Back to Journals » International Journal of General Medicine » Volume 16
One-Year Survival for Developing Acute Kidney Injury in Adult Patients with AMI Cardiogenic Shock Receiving Venoarterial Extracorporeal Membrane Oxygenation
Authors Chen W, Pei M, Chen C, Wang B, Shi L, Qiu G, Duan W , Chen S, Wei Q, Zeng X, Pang H, Wei Y, Wu R, Zhu R, Ji Q, Lyu L
Received 10 July 2023
Accepted for publication 13 September 2023
Published 5 October 2023 Volume 2023:16 Pages 4537—4548
DOI https://doi.org/10.2147/IJGM.S427999
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Scott Fraser
Wan Chen,1,* Mingyu Pei,1,* Chunxia Chen,2,* Bo Wang,1,* Lei Shi,1,* Guozheng Qiu,1 Wenlong Duan,1 Shengxin Chen,1 Qiao Wei,1 Xi Zeng,1 Huifeng Pang,1 Yanlin Wei,1 Ruihua Wu,1 Ruikai Zhu,1 Qingwei Ji,3 Liwen Lyu1
1Department of Emergency, the People’s Hospital of Guangxi Zhuang Autonomous Region& Research Center of Cardiovascular Disease, Guang Xi Academy of Medical Sciences, Nanning, People’s Republic of China; 2Department of Pharmacy, the People’s Hospital of Guangxi Zhuang Autonomous Region, Nanning, People’s Republic of China; 3Department of Cardiovascular Medicine, The People’s Hospital of Guang Xi Zhuang Autonomous Region& Research Center of Cardiovascular Disease, Guangxi Academy of Medical Sciences Nanning, Nanning, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Liwen Lyu, Department of Emergency, The People’s Hospital of Guangxi Zhuang Autonomous Region, Research Center of Cardiovascular Disease, Guangxi Academy of Medical Sciences, Nanning, 530021, People’s Republic of China, Email [email protected] Qingwei Ji, Department of Cardiology, The People’s Hospital of Guangxi Zhuang Autonomous Region, Research Center of Cardiovascular Disease, Guangxi Academy of Medical Sciences Nanning, Nanning, 530021, People’s Republic of China, Email [email protected]
Objective: The incidence of cardiogenic shock cases treated with veno-arterial extracorporeal membrane oxygenation (VA-ECMO) support has been on the rise. Acute kidney injury (AKI) is a significant complication of cardiogenic shock and a frequent serious complication in patients requiring ECMO-supported therapy. AKI is strongly associated with unfavorable patient prognosis. However, there is a paucity of data on the influence of AKI on the prognosis of patients with acute myocardial infarction complicated by cardiogenic shock (AMI-CS) who are receiving ECMO support, particularly with regard to long-term outcomes.
Methods: This retrospective observational study included 103 patients in the People’s Hospital of Guangxi Zhuang Autonomous Region from January 2017 and June 2022. AKI was defined according to Kidney Disease Improving Global Outcome (KDIGO) criteria. Cox regression and logistic regression were used to identify risk factors.
Results: In this study, the incidence of AKI was 63.11%, with AKI stage 1, 2, and 3 accounting for 21.36%, 12.62%, and 29.13%, respectively. Patients with severe AKI had significantly higher in-hospital mortality (43.33% vs 27.40%, P < 0.001), 30-day mortality (60.00% vs 31.51%, P = 0.001), and 1-year mortality (63.67% vs 34.25%, P< 0.001) than those without severe AKI. Furthermore, severe AKI significantly increased the risk of one-year mortality (HR 10.816, CI 3.118– 37.512, P< 0.001). Baseline serum creatinine, baseline platelet, and active cardiopulmonary resuscitation were independent predictors of one-year mortality. In addition, baseline white blood cell count, baseline aspartate aminotransferase, baseline alanine aminotransferase (ALT), baseline serum creatinine, preoperative lactate, and postoperative mean arterial pressure were independent risk factors of severe AKI during hospitalization.
Conclusion: In patients with AMI-CS receiving ECMO support, AKI is highly prevalent. Development of severe AKI significantly increased the risk of one-year mortality.
Keywords: acute kidney injury, extracorporeal membrane oxygenation, prognosis, risk factors
Introduction
The mortality rate in acute myocardial infarction complicated by cardiogenic shock (AMI-CS) is more than 50%.1,2 With the clinical application and development of extracorporeal circulatory support therapy such as ECMO, the short-term adverse prognosis of cardiogenic shock has decreased significantly compared with the previous period. However, complications during ECMO support therapy have become an important influencing factor on the short-term and long-term prognosis of surviving patients.3–5 Acute kidney injury (AKI) is both a major complication of cardiogenic shock and one of the most common serious complications in patients requiring ECMO support therapy. The incidence of AKI in patients during ECMO support is as high as 55–70%.6,7 The incidence of AKI in AMI-CS during ECMO therapy in patients is influenced by multiple factors, including the effects of pro-inflammatory mediator release, nephrotoxic drug use, oxidative stress or high-risk surgery on the kidney, in addition to renal sensitivity to ischemia.8
 |
Figure 1 Study flow chart. |
Previous research studies in patients with AMI have shown that acute kidney injury increases the risk of developing chronic kidney disease (CKD) and end-stage renal disease (ESRD) in survivors and severely affects the long-term prognosis of patients.9,10 Coca et al searched the MEDLINE and EMBASE databases between January 1985 and February 2011 to summarize the relationship between CKD, ESRD, death, and AKI. They found that patients with AKI had an 8.8-fold higher risk of CKD, a 3.1-fold higher risk of ESRD, and twice the risk of premature death compared to patients without AKI.11 The risk of death increased with increasing severity of AKI.12 However, this does not fully account for several important issues regarding the prognosis of patients with AKI occurring in patients treated with ECMO support.
Therefore, more studies are urgently needed to clarify the impact of AKI on the prognosis, especially long-term prognosis, of AMI-CS patients treated with ECMO support.
Materials and Methods
Study Design and Setting
This paragraph describes a retrospective review of prospectively collected data from an institutional database of all VA-ECMO patients at the People’s Hospital of Guangxi Zhuang Autonomous Region between January 2017 and June 2022. The study was approved by the Institutional Review Board of the People’s Hospital of Guangxi Zhuang Autonomous Region (No. LL-KY-SY-2020-08) and performed according to the declaration of Helsinki. Data were extracted from the electronic Clinical Management System (CMS) of the People’s Hospital of Guangxi Zhuang Autonomous Region between 2017 and 2022. Patients were categorized into two groups depending on whether they occur AKI during ECMO. AKI was defined according to the KDIGO criteria and staging (see Table 1).13 Severe AKI was defined as AKI stage 3 whose definition is an increase in serum creatinine (SCr) to 4.0 mg/dl or by 3 times or initiation of renal replacement therapy (RRT) within the first 48 h following the ECMO onset.14 Patients were excluded if they had a known history of end-stage renal disease, had received a recent kidney transplant, had <3 serum creatinine (Scr) determinations during admission, had Scr levels returned to baseline values within 48 h, or were admitted for nephrectomy or dialysis initiation. The study also performed adjudication to define baseline Scr level with the priority of 1) the lowest Scr level prior to ECMO support; if not available, 2) the lowest Scr level during hospitalization, or 3) published reference value of age-specific normal Scr when patients lacked Scr data during admission. Considering the difficulties involved in establishing a baseline Scr level in some ECMO support patients, we performed adjudication to define baseline Scr level with the priority of 1) the lowest Scr level prior to ECMO support; if not available, 2) the lowest Scr level during hospitalization, or 3) published reference value of age-specific normal Scr when patients lacked Scr data during admission. The flow chart of the current study was shown in Figure 1.
 |
Table 1 Defining Acute Kidney Injury |
Outcomes
The primary outcome was all-cause mortality in 1 year for patients with AKI. The secondary endpoint was newly diagnosed ESRD requiring RRT within 365 days after initiation of ECMO support. All participants were followed up by office visits or telephone interviews at 1 month, 6 months and 1 year after enrollment. Follow-up data were monitored and recorded by trained nurses through outpatient interviews and telephone.
Statistical Analysis
Baseline characteristics are presented as means ± standard deviations, medians (interquartile range [IQR]), or frequencies and proportions depending on variable type and distribution. Normality of continuous data was assessed using the Shapiro–Wilk test. Differences in continuous characteristics across the two groups were assessed with a t-test or nonparametric Wilcoxon Rank Sum test. Differences in categorical variables across the two groups were assessed with a chi-square test. The authors then fit univariate and multivariate Cox regression models after confirming the proportional hazards assumption was met. Hazard ratios and their associated 95% confidence intervals were reported. Kaplan-Meier curves were reported for time to death, stratified by the two groups of interest. For all analyses, two-sided p values < 0.05 were considered statistically significant. SPSS statistical software (version 20, IBM, USA) was used for all analyses.
Results
Baseline Clinical Characteristics
Between January 2017 and June 2022, A total of 103 patients were eligible for inclusion in the study and received VA-ECMO for CS therapy during hospital course. Of these, 65 patients (63.11%) experienced some stage of acute kidney injury. Of these, 22 (21.36%) patients experienced stage 1 AKI, 13 (12.62%) experienced stage 2 AKI, and 30 (29.13%) were classified as Stage 3, 38 (36.89%) patients experienced no AKI (see Table 1).
Comparing the severe AKI group and the non-severe group, the non-severe group comprised 73 (70.87%) patients, of which 50 were men and 23 women with an average age of 52.16 ± 14.84 years. The average age of the 30 (29.13%) patients with AKI group was 50.53 ± 14.03 years, of which 24 were men and 6 women. However, the prevalence of hypertension, history of previous Coronary heart disease, Hyperlipidemia, Cerebrovascular accident, COPD and Type 2 diabetes mellitus were not significantly different between the groups (Table 2).
 |
Table 2 Basic Characteristics of the Study Population |
Overall, Patients with severe AKI were received more active CPR, and the severe AKI group patients had higher Bun, Lac and relatively lower MAP levels before received VA-ECMO. As well as higher Lac and relatively lower OI count levels were more prevalent in patients with severe AKI group than those in non-severe group for VA-ECMO therapy after 24h.
In-Hospital Clinical Outcomes, 30-Day Clinical Outcome and 1-Year Clinical Outcome
Patients with severe-AKI had a significantly higher rate of in-hospital mortality (43.33% vs 27.40%, P<0.001) and 30-days mortality (60.00% vs 31.51%, P = 0.001). But the ECMO support time, ICU hospitalization time and Total length of hospital stays were not significantly different between the groups. The 1-year all-cause mortality rate of the overall cohort was 42.72% (44/103), 63.67% (19/30) in the severe AKI group, and 34.25% (25/73) in the non-severe AKI group (P<0.001, Table 3). The 1-year Kaplan-Meier survival estimates were 49.2% (CI: 41–57%) in the non-severe AKI group, and 27.3% (CI: 19–37%) survival in the severe AKI group (log-rank p<0.001, Figure 2).
 |
Table 3 Outcomes of the Study |
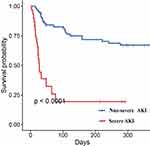 |
Figure 2 Cumulative survival rate for severe AKI group vs non-severe AKI group. |
Cox Regression Analysis and Logistic Regression Analysis
In the multivariable Cox regression analysis of 103 patients who were followed up for 1- year, severe AKI was proved to be an independent predictor of 1-year outcome. As shown in Table 4, the risk of 1-year all-cause mortality in severe AKI patients increased by 10.816-fold compared with that in non-severe AKI patients (HR 10.816, CI 3.118~37.512, P<0.001). Additional covariates, identified as independent predictors of 1-year all-cause mortality in multivariable Cox regression analyses, were baseline Scr (HR 0.996, CI 0.992~1.000, P=0.031), baseline PLT (HR 1.025, CI 1.001~1.050, P=0.042), and active CPR (HR 2.926, CI 1.344~6.372, P=0.007).
 |
Table 4 Predictors of 1-Year Mortality (Cox Regression) |
Multivariable logistic regression model identified baseline WBC count (OR 0.791, CI 0.641~0.977, P=0.029), baseline AST (OR 1.967, CI 1.118~3.461, P=0.019), baseline ALT (OR 0.614, CI 0.383~0.984, P=0.043), baseline Scr (OR 1.034, CI 1.008~1.061, P=0.010), preoperative Lac (OR 1.717, CI 1.034~2.852, P=0.037), and postoperative MAP (OR 0.882, CI 0.785~0.991, P=0.034) as independent predictors of severe AKI during hospital course (Table 5).
 |
Table 5 Predictors of Severe AKI (Logistic Regression) |
Subgroup Analyses
In order to investigate the impact of less severe AKI on survival, firstly, the overall cohort was sub-divided into non- AKI and AKI groups, the Kaplan-Meier survival analysis confirmed that the adjusted 1-year all-cause mortality rate of patients with AKI was comparable with that of patients without AKI (log-rank p=0.096, Figure 3). Secondly, the non-severe AKI group was sub-divided into Stage 0, 1, 2, and 3 AKI groups. The results of the Kaplan-Meier survival analysis showed that the adjusted 1-year all-cause mortality rate of patients with AKI Stage 0, 1, 2 were similarity, there was no significant difference. However, there tended to be a difference in the Patients with severe AKI had the highest mortality (log-rank p<0.001, Figure 4).
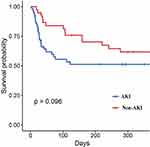 |
Figure 3 Cumulative survival rate for AKI group vs non-AKI group. |
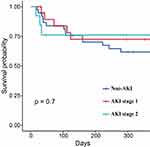 |
Figure 4 Cumulative survival rate for patients without AKI, AKI stage 1 and AKI stage 2. |
Discussion
The purpose of this study was to define AKI using the KDIGO guidelines and to investigate the long-term prognostic impact on AMI-CS patients requiring ECMO therapy. The study found that in AMI-CS patients requiring VA-ECMO supportive therapy, the incidence of AKI was 63.11%, with 21.36% being AKI grade 1, 12.62% being AKI grade 2, and 29.13% being AKI grade 3. The severe AKI group (AKI grade 3) had higher in-hospital mortality, 30-day mortality and 1-year mortality than the non-severe AKI group (AKI grade 0, 1, 2). Severe AKI significantly increased the risk of 1-year mortality in AMI-CS patients treated with ECMO support.
AKI is a common complication in patients with AMI, with an incidence of approximately 9–19.4% reported in previous literature.15–17 The incidence of AKI in AIM-CS patients reaches 25–35%. However, there is no report on the incidence of AKI in AMI-CS patients treated with ECMO support. The incidence of AKI in ECMO-supported patients was as high as 50–85% depending on the AKI grading criteria.6,8,18 In this study, the incidence of AKI in AMI-CS patients treated with ECMO support was high. AKI is a serious complication during ECMO-supported therapy, and its pathophysiological mechanisms are not fully understood.7,19
Korbinian Lackermair et al reported a 12-month all-cause mortality rate of 19% in patients with AMI-CS treated with extracorporeal life support.20 In contrast, the 12-month all-cause mortality rate in this study was 42.72%. Possible reasons for this are that this study had more cases and most of these cases were acute AMI-CS cases acquired in the emergency resuscitation unit or cases that developed CS during PCI, were treated with ECMO support before hemodynamic reconstruction, and 12% of patients experienced CPR. All cases in the other study were given extracorporeal life support according to the patient’s condition after PCI hemodynamic reconstruction support.
Studies in the AMI population have shown that AKI is an independent influence on the prognosis of AMI patients.21 Even “transient AKI” (AKI that resolves within 72 hours of onset) can significantly increase the risk of death in hospitalized patients.22 In this study, the 1-year risk of death was significantly increased in patients with severe AKI and not in patients without severe AKI (AKI grades 0, 1, and 2) in AMI-CS patients requiring ECMO-supported therapy. It is suggested that in AMI-CS patients treated with ECMO support, only severe AKI significantly affects prognosis. This finding is contrary to several previous studies that concluded21 that mild AKI can also affect the short- and long-term prognosis of AMI patients. It is speculated that this may be related to the following factors: 1. Previous studies have suggested that the relationship between AKI and mortality is through indirect pathophysiological pathways leading to death, in which the conversion of AKI to CKD or even ESRD is an important influencing factor, and that adverse events such as hyperkalemia, acidosis and volume overload that occur in chronic renal insufficiency affect the long-term prognosis of patients;23 2. ECMO can rapidly restore renal ischemia and hypoxia in AMI-CS patients and promote early recovery of renal function;24–29 3. In some previous studies, patients with a previous CKD base were included, whereas this group of patients has been excluded from this study, making the prognostic outcome not comparable.
In this study, Scr, PLT, and CPR were found to be independent predictors affecting the 1-year prognosis of acute myocardial infarction complicated by AMI-CS patients requiring ECMO therapy. Several previous studies have demonstrated that baseline renal function is an independent influential factor affecting the occurrence of AKI or prognosis in patients with critical illness, PCI, cardiac disease, and AMI-CS,30–33 and the findings of this study are similar. In the present study, patients had mostly normal baseline renal function, and it has been suggested that a slight elevation of Scr (subclinical changes) in CS patients also affects patient prognosis, which may be related to the fact that most renal impairment in CS patients results in structural changes in the renal unit and deserves further investigation.34
CPR is an independent influence on the prognosis of AMI-CS patients. Among AMI-CS patients, those who underwent CPR before PCI had four times higher in-hospital mortality than those who did not experience CPR.35 It has also been noted that a CPR time of >12.5 minutes was an independent predictor of 30-day mortality in AMI-CS patients treated with ECMO support (adjusted hazard ratio, 4.71; 95% confidence interval, 1.30–17.406; p=0.018).
Previous studies have shown that decreased PLT count is an important influencing factor for poor prognosis in critically ill patients, patients with cardiovascular disease, and is even strongly associated with the risk of long-term cardiovascular disease.36,37 Existing studies have concluded that during ECMO support therapy, ECMO mechanical support devices, hemodynamic instability, renal failure, infection, and disseminated intravascular coagulation (DIC) can cause a decrease in PLT counts for a variety of reasons. A decrease in PLT counts increases the incidence of bleeding/thrombotic events in patients on ECMO support therapy and is an important influencing factor on the short- and long-term prognosis of patients.38 It has also been suggested that reduced platelet counts are a marker of excessive platelet activation and excessive destruction. Platelet activation mediates the recruitment and release of inflammatory cells that play a key role in local and systemic inflammation in many pathological settings such as glomerulonephritis, sepsis, and extensive atherosclerosis, leading to exacerbation of infection in patients.39,40 The above studies are all dynamic changes during clinical treatment, and there was no statistically significant difference in baseline PLT between the two groups of patients in this study. Whether minor changes in PLT prior to ECMO treatment have an impact on prognosis needs further clinical exploration and validation.
Limitations
The study was limited to the clinical experience of a single center, and due to differences in indications and management of VA-ECMO among centers, including differences in anticoagulation, anti-infection, and withdrawal processes during treatment, may significantly affect patient prognostic outcomes. Secondly, because the study is retrospective, methodological limitations make it possible that the data collected on the variables may not be accurate with the actual medical status of the patients. Third, this study used the KDIGO criteria to define AKI, but urine volume information was not available in all samples, and no information was collected on the regression of AKI in patients. Finally, due to the small sample size number and outcome events number, some other confounders were not fully adjusted, including (1) the impact of COVID-19 pandemic;41,42 (2) the approach of PCI;43 (3) impact of operators’ experience;44 (4) PCI or ECMO in off-hour;45 (5) some complications peri-ECMO support and so on. However, the proportions of these confounding factors were also quite small, and the influence could be ignored in this study. The influence of these confounders should to be further explored in future studies with large samples.
Conclusion
Overall, the data further and strengthen clarify that there is an extremely high incidence of AKI in AMI-CS patients requiring VA-ECMO-supported therapy, with a significantly increased 1-year risk of death in patients with severe AKI compared with those without severe AKI and no significant difference in the 1-year risk of death in patients without severe AKI (AKI grades 0, 1, 2). Finally, there is an urgent need to improve prevention and treatment strategies for severe AKI in AMI-CS patients treated with VA-ECMO support and to enhance early identification of this high-risk population.
Data Sharing Statement
The datasets analyzed during the current study will be available from the corresponding author upon reasonable request.
Ethical Approval and Consent to Participate
This study was conducted in accordance with the Declaration of Helsinki and was approved by the Ethics Committee of the People’s Hospital of Guangxi Zhuang Autonomous Region approved this study protocol (No.LL-KY-SY-2020-08). All subjects provided written informed consent.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
This study was supported by the National Natural Science Foundation of China (81960246, 81701089 and 82260258), the Guangxi Natural Science Foundation (2020GXNSFAA238003 and 2017GXNSFBA198010), the Guangxi Medical and Health Appropriate Technology Research and Development Project (S2020076, S2020080), the Science Research and Technology Development Program of Guangxi (2017AB45111), Guangxi Science and Technology Program (AB17292091), Specific Research Project of Guangxi for Research Bases and Talents (GKAD17129026) and Guangxi Science and Technology Specialized Project (GKG14124003-9).
Disclosure
The authors declare that there are no conflicts of interest in this work.
References
1. Takayama H, Truby L, Koekort M, et al. Clinical outcome of mechanical circulatory support for refractory cardiogenic shock in the current era. J Heart Lung Transplant. 2013;32(1):106–111. doi:10.1016/j.healun.2012.10.005
2. Samsky MD, Morrow DA, Proudfoot AG, Hochman JS, Thiele H, Rao SV. Cardiogenic shock after acute myocardial infarction: a review. JAMA. 2021;326(18):1840–1850. doi:10.1001/jama.2021.18323
3. Schmidt M, Burrell A, Roberts L, et al. Predicting survival after ECMO for refractory cardiogenic shock: the survival after veno-arterial-ECMO (SAVE)-score. Eur Heart J. 2015;36(33):2246–2256. doi:10.1093/eurheartj/ehv194
4. Schmidt M, Bréchot N, Hariri S, et al. Nosocomial infections in adult cardiogenic shock patients supported by venoarterial extracorporeal membrane oxygenation. Clin Infect Dis. 2012;55(12):1633–1641. doi:10.1093/cid/cis783
5. Sutter R, Tisljar K, Marsch S. Acute neurologic complications during extracorporeal membrane oxygenation: a systematic review. Crit Care Med. 2018;46(9):1506–1513. doi:10.1097/ccm.0000000000003223
6. Lorusso R, Gelsomino S, Parise O, et al. Neurologic injury in adults supported with veno-venous extracorporeal membrane oxygenation for respiratory failure: findings from the extracorporeal life support organization database. Crit Care Med. 2017;45(8):1389–1397. doi:10.1097/ccm.0000000000002502
7. Kilburn DJ, Shekar K, Fraser JF. The complex relationship of extracorporeal membrane oxygenation and acute kidney injury: causation or association? Biomed Res Int. 2016;2016:1094296. doi:10.1155/2016/1094296
8. Gu M, Mei XL, Zhao YN. A review on extracorporeal membrane oxygenation and kidney injury. J Biochem Mol Toxicol. 2021;35(3):e22679. doi:10.1002/jbt.22679
9. Kooiman J, Seth M, Nallamothu BK, Heung M, Humes D, Gurm HS. Association between acute kidney injury and in-hospital mortality in patients undergoing percutaneous coronary interventions. Circ Cardiovasc Interv. 2015;8(6):e002212. doi:10.1161/circinterventions.114.002212
10. Mitchell AM, Kline JA, Jones AE, Tumlin JA. Major adverse events one year after acute kidney injury after contrast-enhanced computed tomography. Ann Emerg Med. 2015;66(3):267–274.e264. doi:10.1016/j.annemergmed.2015.04.028
11. Coca SG, Singanamala S, Parikh CR. Chronic kidney disease after acute kidney injury: a systematic review and meta-analysis. Kidney Int. 2012;81(5):442–448. doi:10.1038/ki.2011.379
12. Sun Y-B, Tao Y, Yang M. Assessing the influence of acute kidney injury on the mortality in patients with acute myocardial infarction: a clinical trail. Ren Fail. 2018;40(1):75–84. doi:10.1080/0886022x.2017.1419969
13. Stevens PE. Evaluation and management of chronic kidney disease: synopsis of the kidney disease: improving global outcomes 2012 clinical practice guideline. Ann Intern Med. 2013;158(11):825–830. doi:10.7326/0003-4819-158-11-201306040-00007
14. Mou Z, He J, Guan T, Chen L. Acute kidney injury during extracorporeal membrane oxygenation: VA ECMO versus VV ECMO. J Intensive Care Med. 2022;37(6):743–752. doi:10.1177/08850666211035323
15. Parikh CR, Coca SG, Wang Y, Masoudi FA, Krumholz HM. Long-term prognosis of acute kidney injury after acute myocardial infarction. Arch Intern Med. 2008;168(9):987–995. doi:10.1001/archinte.168.9.987
16. Goldberg A, Hammerman H, Petcherski S, et al. Inhospital and 1-year mortality of patients who develop worsening renal function following acute ST-elevation myocardial infarction. Am Heart J. 2005;150(2):330–337. doi:10.1016/j.ahj.2004.09.055
17. Jose P, Skali H, Anavekar N, et al. Increase in creatinine and cardiovascular risk in patients with systolic dysfunction after myocardial infarction. J Am Soc Nephrol. 2006;17(10):2886–2891. doi:10.1681/asn.2006010063
18. Cheng R, Hachamovitch R, Kittleson M, et al. Complications of extracorporeal membrane oxygenation for treatment of cardiogenic shock and cardiac arrest: a meta-analysis of 1866 adult patients. Ann Thorac Surg. 2014;97(2):610–616. doi:10.1016/j.athoracsur.2013.09.008
19. Askenazi DJ, Selewski DT, Paden ML, et al. Renal replacement therapy in critically ill patients receiving extracorporeal membrane oxygenation. Clin J Am Soc Nephrol. 2012;7(8):1328–1336. doi:10.2215/cjn.12731211
20. Lackermair K, Brunner S, Orban M, et al. Outcome of patients treated with extracorporeal life support in cardiogenic shock complicating acute myocardial infarction: 1-year result from the ECLS-shock study. Clin Res Cardiol. 2021;110(9):1412–1420. doi:10.1007/s00392-020-01778-8
21. Meng Z, Zhao Y, Zheng X, He Y. The relationship between AKI in patients with STEMI and short-term mortality: a propensity score matching analysis. Angiology. 2021;72(8):733–739. doi:10.1177/0003319721998567
22. Uchino S, Bellomo R, Bagshaw SM, Goldsmith D. Transient azotaemia is associated with a high risk of death in hospitalized patients. Nephrol Dial Transplant. 2010;25(6):1833–1839. doi:10.1093/ndt/gfp624
23. Kaltsas E, Chalikias G, Tziakas D. The incidence and the prognostic impact of acute kidney injury in acute myocardial infarction patients: current preventive strategies. Cardiovasc Drugs Ther. 2018;32(1):81–98. doi:10.1007/s10557-017-6766-6
24. Abadeer AI, Kurlansky P, Chiuzan C, et al. Importance of stratifying acute kidney injury in cardiogenic shock resuscitated with mechanical circulatory support therapy. J Thorac Cardiovasc Surg. 2017;154(3):856–864.e854. doi:10.1016/j.jtcvs.2017.04.042
25. Ostermann M, Connor M, Kashani K. Continuous renal replacement therapy during extracorporeal membrane oxygenation: why, when and how? Curr Opin Crit Care. 2018;24(6):493–503. doi:10.1097/mcc.0000000000000559
26. Lee CC, Chen SW, Cheng YL, et al. The impact of CRRT modality in patients with AKI receiving ECMO: a nationwide registry study in Taiwan. J Crit Care. 2020;57:102–107. doi:10.1016/j.jcrc.2020.02.006
27. Alba AC, Foroutan F, Buchan TA, et al. Mortality in patients with cardiogenic shock supported with VA ECMO: a systematic review and meta-analysis evaluating the impact of etiology on 29,289 patients. J Heart Lung Transplant. 2021;40(4):260–268. doi:10.1016/j.healun.2021.01.009
28. Hong D, Choi KH, Cho YH, et al. Multidisciplinary team approach in acute myocardial infarction patients undergoing veno-arterial extracorporeal membrane oxygenation. Ann Intensive Care. 2020;10(1):83. doi:10.1186/s13613-020-00701-8
29. Basir MB, Pinto DS, Ziaeian B, et al. Mechanical circulatory support in acute myocardial infarction and cardiogenic shock: challenges and importance of randomized control trials. Catheter Cardiovasc Interv. 2021;98(7):1264–1274. doi:10.1002/ccd.29593
30. Parr CJ, Sharma R, Arora RC, Singal R, Hiebert B, Minhas K. Outcomes of extracorporeal membrane oxygenation support in the cardiac catheterization laboratory. Catheter Cardiovasc Interv. 2020;96(3):547–555. doi:10.1002/ccd.28492
31. Fox KA, Dabbous OH, Goldberg RJ, et al. Prediction of risk of death and myocardial infarction in the six months after presentation with acute coronary syndrome: prospective multinational observational study (GRACE). BMJ. 2006;333(7578):1091. doi:10.1136/bmj.38985.646481.55
32. Hannan EL, Farrell LS, Wechsler A, et al. The New York risk score for in-hospital and 30-day mortality for coronary artery bypass graft surgery. Ann Thorac Surg. 2013;95(1):46–52. doi:10.1016/j.athoracsur.2012.08.047
33. Peterson ED, Dai D, DeLong ER, et al. Contemporary mortality risk prediction for percutaneous coronary intervention: results from 588,398 procedures in the National Cardiovascular Data Registry. J Am Coll Cardiol. 2010;55(18):1923–1932. doi:10.1016/j.jacc.2010.02.005
34. Auer J, Verbrugge FH, Lamm G. Editor’s choice- what do small serum creatinine changes tell us about outcomes after acute myocardial infarction? Eur Heart J Acute Cardiovasc Care. 2018;7(8):739–742. doi:10.1177/2048872617728721
35. Schwarz B, Abdel-Wahab M, Robinson DR, Richardt G. Predictors of mortality in patients with cardiogenic shock treated with primary percutaneous coronary intervention and intra-aortic balloon counterpulsation. Med Klin Intensivmed Notfmed. 2016;111(8):715–722. doi:10.1007/s00063-015-0118-8
36. Takano AM, Iwata H, Miyosawa K, et al. Reduced number of platelets during intra-aortic balloon pumping counterpulsation predicts higher cardiovascular mortality after device removal in association with systemic inflammation. Int Heart J. 2020;61(1):89–95. doi:10.1536/ihj.19-349
37. Vanderschueren S, De Weerdt A, Malbrain M, et al. Thrombocytopenia and prognosis in intensive care. Crit Care Med. 2000;28(6):1871–1876. doi:10.1097/00003246-200006000-00031
38. Guimbretière G, Anselmi A, Roisne A, et al. Prognostic impact of blood product transfusion in VA and VV ECMO. Perfusion. 2019;34(3):246–253. doi:10.1177/0267659118814690
39. Losito I, Conte E, Cataldi TR, Cioffi N, Megli FM, Palmisano F. The phospholipidomic signatures of human blood microparticles, platelets and platelet-derived microparticles: a comparative HILIC-ESI-MS investigation. Lipids. 2015;50(1):71–84. doi:10.1007/s11745-014-3975-7
40. Winnersbach P, Rossaint J, Buhl EM, et al. Platelet count reduction during in vitro membrane oxygenation affects platelet activation, neutrophil extracellular trap formation and clot stability, but does not prevent clotting. Perfusion. 2022;37(2):134–143. doi:10.1177/0267659121989231
41. De Luca G, Silverio A, Verdoia M, et al. Angiographic and clinical outcome of SARS-CoV-2 positive patients with ST-segment elevation myocardial infarction undergoing primary angioplasty: a collaborative, individual patient data meta-analysis of six registry-based studies. Eur J Intern Med. 2022;105:69–76. doi:10.1016/j.ejim.2022.08.021
42. Tokarek T, Dziewierz A, Malinowski KP, et al. Treatment delay and clinical outcomes in patients with ST-segment elevation myocardial infarction during the COVID-19 pandemic. J Clin Med. 2021;10(17). doi:10.3390/jcm10173920
43. Tokarek T, Dziewierz A, Plens K, Rakowski T, Dudek D, Siudak Z. Radial approach reduces mortality in patients with ST-segment elevation myocardial infarction and cardiogenic shock. Pol Arch Intern Med. 2021;131(5):421–428. doi:10.20452/pamw.15886
44. Zabojszcz M, Januszek R, Siudak Z, et al. Association between the mortality rate and operator volume in patients undergoing emergency or elective percutaneous coronary interventions. Kardiol Pol. 2020;78(2):138–146. doi:10.33963/kp.15123
45. Tokarek T, Dziewierz A, Plens K, et al. Percutaneous coronary intervention during on- and off-hours in patients with ST-segment elevation myocardial infarction. Hellenic J Cardiol. 2021;62(3):212–218. doi:10.1016/j.hjc.2021.01.011
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
