaucoma is the main source of morbidity and often requires angle-bypass surgery to obtain IOP control.10 However, in children affected with ARS, it is critical to also simultaneously address refractive error, amblyopia, and strabismus to optimize visual outcomes.101,102 Since ARS is inherited in an autosomal dominant pattern, biological parents of affected children should be examined as there can be phenotypic variation and eye findings may be subtle. Genetic testing can help confirm the diagnosis, but lack of identification of a gene mutation does not rule out this clinical diagnosis. The discovery of additional genes associated with ARS will improve our understanding of molecular pathways involved in craniofacial and ocular neural crest cell development.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Shields MB. Axenfeld-Rieger syndrome: a theory of mechanism and distinctions from the iridocorneal endothelial syndrome. Trans Am Ophthalmol Soc. 1983;81:736–784.
2. Shields MB, Buckley E, Klintworth GK, Thresher R. Axenfeld-Rieger syndrome. A spectrum of developmental disorders. Surv Ophthalmol. 1985;29(6):387–409. doi:10.1016/0039-6257(85)90205-X
3. Chang TC, Summers CG, Schimmenti LA, Grajewski AL. Axenfeld-Rieger syndrome: new perspectives. Br J Ophthalmol. 2012;96(3):318–322. doi:10.1136/bjophthalmol-2011-300801
4. Seifi M, Walter MA. Axenfeld-Rieger syndrome. Clin Genet. 2018;93:1123–1130. doi:10.1111/cge.13148
5. Axenfeld TH. Embryotoxon corneaposterius. Klin Monbl Augenheilkd. 1920;65:381–382.
6. Rieger H. Dysgenesis mesodermalis corneae et iridis. Z Augenheilkd. 1935;86:333.
7. Lines MA, Kozlowski K, Walter MA. Molecular genetics of Axenfeld-Rieger malformations. Hum Mol Genet. 2002;11(10):1177–1184. doi:10.1093/hmg/11.10.1177
8. Hjalt TA, Semina EV. Current molecular understanding of Axenfeld-Rieger. Expert Rev Mol Med. 2005;7(25):1–17. doi:10.1017/S1462399405010082
9. Leis LM, Tyler RC, Volkmann Kloss BA, et al. PITX2 and FOXC1 spectrum of mutations in ocular syndromes. Eur J Hum Genet. 2012;20(12):1224–1233. doi:10.1038/ejhg.2012.80
10. Zepeda EM, Branham K, Moroi SE, Bohnsack BL. Surgical outcomes of glaucoma associated with Axenfeld-Rieger syndrome. BMC Ophthalmol. 2020;20:172. doi:10.1186/s12886-020-01417-w
11. Hjalt TA, Semina EV, Amendt BA, Murray JC. The Pitx2 protein in mouse development. Dev Dyn. 2000;218(1):195–200. doi:10.1002/(SICI)1097-0177(200005)218:1<195::AID-DVDY17>3.0.CO;2-C
12. Kulak SC, Kozlowski K, Semina EV, Pearce WG, Walter MA. Mutation in the RIEG1 gene in patients with iridogoniodysgenesis syndrome. Hum Mol Genet. 1998;7(7):1113–1117. doi:10.1093/hmg/7.7.1113
13. Smith RS, Zabealeta A, Kume T, et al. Haploinsufficiency of the transcription factors FOXC1 and FOXC2 results in aberrant ocular development. Hum Mol Genet. 2000;9(7):1021–1032. doi:10.1093/hmg/9.7.1021
14. Saadi I, Semina EV, Amendt BA, et al. Identification of a dominant negative homeodomain mutation in Rieger syndrome. J Biol Chem. 2001;276:23034–23041. doi:10.1074/jbc.M008592200
15. Strungaru MH, Dinu I, Walter MA. Genotype-phenotype correlations in Axenfeld-Rieger malformation and glaucoma patients with FOXC1 and PITX2 mutations. Invest Ophthalmol Vis Sci. 2007;48:228–237. doi:10.1167/iovs.06-0472
16. Kaur K, Ragge NK, Ragoussis J. Molecular analysis of FOXC1 in subjects presenting with severe developmental eye anomalies. Mol Vision. 2009;15:1366–1373.
17. Suzuki K, Nakamura M, Amano E, Mokuno K, Shirai S, Terasaki H. Case of chromosome 6p25 terminal deletion associated with Axenfeld-Rieger syndrome and persistent hyperplastic primary vitreous. Am J Med Genet A. 2006;140:503–508. doi:10.1002/ajmg.a.31085
18. Ferguson JG, Hicks EL. Rieger’s anomaly and glaucoma associated with partial trisomy 16q. Case report. Arch Ophthalmol. 1987;105(3):323. doi:10.1001/archopht.1987.01060030037015
19. Phillips JC, Del Bono EA, Haines JL, et al. A second locus for Rieger syndrome maps to chromosome 13q14. Am J Hum Genet. 1996;59(3):613–619.
20. Idrees F, Vaideanu D, Fraser SG, Sowden JC, Khaw PT. A review of anterior segment dysgeneses. Surv Ophthalmol. 2006;51(3):213–231. doi:10.1016/j.survophthal.2006.02.006
21. Tanwar M, Dada T, Dada R. Axenfeld-Rieger Syndrome Associated with Congenital Glaucoma and Cytochrome P4501B1 Gene Mutations. Case Rep Med. 2010;2010:1–6. doi:10.1155/2010/212656
22. Reis LM, Semina EV. Conserved genetic pathways associated with microphthalmia, anophthalmia, and coloboma. Birth Defects Res C Embryo Today. 2015;105:96–113. doi:10.1002/bdrc.21097
23. Ma A, Yousoof S, Grigg JR, et al. Revealing hidden genetic diagnoses in the ocular anterior segment disorders. Genet Med. 2020;22(10):1623–1632. doi:10.1038/s41436-020-0854-x
24. Ozeki H, Shirai S, Ikeda K, Ogura Y. Anomalies associated with Axenfeld-Rieger syndrome. Graefes Arch Clin Exp Ophthalmol. 1999;237(9):730–734. doi:10.1007/s004170050304
25. Perveen R, Lloyd IC, Clayton-Smith J, et al. Phenotypic variability and asymmetry of Rieger syndrome associated with PITX2 mutations. Invest Ophthalmol Vis Sci. 2000;41(9):2456–2460.
26. Phillips JC. Four novel mutations in the PITX2 gene in patients with Axenfeld-Rieger syndrome. Ophthalmic Res. 2002;34(5):324–326. doi:10.1159/000065602
27. Lines MA, Kozlowski K, Kulak SC, et al. Characterization and prevalence of PITX2 microdeletions and mutations in Axenfeld-Rieger malformations. Invest Ophthalmol Vis Sci. 2004;45(3):828–833. doi:10.1167/iovs.03-0309
28. Fetterman CD, Mirazayan F, Walter MA. Characterization of a novel FOXC1 mutation, P297S, identified in two individuals with anterior segment dysgenesis. Clin Genet. 2009;76:296–299. doi:10.1111/j.1399-0004.2009.01210.x
29. Footz T, Idrees F, Acharya M, Kozlowski K, Walter MA. Analysis of mutations of the Pitx2 transcription factor found in patients with Axenfeld-Rieger Syndrome. Invest Ophthalmol Vis Sci. 2009;50:2599–2606. doi:10.1167/iovs.08-3251
30. Tumer Z, Bach-Holm D. Axenfeld-Rieger syndrome and spectrum of Pitx2 and Foxc1 mutations. Eur J Hum Genet. 2009;17:1527–1539. doi:10.1038/ejhg.2009.93
31. D’haene B, Meire F, Claerhout I, et al. Expanding the spectrum of FOXC1 and PITX2 mutations and copy number changes in patients with anterior segment malformations. Invest Ophthalmol Vis Sci. 2011;52(1):324–333. doi:10.1167/iovs.10-5309
32. French CR. Mechanistic insights into Axenfeld-Rieger syndrome from zebrafish foxc1 and pitx2 mutants. Int J Mol Sci. 2021;22(18):10001. doi:10.3390/ijms221810001
33. Idrees F, Bloch-Zupan A, Free SL, et al. A novel homeobox mutation in the PITX2 gene in a family of Axenfeld-Rieger syndrome associated with brain, ocular, and dental phenotypes. Am J Med Genet B Neuropsychiatr Genet. 2006;141B(2):184–191. doi:10.1002/ajmg.b.30237
34. Dressler S, Meyer-Marcotty P, Weisschuh N, et al. Dental and craniofacial anomalies associated with Axenfeld-Rieger syndrome with PITX2 mutation. Case Rep Med. 2010;2010:621984. doi:10.1155/2010/621984
35. Prem Senthil M, Knight LSW, Taranath D, et al. Comparison of Anterior Segment Abnormalities in Individuals With FOXC1 and PITX2 Variants. Cornea. 2022;41(8):1009–1015. doi:10.1097/ICO.0000000000003020
36. Hittner HM, Kretzer FL, Antoszyk JH, Ferrell RE, Mehta RS. Variable expressivity of autosomal dominant anterior segment mesenchymal dysgenesis in six generations. Am J Ophthalmol. 1982;93(1):57–70. doi:10.1016/0002-9394(82)90700-0
37. Gage PJ, Suh H, Camper SA. Dosage requirement of Pitx2 for development of multiple organs. Development. 1999;126(20):4643–4651. doi:10.1242/dev.126.20.4643
38. Kitamura K, Miura H, Miyagawa-Tomita S, et al. Mouse Pitx2 deficiency leads to anomalies of the ventral body wall, heart, extra- and periocular mesoderm and right pulmonary isomerism. Development. 1999;126:5749–5758. doi:10.1242/dev.126.24.5749
39. Kume T, Deng K, Hogan BL. Murine forkhead/winged helix genes Foxc1 (Mf1) and Foxc2 (Mfh) are required for the early organogenesis of the kidney and urinary tract. Development. 2000;127:1387–1395. doi:10.1242/dev.127.7.1387
40. Evans AL, Gage PJ. Expression of the homeobox gene Pitx2 in neural crest is required for optic stalk and ocular anterior segment development. Hum Mol Genet. 2005;14:3347–3359. doi:10.1093/hmg/ddi365
41. Bohnsack BL, Kasprick DS, Kish PE, Goldman D, Kahana A. A zebrafish model of axenfeld-Rieger syndrome reveals that pitx2 regulation by retinoic acid is essential for ocular and craniofacial development. Invest Ophthalmol Vis Sci. 2012;53(1):7–22. doi:10.1167/iovs.11-8494
42. French CR, Seshadri S, Destefano AL, et al. Mutation of FOXC1 and PITX2 induces cerebral small-vessel disease. J Clin Invest. 2014;124(11):4877–4881. doi:10.1172/JCI75109
43. Chen L, Gage PJ. Heterozygous Pitx2 null mice accurately recapitulate the ocular features of Axenfeld-Rieger Syndrome and congenital glaucoma. Invest Ophthalmol Vis Sci. 2016;57(11):5023–5030. doi:10.1167/iovs.16-19700
44. Seo S, Chen L, Liu W, et al. Foxc1 and Foxc2 in the neural crest are required for ocular anterior segment development. Invest Ophthalmol Vis Sci. 2017;58(3):1368–1377. doi:10.1167/iovs.16-21217
45. Hendee KE, Sorokina EA, Muheisen SS, et al. PITX2 deficiency and associated human disease: insights from the zebrafish model. Hum Mol Genet. 2018;27(10):1675–1695. doi:10.1093/hmg/ddy074
46. Barembaum M, Bronner-Fraser M. Early steps in neural crest specification. Semin Cell Dev Biol. 2005;16(6):642–646. doi:10.1016/j.semcdb.2005.06.006
47. Minoux M, Rijli FM. Molecular mechanisms of cranial neural crest cell migration and patterning in craniofacial development. Development. 2010;137(16):2605–2621. doi:10.1242/dev.040048
48. Bhattacharya D, Khan B, Simoes-Costa M. Neural crest metabolism: at the crossroads of development and disease. Dev Biol. 2021;475:245–255. doi:10.1016/j.ydbio.2021.01.018
49. Williams AL, Bohnsack BL. Neural crest derivatives in ocular development: discerning the eye of the storm. Birth Defects Res C Embryo Today. 2015;105(2):87–95. doi:10.1002/bdrc.21095
50. Weigele J, Bohnsack BL. Genetics underlying the interactions between neural crest cells and eye development. J Dev Biol. 2020;8(4):26. doi:10.3390/jdb8040026
51. Williams AL, Bohnsack BL. The ocular neural crest: specification, migration, and then what? Front Cell Dev Biol. 2020;8:595896. doi:10.3389/fcell.2020.595896
52. Le Lièvre CS, Le Douarin NM. Mesenchymal derivatives of the neural crest: analysis of chimaeric quail and chick embryos. J Embryol Exp Morphol. 1975;34(1):125–154.
53. Johnston MC, Noden DM, Hazelton RD, Coulombre JL, Coulombre AJ. Origins of avian ocular and periocular tissues. Exp Eye Res. 1979;29(1):27–43. doi:10.1016/0014-4835(79)90164-7
54. Jiang X, Iseki S, Maxson RE, Sucov HM, Morriss-Kay GM. Tissue origins and interactions in the mammalian skull vault. Dev Biol. 2002;241(1):106–116. doi:10.1006/dbio.2001.0487
55. Gage PJ, Rhoades W, Prucka SK, Hjalt T. Fate maps of neural crest and mesoderm in the mammalian eye. Invest Ophthalmol Vis Sci. 2005;46(11):4200–4208. doi:10.1167/iovs.05-0691
56. Piacentino ML, Li Y, Bronner ME. Epithelial-to-mesenchymal transition and different migration strategies as viewed from the neural crest. Curr Opin Cell Biol. 2020;66:43–50. doi:10.1016/j.ceb.2020.05.001
57. Milmoe NJ, Tucker AS. Craniofacial transitions: the role of EMT and MET during head development. Development. 2021;148(4). doi:10.1242/dev.196030
58. Stoller JZ, Epstein JA. Cardiac neural crest. Semin Cell Dev Biol. 2005;16(6):704–715. doi:10.1016/j.semcdb.2005.06.004
59. Yamagishi H. Cardiac Neural Crest. Cold Spring Harb Perspect Biol. 2021;13(1). doi:10.1101/cshperspect.a036715
60. Chawla B, Schley E, Williams AL, Bohnsack BL. Retinoic acid and Pitx2 regulate early neural crest survival and migration in craniofacial and ocular development. Birth Defects Res B Dev Reprod Toxicol. 2016;107(3):126–135. doi:10.1002/bdrb.21177
61. Berry FB, Lines MA, Oas JM, et al. Functional interactions between FOXC1 and PITX2 underlie the sensitivity to FOXC1 gene dose in Axenfeld-Rieger syndrome and anterior segment dysgenesis. Hum Mol Genet. 2006;15(6):905–919. doi:10.1093/hmg/ddl008
62. Reis LM, Maheshwari M, Capasso J, et al. Axenfeld-Rieger syndrome: more than meets the eye. J Med Genet;2022. jmedgenet-2022–108646. doi:10.1136/jmg-2022-108646
63. Alwadani S, Alward WLM, Syed NA, Bouhenni RA, Brownstein S, Edward DP. Posterior Embryotoxon Revisited: an Immunohistologic Study. Ophthalmol Glaucoma. 2022;5(4):396–401. doi:10.1016/j.ogla.2022.01.003
64. Alward WL. Axenfeld-Rieger syndrome in the age of molecular genetics. Am J Ophthalmol. 2000;130:107–115. doi:10.1016/S0002-9394(00)00525-0
65. Guerriero S, L’Abbate M, La Tegola MG, Alessio G, Sborgia G. Combined aniridia ring implantation and cataract surgery in an Axenfeld-Rieger syndrome: a UBM report. Eye Contact Lens. 2011;37(1):45–47. doi:10.1097/ICL.0b013e3182050472
66. Ma Y, Wu X, Ni S, Chen X, He S, Xu W. The diagnosis and phacoemulsification in combination with intraocular lens implantation for an Axenfeld-Rieger syndrome patient with small cornea: a case report. BMC Ophthalmol. 2020;20(1):148. doi:10.1186/s12886-020-01406-z
67. Honkanen RA, Nishimura DY, Swiderski RE, et al. A family with Axenfeld-Rieger syndrome and Peters Anomaly caused by a point mutation (Phe112Ser) in the FOXC1 gene. Am J Ophthalmol. 2003;135(3):368–375. doi:10.1016/S0002-9394(02)02061-5
68. Qin Y, Gao P, Yu S, et al. A large deletion spanning PITX2 and PANCR in a Chinese family with Axenfeld-Rieger syndrome. Mol Vis. 2020;26:670–678.
69. Meng Y, Lu G, Xie Y, Sun X, Huang L. Case report of the rare Peters’ anomaly complicated with Axenfeld-Rieger syndrome: a case report and brief review of the literature. Medicine. 2022;101(2):e21213. doi:10.1097/MD.0000000000021213
70. Jacobson A, Bohnsack BL. Posterior segment findings in Axenfeld-Rieger syndrome. J Aapos. 2022;26(6):320–322. doi:10.1016/j.jaapos.2022.08.263
71. Golaszewska K, Dub N, Saeed E, Mariak Z, Konopińska J. Axenfeld-Rieger syndrome combined with a foveal anomaly in a three generation family: a case report. BMC Ophthalmol. 2021;21(1):154. doi:10.1186/s12886-021-01899-2
72. Ramesh PV, Devadas AK, Varsha V, et al. A rare case of unilateral Axenfeld-Rieger anomaly associated with optic disc coloboma: a multimodal imaging canvas. Indian J Ophthalmol. 2022;70(7):2645–2647. doi:10.4103/ijo.IJO_2950_21
73. Papadopoulos M, Cable N, Rahi J, Khaw PT, Investigators BES. The British Infantile and Childhood Glaucoma (BIG) eye study. Invest Ophthalmol Vis Sci. 2007;48(9):100–106. doi:10.1167/iovs.06-1350
74. Karaconji T, Zagora S, Grigg JR. Approach to childhood glaucoma: a review. Clin Exp Ophthalmol. 2022;50(2):232–246. doi:10.1111/ceo.14039
75. Mears AJ, Mirzayans F, Gould DB, Pearce WG, Walter MA. Autosomal dominant iridogoniodysgenesis anomaly maps to 6p25. Am J Hum Genet. 1996;59(6):1321–1327.
76. Walter MA, Mirzayans F, Mears AJ, Hickey K, Pearce WG. Autosomal-dominant iridogoniodysgenesis and Axenfeld-Rieger syndrome are genetically distinct. Ophthalmology. 1996;103(11):1907–1915. doi:10.1016/S0161-6420(96)30408-9
77. Jordan T, Ebenezer N, Manners R, McGill J, Bhattacharya S. Familial glaucoma iridogoniodysplasia maps to a 6p25 region implicated in primary congenital glaucoma and iridogoniodysgenesis anomaly. Am J Hum Genet. 1997;61(4):882–888. doi:10.1086/514874
78. Kozlowski K, Walter MA. Variation in residual PITX2 activity underlies the phenotypic spectrum of anterior segment developmental disorders. Hum Mol Genet. 2000;9(14):2131–2139. doi:10.1093/hmg/9.14.2131
79. DeBry PW, Perkins TW, Heatley G, Kaufman P, Brumback LC. Incidence of late-onset bleb-related complications following trabeculectomy. Arch Ophthalmol. 2002;120(3):297–300. doi:10.1001/archopht.120.3.297
80. Kim EA, Law SK, Coleman AL, et al. Long-term bleb-related infections after trabeculectomy: incidence, risk factors, and influence of bleb revision. Am J Ophthalmol. 2015;159(6):1082–1091. doi:10.1016/j.ajo.2015.03.001
81. Luebke J, Neuburger M, Jordan JF, et al. Bleb-related infections and long-term follow-up after trabeculectomy. Int Ophthalmol. 2019;39:571–577. doi:10.1007/s10792-018-0851-0
82. Donahue SP, Keech RV, Munden P, Scott WE. Baerveldt implant surgery in the treatment of advanced childhood glaucoma. J AAPOS. 1997;1:41–45. doi:10.1016/S1091-8531(97)90022-7
83. Djodeyre MR, Peralta Calvo J, Abelairas Gomez J. Clinical evaluation and risk factors of time to failure of Ahmed Glaucoma Valve implant in pediatric patients. Ophthalmology. 2001;108:614–620. doi:10.1016/S0161-6420(00)00603-5
84. Budenz DL, Gedde SJ, Brandt JD, Kira D, Feuer W, Larson E. Baerveldt glaucoma implant in the management of refractory childhood glaucomas. Ophthalmology. 2004;111:2204–2210. doi:10.1016/j.ophtha.2004.05.017
85. Tai AX, Song JC. Surgical outcomes of Baerveldt implants in pediatric glaucoma patients. J AAPOS. 2014;18:550–553. doi:10.1016/j.jaapos.2014.08.003
86. Al-Haddad C, Al-Salem K, Ismail K, Noureddin B. Long-term outcomes of Ahmed tube implantation in pediatric glaucoma after multiple surgeries. Int Ophthalmol. 2018;38:2649–2658. doi:10.1007/s10792-017-0743-8
87. Senthil S, Turaga K, Mohammed HA, et al. Outcomes of silicone Ahmed glaucoma valve implantation in refractory pediatric glaucoma. J Glaucoma. 2018;27(9):769–775. doi:10.1097/IJG.0000000000001032
88. Jacobson A, Besirli CG, Bohnsack BL. Outcomes of Baerveldt glaucoma drainage devices in pediatric eyes. J Glaucoma. 2021;31(6):468–477. doi:10.1097/IJG.0000000000001970
89. Medert CM, Cavuoto KM, Vanner EA, Grajewski AL, Chang TC. Risk factors for glaucoma drainage device failure and complication in the pediatric population. Ophthalmol Glaucoma. 2021;4:63–70. doi:10.1016/j.ogla.2020.07.006
90. Jacobson A, Bohnsack BL. Ologen augmentation of Ahmed valves in pediatric glaucomas. J AAPOS. 2022;263:e1–122.e126.
91. Ayyala RS, Zurakowski D, Smith JA, et al. A clinical study of the Ahmed glaucoma valve implant in advanced glaucoma. Ophthalmology. 1998;105(10):1968–1976. doi:10.1016/S0161-6420(98)91049-1
92. Tsai JC, Johnson CC, Kammer JA, Dietrich MS. The Ahmed shunt versus the Baerveldt shunt for refractory glaucoma II: longer-term outcomes from a single surgeon. Ophthalmology. 2006;113:913–917. doi:10.1016/j.ophtha.2006.02.029
93. Minckler DS, Francis BA, Hodapp EA, et al. Aqueous shunts in glaucoma: a report by the American Academy of Ophthalmology. Ophthalmology. 2008;115(6):1089–1098. doi:10.1016/j.ophtha.2008.03.031
94. Gedde SJ, Schiffman JC, Feuer WJ, et al. Treatment outcomes in the tube versus trabeculectomy (TVT) study after five years of follow-up. Am J Ophthalmol. 2012;153(5):789–803e782. doi:10.1016/j.ajo.2011.10.026
95. Bailey AK, Sarkisian SR. Complications of tube implants and their management. Curr Opin Ophthalmol. 2014;25(2):148–153. doi:10.1097/ICU.0000000000000034
96. Banitt MR, Sidoti PA, Gentile RC, et al. Pars plana Baerveldt implantation for refractory childhood glaucomas. J Glaucoma. 2009;18:412–417. doi:10.1097/IJG.0b013e31818624bd
97. Ozgonul C, Besirli CG, Bohnsack BL. Combined vitrectomy and glaucoma drainage device implantation surgical approach for complex pediatric glaucomas. J AAPOS. 2017;21(2):121–126. doi:10.1016/j.jaapos.2017.02.001
98. Jacobson A, Besirli CG, Bohnsack BL. Outcomes of combined endoscopic vitrectomy and posteriorly placed glaucoma drainage devices in pediatric patients. BMC Ophthalmol. 2022;22(1):149. doi:10.1186/s12886-022-02373-3
99. Dolezal KA, Besirli CG, Mian SI, Sugar A, Moroi SE, Bohnsack BL. Glaucoma and cornea surgery outcomes in Peters Anomaly. Am J Ophthalmol. 2019;208:367–375. doi:10.1016/j.ajo.2019.08.012
100. Tansuebchueasai N, Kiddee W, Wangsupadilok B. Clinical characteristics and prognostic factors of visual outcomes in childhood glaucoma. J Pediatr Ophthalmol Strabismus. 2020;57(5):283–291. doi:10.3928/01913913-20200701-01
101. Surukrattanaskul S, Suvannachart P, Chansangpetch S, Manassakorn A, Tantisevi V, Rojanapongpun P. Characteristics and long-term outcomes of childhood glaucoma: a retrospective-cohort study. F1000Res. 2021;10:165. doi:10.12688/f1000research.51256.1
102. Tam EK, Elhusseiny AM, Shah AS, Mantagos IS, VanderVeen DK. Etiology and outcomes of childhood glaucoma at a tertiary referral center. J Aapos. 2022;26(3):117.e111–117.e116. doi:10.1016/j.jaapos.2021.12.009
103. Maclean K, Smith J, St Heaps L, et al. Axenfeld-Rieger malformation and distinctive facial features: clues to a recognizable 6p25 microdeletion syndrome. Am J Med Genet A. 2005;132a(4):381–385. doi:10.1002/ajmg.a.30274
104. Berenstein-Aizman G, Hazan-Molina H, Drori D, Aizenbud D. Axenfeld-Rieger syndrome: dentofacial manifestation and oral rehabilitation considerations. Pediatr Dent. 2011;33(5):440–444.
105. Yang Y, Zhu J, Chiba Y, Fukumoto S, Qin M, Wang X. Enamel defects of Axenfeld-Rieger syndrome and the role of PITX2 in its pathogenesis. Oral Dis. 2022. doi:10.1111/odi.14315
106. Cazzolla AP, Testa NF, Spirito F, et al. Axenfeld-Rieger syndrome: orthopedic and orthodontic management in a pediatric patient: a case report. Head Face Med. 2022;18(1):25. doi:10.1186/s13005-022-00329-y
107. Tsai JC, Grajewski AL. Cardiac valvular disease and Axenfeld-Rieger syndrome. Am J Ophthalmol. 1994;118(2):255–256. doi:10.1016/S0002-9394(14)72910-1
108. Gripp KW, Hopkins E, Jenny K, Thacker D, Salvin J. Cardiac anomalies in Axenfeld-Rieger syndrome due to a novel FOXC1 mutation. Am J Med Genet A. 2013;161a(1):114–119. doi:10.1002/ajmg.a.35697
109. Valikodath N, Johns JA, Godown J. Cardiac anomalies in Axenfeld-Rieger syndrome. Cardiol Young. 2022;1–3. doi:10.1017/S1047951122003857
110. Kannu P, Oei P, Slater HR, Khammy O, Aftimos S. Epiphyseal dysplasia and other skeletal anomalies in a patient with the 6p25 microdeletion syndrome. Am J Med Genet A. 2006;140(18):1955–1959. doi:10.1002/ajmg.a.31411
111. Ali Z, Charan P, Said JM, Stark Z. Axenfeld-Rieger syndrome as rare cause of umbilical abnormality. Ultrasound Obstet Gynecol. 2019;54(2):276–277. doi:10.1002/uog.20129
112. Yamazaki H, Nakamura T, Hosono K, et al. Sensorineural hearing loss and hypoplastic cochlea in Axenfeld-Rieger syndrome with FOXC1 mutation. Auris Nasus Larynx. 2021;48(6):1204–1208. doi:10.1016/j.anl.2020.07.006
113. Akcay BIS, Kardes E, Limon U. Axenfeld-Rieger syndrome in monozygotic twin brothers: case report. North Clin Istanb. 2022;9(4):411–413. doi:10.14744/nci.2021.89577
114. Lowry RB, Gould DB, Walter MA, Savage PR. Absence of PITX2, BARX1, and FOXC1 mutations in De Hauwere syndrome (Axenfeld-Rieger anomaly, hydrocephaly, hearing loss): a 25-year follow up. Am J Med Genet A. 2007;143a(11):1227–1230. doi:10.1002/ajmg.a.31732
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

