Back to Journals » Drug Design, Development and Therapy » Volume 11
Onychomadesis associated with chemotherapy: case report and mini literature review
Authors Li A, Li Y, Ge L , Li P, Li W
Received 14 April 2017
Accepted for publication 6 July 2017
Published 14 August 2017 Volume 2017:11 Pages 2373—2376
DOI https://doi.org/10.2147/DDDT.S139643
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Sukesh Voruganti
Ang Li,1,2,* Yanqiong Li,1,3,* Lingzhi Ge,4 Ping Li,1,3 Wenfei Li1
1Department of Dermatology, Qianfoshan Hospital, Shandong University, Jinan, People’s Republic of China; 2Department of Clinical Medicine, Queen Mary School, Medical School, Nanchang University, Nanchang, People’s Republic of China; 3Department of Clinical Medicine, Taishan Medical college, Tai’an, People’s Republic of China; 4Department of Dermatology, Affiliated First Hospital of Taishan Medical college, Tai’an, People’s Republic of China
*These authors contributed equally to this work
Abstract: The side effects of chemotherapy drugs have increased in recent years, and some side effects can lead to onychomadesis. A 72-year-old woman who was diagnosed with an invasive ductal carcinoma of the right breast underwent a modified radical mastectomy in April 2015, followed by chemotherapy with capecitabine and nanoparticle albumin-bound paclitaxel (nab-paclitaxel). Subsequently, the patient experienced palmoplantar redness, pain, onycholysis, a transparent serous exudate, and onychomadesis. The chemotherapy was discontinued, and the patient was treated with oral vitamin B6, a polymyxin ointment, and a high-energy red light. The palmoplantar redness and pain were alleviated after 1 month. However, although her fingernails improved, dysesthesia symptoms remained, and all her toenails exhibited defects or deformities at a 24-month follow-up. The symptoms of this disorder should be recognized by dermatologists.
Keywords: capecitabine and nab-paclitaxel, side effects, onychomadesis loss of nail
Introduction
With new treatment strategies improving the treatment outcomes of cancer,1 an increasing number of chemotherapy drugs such as capecitabine (Roche, Shanghai, People’s Republic of China) and nanoparticle albumin-bound paclitaxel (nab-paclitaxel) (Fresenius Kabi USA, LLC., MelrosePark, IL, USA) have been applied in clinical practice. Capecitabine has proven activity against several types of cancers,2 especially for breast and colon cancers. Nab-paclitaxel is a combination of paclitaxel and an albumin preparation, and paclitaxel is one of several cytoskeletal medications used to treat several types of cancer. Chemotherapy drugs such as capecitabine and nab-paclitaxel often have clinical side effects including hand-foot syndrome (HFS), acute paronychia, exudative hyponychial dermatitis, neutropenia, and hyperbilirubinemia.
Here, we describe a case of specific onychomadesis of all the toenails of a woman after she was treated with capecitabine and nab-paclitaxel. To the best of our knowledge, only five patients with onychomadesis associated with chemotherapy have been described in the English literature.3–6 Our patient had special clinical characteristics, and these features differed from those reported previously in the literature. This study was approved by Qianfoshan Hospital, Shandong University, and written informed consent has been provided by the patient to have the case details and any accompanying images published.
Case report
A 72-year-old woman who was diagnosed with an invasive ductal carcinoma of the right breast underwent a modified radical mastectomy in April 2015, followed by chemotherapy with 1,500 mg of oral capecitabine (Xeloda Roche, Shanghai, People’s Republic of China) twice daily for 2 weeks at 3-week intervals and 300 mg of intravenous nab-paclitaxel every 3 weeks. The patient noticed dysesthesia and desquamation of her hands and feet, and thinning toenails after 1 month of chemotherapy. Three months later (1 week after the fourth round of chemotherapy), she visited our department while exhibiting palmoplantar redness, pain, onycholysis, a transparent serous exudate, and onychomadesis.
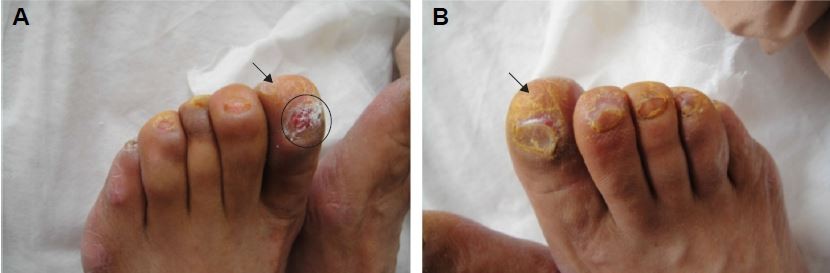
Examination of the patient revealed toenail changes including subungual hyperkeratosis, onycholysis, onychomadesis, and a transparent serous exudate emanating from all the nail beds (Figure 1). Her hands had desquamation and redness, and fingernail changes included dark pigmentations, Beau’s lines, and subungual hyperkeratosis (Figure 2). The patient also complained of significant pain in the heels of her feet. In addition, numbness and dysesthesia of the hands and feet were noticed. Her dorsalis pedis pulse was normal, and a fungal microscopic exam was negative. The patient suffered from hypertension for 3 years, and she underwent coronary artery stenting in 2012. She was not diabetic, and she denied taking any drugs except aspirin and lovastatin.
  | Figure 1 Subungual hyperkeratosis (arrow) (A, B), onycholysis, onychomadesis, and a transparent serous exudate emanating from the toenail bed (circle) (A). |
  | Figure 2 The patient’s hands became desquamated and red (B). Fingernail changes included dark pigmentations, Beau’s lines, and subungual hyperkeratosis (A). |
The chemotherapy was discontinued. The patient was treated with oral vitamin B6 (Beijing Double Crane Pharmaceutical Company Limited, Beijing, People’s Republic of China), a polymyxin ointment (Zhejiang Rishengchang Pharmaceutical Company Limited, Dongyang, People’s Republic of China), and high-energy red light (126 J/cm2 at a wavelength of 633 nm for 20 min every day). The exudation in the nail bed disappeared after 6 days. The palmoplantar redness and pain were alleviated after 1 month. The dysesthesia symptoms remained, and all the toenails exhibited defects or deformities (Figure 3); however, her hands returned to normal and her fingernails improved at a 24-month follow-up (Figure 4).
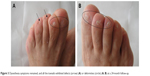  | Figure 3 Dysesthesia symptoms remained, and all the toenails exhibited defects (arrow) (A) or deformities (circle) (A, B) at a 24-month follow-up. |
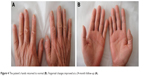  | Figure 4 The patient’s hands returned to normal (B). Fingernail changes improved at a 24-month follow-up (A). |
Discussion
Since onychomadesis caused by chemotherapeutic agents was originally described by Kochupillai et al,3 only five cases have been reported. The clinical features of these onychomadesis patients are listed in Table 1. Our patient presented palmoplantar redness, pain, and onychomadesis after she was treated with oral capecitabine and intravenous nab-paclitaxel. With the increasing number of chemotherapy treatments and increases in chemotherapy drug doses, her nail condition worsened. Additionally, her fingernail changes included dark pigmentation, Beau’s lines, and trachyonychia. The nail changes that our patient presented with in this case study are rare. The severe toenail changes of grade 3 nail toxicity and HFS were compatible with grade 3 according to the National Cancer Institute Common Toxicity Criteria.
Our patient did not have a history of onychomycosis, pemphigus vulgaris, critical illness, or other conditions, and her fungal examination test was negative; thus, her onychomadesis was associated with the chemotherapeutic agents. The mechanism underlying onychomadesis from chemotherapy is not well understood, although several mechanisms have been proposed.7 Studies have shown that systemic chemotherapy may arrest mitotic activity in the nail matrix and the nail, resulting in nail separation and shedding.8,9 The spectrum of possible causes of onychomadesis, for example, Beau’s lines, onycholysis, and shedding, is related to the weak or strong mitotic arresting activity of chemotherapy.
Although her onychomycosis was associated with capecitabine and nab-paclitaxel chemotherapy, we believe that capecitabine was probably responsible for the nail pathology, in consideration of previous cases. The HFS of our patient was compatible with grade 3. In association with mitotane, HFS was originally described in 1974.10 Patients with HFS may experience a well-demarcated plaque, palmoplantar dysesthesia, and burning pain. There are some reports of HFS or nail toxicity due to chemotherapy, but reports of the simultaneous occurrence of onychomadesis and HFS are rare.
Even after many years of reports related to these side effects, there has been little information regarding effective treatments or prevention.11,12 Our patient was administered a high-energy red light after wound cleaning in the first week, while being given oral vitamin B6 and polymyxin ointment. High-energy red light can repair damaged tissue and has anti-inflammatory and analgesic activities. Exudation in the nail bed disappeared after 6 days. The significant pain symptoms were alleviated after receiving high-energy red light therapy for 1 month. Although her fingernails improved, dysesthesia symptoms remained, and all her toenails exhibited defects or deformities at a 24-month follow-up.
Acknowledgments
We would like to thank Drs Furen Zhang, Hong Liu, and Baoqi Yang in Shandong Provincial Hospital for Skin Diseases, Shandong University, for their help. This study was supported by the grant from Natural Science Foundation of Shandong (No Y2008C160 and No ZR2011HL056).
Disclosure
The authors report no conflicts of interest in this work.
References
Rashad N, Abdel-Rahman O. Differential clinical pharmacology of rolapitant in delayed chemotherapy-induced nausea and vomiting (CINV). Drug Des Devel Ther. 2017;11:947–954. | ||
van Pelt-Sprangers MJ, Geijteman EC, Alsma J, et al. Oromandibular dystonia: a serious side effect of capecitabine. BMC Cancer. 2015;15:115. | ||
Kochupillai V, Prabhu M, Bhide NK. Cancer chemotherapy and nail loss (onychomadesis). Acta Haematol. 1983;70(2):137. | ||
Cetin M, Utas S, Unal A, et al. Shedding of the nails due to chemotherapy (onychomadesis). J Eur Acad Dermatol Venereol. 1998;11(2):193–194. | ||
Chen GY, Chen YH, Hsu MM, et al. Onychomadesis and onycholysis associated with capecitabine. Br J Dermatol. 2001;145(3):521–522. | ||
Vaccaro M, Barbuzza O, Guarneri F, et al. Nail and periungual toxicity following capecitabine therapy. Br J Clin Pharmacol. 2008;66(2):325–326. | ||
Hardin J, Haber RM. Onychomadesis: literature review. Br J Dermatol. 2015;172(3):592–596. | ||
Hussain S, Anderson DN, Salvatti ME, et al. Onycholysis as a complication of systemic chemotherapy: report of five cases associated with prolonged weekly paclitaxel therapy and review of the literature. Cancer. 2000;88(10):2367–2371. | ||
Maino KL, Norwood C, Stashower ME. Onycholysis with the appearance of a “sunset” secondary to capecitabine. Cutis. 2003;72(3):234–236. | ||
Zuehlke RL. Erythematous eruption of the palms and soles associated with mitotane therapy. Dermatologica. 1974;148(2):90–92. | ||
Scotte F, Banu E, Medioni J, et al. Matched case-control phase 2 study to evaluate the use of a frozen sock to prevent docetaxel-induced onycholysis and cutaneous toxicity of the foot. Cancer. 2008;112(7):1625–1631. | ||
Macedo LT, Lima JP, dos Santos LV, et al. Prevention strategies for chemotherapy-induced hand-foot syndrome: a systematic review and meta-analysis of prospective randomised trials. Support Care Cancer. 2014;22(6):1585–1593. |
 © 2017 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2017 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

