Back to Journals » Drug Design, Development and Therapy » Volume 14
One-Pot Synthesis of Novel Thiazoles as Potential Anti-Cancer Agents
Authors Sayed AR, Gomha SM , Taher EA , Muhammad ZA, El-Seedi HR, Gaber HM , Ahmed MM
Received 28 June 2019
Accepted for publication 12 March 2020
Published 3 April 2020 Volume 2020:14 Pages 1363—1375
DOI https://doi.org/10.2147/DDDT.S221263
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Prof. Dr. Cristiana Tanase
Abdelwahed R Sayed,1,2 Sobhi M Gomha,3,4 Eman A Taher,5,6 Zeinab A Muhammad,5 Hesham R El-Seedi,6,7 Hatem M Gaber,5 Mahgoub M Ahmed8
1Department of Chemistry, Faculty of Science, KFU, Hofuf, Saudi Arabia; 2Department of Chemistry, Faculty of Science, Beni-suef University, Beni-suef, Egypt; 3Department of Chemistry, Faculty of Science, Cairo University, Giza 12613, Egypt; 4Department of Chemistry, Faculty of Science, Islamic University in Almadinah Almonawara, Almadinah Almonawara 42351, Saudi Arabia; 5Department of Pharmaceutical Chemistry, National Organization for Drug Control and Research (NODCAR), Giza 12311, Egypt; 6Chemistry Department, Faculty of Science, El-Menoufia University, Shebin El-Kom 32512, Egypt; 7Division of Pharmacognosy, Department of Medicinal Chemistry, Uppsala University, Biomedical Centre, Uppsala SE-75123, Sweden; 8Molecular Drug Evaluation Department, National Organization for Drug Control and Research (NODCAR), Giza 12311, Egypt
Correspondence: Sobhi M Gomha Tel +20 100-164-9576
Fax +20 25685799
Email [email protected]
Background: Thiazole and thiosemicarbazone derivatives are known to have potential anticancer activity with a mechanism of action related to inhibition of matrix metallo-proteinases, kinases and anti-apoptotic BCL2 family proteins.
Materials and Methods: A novel three series of 5-(1-(2-(thiazol-2-yl)hydrazono)ethyl)thiazole derivatives were prepared in a one-pot three-component reaction using 2-(2-benzylidene hydrazinyl)-4-methylthiazole as a starting precursor. MS, IR, 1H-NMR and 13C-NMR were used to elucidate the structures of the synthesized compounds. Most of the synthesized products were evaluated for their in vitro anticancer screening against HCT-116, HT-29 and HepG2 using the MTT colorimetric assay.
Results: The results indicated that compounds 4c, 4d and 8c showed growth inhibition activity against HCT-116 with IC50 values of 3.80 ± 0.80, 3.65 ± 0.90 and 3.16 ± 0.90 μM, respectively, compared to harmine (IC50 = 2.40 ± 0.12 μM) and cisplatin (IC50 = 5.18 ± 0.94 μM) reference drugs. Also, compounds 8c, 4d and 4c showed promising IC50 values of 3.47 ± 0.79, 4.13 ± 0.51 and 7.24 ± 0.62 μM, respectively, against the more resistant human colorectal cancer (HT-29) cell line compared with harmine (IC50 = 4.59 ± 0.67 μM) and cisplatin (IC50 = 11.68 ± 1.54 μM). On the other hand, compounds 4d, 4c, 8c and 11c were the most active (IC50 values of 2.31± 0.43, 2.94 ± 0.62, 4.57 ± 0.85 and 9.86 ± 0.78 μM, respectively) against the hepatocellular carcinoma (HepG2) cell line compared with harmine (IC50 = 2.54 ± 0.82 μM) and cisplatin (IC50 = 41 ± 0.63 μM). The study also suggested that the mechanism of the anticancer action exerted by the most active compounds ( 4c, 4d and 8c) inside HCT-116 cells was apoptosis through the Bcl-2 family.
Conclusion: Thiazole scaffolds 4c, 4d and 8c showed anticancer activities in the micromolar range and are appropriate as a candidate for cancer treatment.
Keywords: hydrazones, hydrazonoyl halides, cyclization, harmine, HCT-116, HepG2, HT-29
Introduction
Colorectal cancer (CRC) is considered one of the most common malignancies with a high incidence and mortality worldwide. It is estimated that 1.4 million individuals were newly diagnosed with CRC in 2012, which resulted in 693,900 mortalities.1 Surgery, chemotherapy and radiation treatment are the three main standard therapies for CRC. Chemotherapy is an important treatment for cancer, particularly in tumors with a propensity to invade adjacent tissues and metastasize to other organs. Challenges remain that require the continued search for novel effective and less toxic chemotherapeutic agents for the treatment of colon cancer.2 Apoptosis is a form of regulated cell death that is triggered in response to developmental cues or cellular stress. This selective cell suicide plays an essential role in numerous physiological and pathological processes including development, immunity and disease where the elimination of damaged or superfluous cells helps to ensure organismal health.3 There are two apoptotic pathways – the extrinsic pathway and the intrinsic (mitochondrial) pathway. The intrinsic apoptotic pathway (the mitochondrion-mediated pathway) is initiated in response to a variety of stress signals,4 and a complex interplay of Bcl-2 (B-cell lymphoma 2) proteins relays this signal to the mitochondrial outer membrane (OM) to initiate Bak and Bax activation, oligomerization and OM damage. Breaching the mitochondrial OM releases apoptogenic factors, including cytochrome c and Smac, which activate a group of aspartate-specific proteases (caspases).5 Caspases, in turn, cleave several hundred cellular proteins to coordinate the destruction of the cell.6 Recent pharmaceutical advances have allowed the specific targeting of protein–protein interactions in the BCL-2 family.5,7
Thiosemicarbazones are a large class of compounds, which represent great therapeutic value against parasitic diseases8,9 and microbial diseases.10 They were also identified to be among the most interesting antitumor inhibitors due to induction of oxidative stress and ROS-mediated cell injury.11,12 Thiazole heterocycles, derivatives of thiosemicarbazone, are scaffolds of many natural, synthetic and semi-synthetic drugs which exhibit numerous remarkable pharmacological activities including antiparasitic, anti-inflammatory and antineoplastic activities.13–17 Thiazole derivatives are also known to have potential anticancer activity with a mechanism of action related to inhibition of matrix metallo-proteinases, kinases and anti-apoptotic BCL2 family proteins.18–21
Multicomponent reactions (MCR) are one-pot processes which always occupy great importance in the repertoire of sustainable synthetic tools because of their high efficiency and atom economy.22,23 As part of our ongoing studies on the synthesis of new heterocyclic compounds via one-pot, multicomponent reactions,24–33 herein this study describes a convenient and rapid method for the synthesis of thiazolyl-hydrazono-ethylthiazole derivatives by one-pot three-component reactions using 2-(2-benzylidenehydrazinyl)-4-methylthiazole (1), thiosemicarbazide (2) and the appropriate hydrazonoyl chlorides (3a–e or 7a–d) or phenacyl bromides (10a–e) in the presence of a catalytic amount of TEA in dioxane. The cytotoxic potential of the newly synthesized compounds was examined against HCT-116, HT-29 and HepG2 cells using the MTT assay to elucidate its underlying mechanisms of action related to their anti-apoptotic BCl2 family proteins.
Materials and Methods
Chemistry
Melting points were measured on an Electrothermal IA 9000 series digital melting point apparatus (Bibby Sci. Lim., Stone, UK). IR spectra were recorded in potassium bromide discs on PyeUnicam SP 3300 and Shimadzu FTIR 8101 PC infrared spectrophotometers (Shimadzu, Tokyo, Japan). NMR spectra were measured on a Varian Mercury VX-300 NMR spectrometer (Varian, Inc., Karlsruhe, Germany). 1H spectra were recorded at 300 MHz and 13C spectra were recorded at 75.46 MHz in deuterated dimethyl sulfoxide (DMSO-d6). Mass spectra were run on a Shimadzu GCMS-QP1000 EX mass spectrometer (Tokyo, Japan) at 70 eV. Elemental analyses were measured using a German-made Elementarvario LIII CHNS analyzer. Biological activities of the synthesized compounds were carried out at the Regional Center for Mycology and Biotechnology at Al-Azhar University, Cairo, Egypt.
General Procedure for Synthesis of Thiazole Derivatives 4a–e and 8a–d
A mixture of 2-(2-benzylidenehydrazinyl)-4-methylthiazole (1) (0.259 g, 1 mmol), thiosemicarbazide (2) (0.091 g, 1 mmol) and the appropriate hydrazonoyl chlorides (3a–e or 7a–d) (1 mmol) in dioxane (20 mL) containing catalytic amounts of TEA was refluxed for 2–4 h (monitored by TLC). The formed precipitate was isolated by filtration, washed with methanol, dried and recrystallized from appropriate solvent to give products 4a–e or 8a–d. The physical properties and spectral data of the obtained products 4a–e and 8a–d are listed below.
Red solid, 70% yield, m.p. 235–237 °C (DMF); IR (KBr): v 3425, 3220 (2NH), 3035, 2914 (C–H) cm−1; 1H-NMR (DMSO-d6): δ 1.91 (s, 3H, CH3), 2.10 (s, 3H, CH3), 2.29 (s, 3H, CH3), 6.97–8.10 (m, 10H, Ar-H), 8.69 (s, 1H, CH=N), 10.58 (brs, 1H, NH), 11.19 (brs, 1H, NH); 13C-NMR (DMSO-d6): δ 8.89, 21.57, 23.56 (3CH3), 45.86, 66.81, 114.62, 115.63, 117.86, 118.99, 121.70, 122.89, 125.60, 126.60, 127.66, 129.81, 137.19, 140.76, 161.91, 165.21 (Ar–C and C=N); MS m/z (%): 474 (M+, 14). Anal. Calcd for C23H22N8S2 (474.14): C, 58.21; H, 4.67; N, 23.61. Found: C, 58.36; H, 4.51; N, 23.47%.
Red solid, 73% yield, m.p. 220–222 °C (DMF); IR (KBr): v 3428, 3210 (2NH), 3030, 2917 (C–H) cm−1; 1H-NMR (DMSO-d6): δ 1.91 (s, 3H, CH3), 2.26 (s, 3H, CH3), 2.36 (s, 3H, CH3), 2.51 (s, 3H, CH3), 7.17–8.13 (m, 9H, Ar–H), 8.66 (s, 1H, CH=N), 10.48 (brs, 1H, NH), 11.23 (brs, 1H, NH); 13C-NMR (DMSO-d6): δ 9.21, 16.78, 18.90, 19.82 (4CH3), 112.97, 114.15, 116.69, 120.79, 128.63, 129.94, 131.57, 132.35, 135.18, 137.05, 139.35, 143.49, 151.36, 156.16, 157.05, 159.27 (Ar–C and C=N); MS m/z (%): 488 (M+, 17). Anal. Calcd for C24H24N8S2 (488.63): C, 58.99; H, 4.95; N, 22.93. Found: C, 59.08; H, 4.78; N, 22.79%.
Red solid, 68% yield, m.p. 215–217 °C (DMF); IR (KBr): v 3429, 3315 (2NH), 3020, 2922 (C–H) cm−1; 1H-NMR (DMSO-d6): δ 1.91 (s, 3H, CH3), 2.30 (s, 3H, CH3), 2.42 (s, 3H, CH3), 7.36–8.11 (m, 9H, Ar–H), 8.70 (s, 1H, CH=N), 10.65 (brs, 1H, NH), 11.40 (brs, 1H, NH); MS m/z (%): 511 (M++2, 6), 509 (M+, 19). Anal. Calcd for C23H21ClN8S2 (509.05): C, 54.27; H, 4.16; N, 22.01. Found: C, 54.14; H, 3.99; N, 21.90%.
Red solid, 78% yield, m.p. 188–190 °C (DMF); IR (KBr): v 3423, 3305 (2NH), 3040, 2916 (C–H) cm−1; 1H-NMR (DMSO-d6): δ 1.91 (s, 3H, CH3), 2.09 (s, 3H, CH3), 2.40 (s, 3H, CH3), 7.39–8.10 (m, 8H, Ar–H), 8.22 (s, 1H, CH=N), 9.70 (s, 1H, NH), 10.71 (s, 1H, NH); MS m/z (%): 543 (M+, 17). Anal. Calcd for C23H20Cl2N8S2 (543.49): C, 50.83; H, 3.71; N, 20.62. Found: C, 50.62; H, 3.55; N, 20.46%.
Red solid, 70% yield, m.p. 172–174 °C (EtOH); IR (KBr): v 3422, 3192 (2NH), 3062, 2916 (C–H) cm−1; 1H-NMR (DMSO-d6): δ 1.91 (s, 3H, CH3), 2.10 (s, 3H, CH3), 2.57 (s, 3H, CH3), 7.43–8.18 (m, 9H, Ar–H), 8.61 (s, CH=N), 10.63 (brs, 1H, NH), 11.10 (brs, 1H, NH); 13C-NMR (DMSO-d6): δ 8.86, 16.91, 18.92 (CH3), 114.09, 118.11, 120.55, 126.03, 127.11, 129.30, 130.08, 131.84, 134.62, 136.11, 141.11, 143.07, 149.73, 154.89, 162.35, 168.51 (Ar–C and C=N); MS m/z (%): 520 (M+, 19). Anal. Calcd for C23H21N9O2S2 (519.60): C, 53.17; H, 4.07; N, 24.26. Found: C, 53.45; H, 3.85; N, 24.00%.
Orange solid, 75% yield, m.p. 232–234 °C (DMF); IR (KBr): v 3426, 3395, 3181 (3NH), 3050, 2924 (C–H), 1684 (C=O) cm−1; 1H-NMR (DMSO-d6): δ 1.82 (s, 3H, CH3), 2.44 (s, 3H, CH3), 6.92–8.11 (m, 10H, Ar–H), 8.52 (s, 1H, CH=N), 10.45 (brs, 1H, NH), 10.80 (brs, 1H, NH), 12.47 (brs, 1H, NH); 13C-NMR (DMSO-d6): δ 12.89, 18.50 (2CH3), 115.07, 119.85, 123.16, 126.11, 126.72, 128.98, 129.38, 129.57, 129.81, 131.31, 133.48, 143.10, 153.77, 157.93, 163.05 (Ar–C and C=N), 172.94 (C=O); MS m/z (%): 476 (M+, 13). Anal. Calcd for C22H20N8OS2 (476.58): C, 55.45; H, 4.23; N, 23.51. Found: C, 55.29; H, 3.98; N, 23.36%.
Orange solid, 69% yield, m.p. 200–202 °C (EtOH); IR (KBr): v 3421, 3280, 3183 (3NH), 3051, 2918 (C–H), 1685 (C=O) cm−1; 1H-NMR (DMSO-d6): δ 2.24 (s, 3H, CH3), 2.45 (s, 3H, CH3), 2.47 (s, 3H, CH3), 7.04–8.09 (m, 9H, Ar–H), 8.11 (s, 1H, CH=N), 10.38 (brs, 1H, NH), 10.78 (brs, 1H, NH), 12.24 (brs, 1H, NH); MS m/z (%): 490 (M+, 37). Anal. Calcd for C23H22N8OS2 (490.60): C, 56.31; H, 4.52; N, 22.84. Found: C, 56.20; H, 4.36; N, 22.65%.
Orange solid, 75% yield, m.p. 205–207 °C (DMF); IR (KBr): v 3427, 3255, 3178 (3NH), 3060, 2926 (C–H), 1684 (C=O) cm−1; 1H-NMR (DMSO-d6): δ 2.44 (s, 3H, CH3), 2.47 (s, 3H, CH3), 7.11–8.11 (m, 9H, Ar–H), 8.53 (s, 1H, CH=N), 10.55 (s, 1H, NH), 10.88 (brs, 1H, NH), 12.21 (brs, 1H, NH); 13C-NMR (DMSO-d6): δ 8.95, 18.87 (CH3), 115.84, 116.59, 125.58, 126.38, 127.07, 129.34, 129.51, 130.09, 134.68, 143.23, 147.14, 153.43, 158.64, 163.83, 167.54 (Ar–C and C=N), 173.93 (C=O); MS m/z (%): 513 (M++2, 5), 511 (M+, 17). Anal. Calcd for C22H19ClN8OS2 (511.02): C, 51.71; H, 3.75; N, 21.93. Found: C, 51.52; H, 3.64; N, 21.79%.
Orange solid, 70% yield, crystal (dioxane); m.p. 176–167 °C (EtOH); IR (KBr): v 3429, 3210, 3180 (3NH), 3050, 2920 (C–H) 1701 (C=O) cm−1; 1H-NMR (DMSO-d6): δ 1.91 (s, 3H, CH3), 2.08 (s, 3H, CH3), 7.38–8.43 (m, 9H, Ar–H), 8.54 (s, 1H, CH=N), 10.26 (brs, 1H, NH), 11.09 (brs, 1H, NH), 12.31 (brs, 1H, NH); 13C-NMR (DMSO-d6): δ 12.10, 19.45 (2CH3), 113.52, 116.62, 118.33, 120.94, 123.75, 125.55, 128.23, 129.99, 130.86, 134.39, 134.96, 147.92, 154.70, 158.09, 162.10 (Ar–C and C=N), 172.59 (C=O); MS m/z (%): 521 (M+, 20). Anal. Calcd for C22H19N9O3S2 (521.57): C, 50.66; H, 3.67; N, 24.17. Found: C, 50.95; H, 3.40; N, 23.77%.
Alternative Synthesis for 4a and 8a
A mixture of 2-(2-benzylidenehydrazinyl)-4-methylthiazole (1) (2.59 g, 10 mmol) and thiosemicarbazide (2) (0.91 g, 10 mmol) in EtOH (20 mL) containing catalytic amounts of HCl was refluxed for 2 h (monitored by TLC). The formed precipitate was isolated by filtration, washed with methanol, dried and recrystallized from EtOH to give thiosemicarbazone derivative 5. Yellow solid, 75% yield, m.p. 190–192 oC; IR (KBr): v 3412, 3245, 3151 (2NH and NH2), 3069, 2960 (C–a H) cm−1; 1H-NMR (DMSO-d6): δ 2.34 (s, 3H, CH3), 2.41 (s, 3H, CH3), 7.12–8.14 (m, 7H, Ar–H and NH2), 8.20 (s, 1H, CH=N), 10.36 (brs, 1H, NH), 11.41 (brs, 1H, NH); MS m/z (%): 332 (M+, 17). Anal. Calcd for C14H16N6S2 (332.44): C, 50.58; H, 4.85; N, 25.28. Found: C, 50.37; H, 4.65; N, 25.07%.
Reaction of 5 with Hydrazonoyl Chlorides 3a and 7a
A mixture of 2-(1-(2-(2-benzylidenehydrazinyl)-4-methylthiazol-5-yl)ethylidene)hydrazine- carbothioamide (5) (0.332 g, 1 mmol) and the appropriate 2-oxo-N’-phenylpropanehydrazonoyl chloride (3a) or ethyl 2-chloro-2-(2-phenylhydrazono)acetate (7a) (1 mmol) in dioxane (20 mL) containing a catalytic amount of TEA was refluxed for 4 h (monitored by TLC). The formed precipitate was isolated by filtration, washed with methanol, dried and recrystallized from DMF to give a product proved to be identical in all respects (m.p., mixed m.p. and IR spectra) with the products 4a or 8a obtained from reaction of 1 + 2 + 3a or 1 +2 + 7a, respectively.
Synthesis of Thiazole Derivatives 11a–e
A mixture of 2-(2-benzylidenehydrazinyl)-4-methylthiazole (1) (0.259 g, 1 mmol), thiosemicarbazide (2) (0.091 g, 1 mmol) and the appropriate phenacyl bromides 10a–e (1 mmol) in EtOH (20 mL) was refluxed for 3–5 h, allowed to cool and the solid formed was filtered off, washed with EtOH, dried and recrystallized from DMF to give the corresponding thiazoles 11a–e. The products 11a–e together with their physical constants are listed below.
Green solid, 68% yield, m.p. 250–252 °C; IR (KBr): v 3429, 3220 (2NH), 3063, 2920 (C–H) cm−1; 1H-NMR (DMSO-d6): δ 1.91 (s, 3H, CH3), 2.08 (s, 3H, CH3), 2.42 (s, 3H, CH3), 7.22–8.05 (m, 9H, Ar–H), 8.13 (s, 1H, thiazole-H5), 8.27 (s, 1H, CH=N), 10.11 (brs, 1H, NH), 10.90 (brs, 1H, NH); 13C-NMR (DMSO-d6): δ 14.45, 18.41, 24.09 (3CH3), 115.75, 116.59, 120.79, 123.22, 125.03, 127.50, 129.36, 133.29, 134.48, 137.93, 142.15, 143.10, 152.10, 156.84, 160.09, 162.64 (Ar–C and C=N); MS m/z (%): 446 (M+, 4). Anal. Calcd for C23H22N6S2 (446.59): C, 61.86; H, 4.97; N, 18.82. Found: C, 61.69; H, 4.75; N, 18.68%.
Green solid, 69% yield, m.p. 203–205 °C; IR (KBr): v 3430, 3330 (2NH), 3110, 2938 (C–H) cm−1; 1H-NMR (DMSO-d6): δ 1.92 (s, 3H, CH3), 2.46 (s, 3H, CH3), 3.76 (s, 3H, OCH3), 7.32–7.79 (m, 10H, Ar–H and thiazole-H5), 8.18 (s, 1H, CH=N), 10.14 (brs, 1H, NH), 11.00 (brs, 1H, NH); MS m/z (%): 462 (M+, 11). Anal. Calcd for C23H22N6OS2 (462.59): C, 59.72; H, 4.79; N, 18.17. Found: C, 59.91; H, 4.58; N, 18.05%.
Green solid, 73% yield, m.p. 240–242 °C; IR (KBr): v 3426, 3260 (2NH), 3030, 2938 (C–H) cm−1; 1H-NMR (DMSO-d6): δ 1.93 (s, 3H, CH3), 2.35 (s, 3H, CH3), 7.39–7.86 (m, 10H, Ar–H and thiazole-H5), 8.25 (s, 1H, CH=N), 10.05 (brs, 1H, NH),10.91 (br s, 1H, NH); MS m/z (%): 467 (M+, 14). Anal. Calcd for C22H19ClN6S2 (467.01): C, 56.58; H, 4.10; N, 18.00. Found: C, 56.88; H, 3.85; N, 17.70%.
Green solid, 71% yield, m.p. 257–259 °C; IR (KBr): v 3429, 3384 (2NH), 3072, 2918 (C–H) cm−1; 1H-NMR (DMSO-d6): δ 2.24 (s, 3H, CH3), 2.27 (s, 3H, CH3), 7.38–8.14 (m, 10H, Ar–-H and thiazole-H5), 8.35 (s, 1H, CH=N), 10.03 (brs, 1H, NH), 10.86 (brs, 1H, NH); 13CNMR (DMSO-d6): δ 13.7, 18.8 (2CH3), 112.6, 119.6, 126.1, 127.8, 128.4, 129.2, 129.9, 130.6, 133.3, 134.8, 136.2, 138.9, 140.8, 142.0, 145.0, 151.4; MS m/z (%): 513 (M++2, 5), 511 (M+, 7). Anal. Calcd for C22H19BrN6S2 (511.46): C, 51.66; H, 3.74; N, 16.43. Found: C, 51.35; H, 3.59; N, 16.31%.
Green solid, 71% yield, m.p. 225–227 °C; IR (KBr): v 3429, 3318 (2NH), 3115, 2920 (C–H) cm−1; 1H-NMR (DMSO-d6): δ 1.91 (s, 3H, CH3), 2.41 (s, 3H, CH3), 7.41–7.72 (m, 9H, Ar–H), 8.06 (s, 1H, thiazole-H5), 8.36 (s, 1H, CH=N), 10.21 (brs, 1H, NH), 11.09 (brs, 1H, NH); 13C-NMR (DMSO-d6): δ 14.06, 18.98 (2CH3), 124.64, 126.80, 129.34, 132.16, 133.18, 134.78, 135.23, 137.82, 141.29, 143.89, 150.70, 151.50, 157.23, 158.76, 164.69, 166.94 (Ar–C and C=N); MS m/z (%): 477 (M+, 10). Anal. Calcd for C22H19N7O2S2 (477.56): C, 55.33; H, 4.01; N, 20.53. Found: C, 55.14; H, 3.94; N, 20.45%.
Alternative Synthesis for 11a
A mixture of thiosemicarbazone derivative 5 (0.332 g, 1 mmol) and 2-bromo-1-(p-tolyl)ethanone (10a) (0.213 g, 1 mmol) in EtOH (20 mL) was refluxed for 3 h. The formed precipitate was isolated by filtration, washed with MeOH, dried and recrystallized from DMF to give 11a.
Anticancer Activity
The cytotoxic potential of the newly synthesized compounds was examined against HCT-116, HT-29 and HepG2 cells using the MTT assay after 24 h of incubation.34 For more details, see the Supplementary Materials.
Mammalian cell line: HCT-116, HT-29 and HepG2 cells were obtained from VACSERA Tissue Culture Unit, Cairo, Egypt.
Mechanistic Study on the Antitumor Activity
The analysis of the apoptotic markers Bax and caspase-3 levels as well as the anti-apoptotic marker Bcl-2 levels were assessed as reported earlier35 for the most potent synthesized compounds on HCT-116 cells using ELISA colorimetric kits according to the manufacturer’s instructions.
Results and Discussion
Chemistry
2-(2-Benzylidenehydrazinyl)-4-methylthiazole (1),36 thiosemicarbazide (2) and the appropriate N-aryl-2-oxopropanehydrazonoyl chlorides (3a–e)37 were allowed to react in a one-pot three-component reaction in refluxing dioxane containing a catalytic amount of TEA to afford the arylazothiazoles 4a–e, respectively (Scheme 1). The structures of these products were established on the basis of elemental analyses as well as spectral data. The 1H-NMR spectra of 4a–e showed generally three singlets at δ ~ 1.91, 2.10 and 2.29 ppm due to the three CH3 groups, a multiplet at δ ~ 6.97–8.10 ppm assignable to the aromatic protons, a singlet (1H) at δ ~ 8.69 ppm assignable to the methine proton, beside two broad singlets (D2O exchangeable) at δ ~ 10.58 and 11.19 ppm due to the two NH protons. IR spectra of the compounds 3a–e showed two absorption bands due to the two NH groups at v ~ 3425 and3220 cm−1. Moreover, the mass spectrum of 4a revealed a molecular ion peak at m/z = 474 which is consistent with its molecular weight.
Compound 4a was alternatively synthesized by reacting 2-(1-(2-(2-benzylidenehydrazinyl)-4-methylthiazol-5-yl)ethylidene)hydrazine-carbothioamide (5) (prepared separately from reaction of acetylthiazole 1 and thiosemicarbazide 2 under reflux in ethanol in the presence of HCl drops for 1 h) with 2-oxo-N’-phenylpropanehydrazonoyl chloride (3a) in refluxing dioxane/TEA (Scheme 1). The obtained product was found to be identical with 4a in all respects (TLC, m.p. and IR spectrum) which affords further evidence to all structures 4a–e.
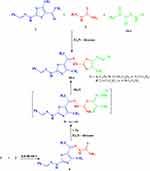 |
Scheme 1 Synthesis of arylazothiazoles 4a–e. |
In a similar way, acetylthiazole 1 reacted with thiosemicarbazide 2 and the appropriate hydrazonoyl chlorides 7a–d34 in a one-pot three-component reaction in refluxing dioxane/TEA to afford the respective arylhydrazothiazolones 8a–d (Scheme 2). The structures of compounds 8a–d were confirmed by 1H-NMR, 13C-NMR, IR and MS. For example, the IR spectra of product 7a showed the exhibited presence of stretching bands for 3NH and C=O groups at the normal wave number v. The mass spectra of the products 8a–d revealed in each case a molecular ion peak m/z at the expected molecular weight calculated for each compound (see Experimental).
In the 1H-NMR spectrum of 7a, six singlet signals were observed (δ 1.82, 2.44, 8.52, 10.45, 10.80, 12.47 ppm) for two CH3 groups, CH=N proton and the three NH groups, respectively, in addition to the expected aromatic protons.
To support this mechanism, compound 5 was allowed to react with ethyl 2-chloro-2-(2-phenylhydrazono)acetate (7a) in refluxing dioxane/TEA to afford a product identically similar to 8a (Scheme 2).
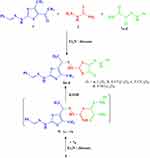 |
Scheme 2 Synthesis of arylhydrazothiazoles 8a–d. |
The chemical reactivity of acetylthiazole 1 toward thiosemicarbazide 2 and a variety of phenacyl bromides 10a–e was also studied aiming to synthesize another series of novel thiazole derivatives. Thus, reaction of compound 1 with 2 and p-substituted phenacyl bromide derivatives 10a–e in EtOH under reflux led to the formation of products 11a–e (Scheme 3). Analytical and spectral data of these reaction products are in complete agreement with their proposed structures. The IR spectra of the products showed two NH absorption bands in 11a at υ = 3429 and 3220 cm−1. The 1H-NMR spectra of compounds 11a–d revealed the characteristic three singlet signals for the 3CH3 at δ 1.91, 2.08 and2.42 ppm, a multiplet group at δ 7.22–8.05 ppm, a singlet signal at δ 8.27 ppm due to thiazole-H5, a singlet signal at δ 8.27 ppm due to CH=N and also two broad singlet signals at δ 10.11 and 10.90 ppm due to 2NH groups.
The structure assigned for products 11 was further evidenced via the alternative method. Thus, reaction of 5 with 2-bromo-1-(p-tolyl)ethanone (10a) in EtOH afforded a product identical in all respects (m.p., mixed m.p. and IR spectra) with compound 11a obtained from reaction of 1 + 2 + 10a (Scheme 3).
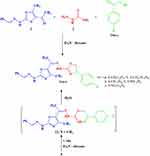 |
Scheme 3 Synthesis of thiazoles 11a–e. |
Anticancer Activity
The pharmacological activities of the synthesized products 4a–e, 5, 8a–d and 11a–e were investigated for their human colon carcinoma cell line in comparison with harmine and cisplatin as reference drugs using the colorimetric MTT assay. The relation between drug concentration and surviving cells is plotted to get the survival curve. The 50% inhibitory concentration (IC50) was obtained and the anti-proliferative activity was expressed as the mean IC50 of 3 independent experiments (μM) ± standard deviation from three replicates.
The outline data presented in Tables 1–3 show that the anticancer activity of the tested compounds depends on their structures and the concentration. The descending order of in vitro inhibitory activity of the tested compounds toward the HCT-116 was as follows: 8c > 4d > 4c > 11c > 11d >11b >11a > 4b > 4a > 8a > 8b > 4e > 8d > 11e > 5. Compounds 4c, 4d and 8c were the most active (IC50 value of 3.80 ± 0.8, 3.65 ± 0.9 and 3.16 ± 0.9 μM, respectively) against the HCT-116 cell line compared with harmine reference drug with IC50 value of 2.40 ± 0.12 μM as well as cisplatin (IC50 = 5.18± 0.94 μM). Compounds 11c, 11d, 11b, 11a, 4b, 4a, 8a, 8b and 4e have moderate inhibitory activity (IC50 = 14.50–31.50 μM) while the other measured compounds 8d, 11e and 5 were inactive against HCT-116 (IC50 value > 48.20 μM).
 |
Table 1 The Anticancer Activity of Compounds 4a–e, 5, 8a–d and 11a–e Against Colon Carcinoma (HCT-116) Cell Line Expressed as IC50 Values (μM) ± Standard Deviation from Three Replicates |
 |
Table 2 The Anticancer Activity of the Synthesized Compounds Against HepG2 Cell Line Expressed as IC50 Values (μM) ± Standard Deviation from Three Replicates |
 |
Table 3 The Anticancer Activity of Compounds 4c, 4d and 8c Against HT-29 Cell Line Expressed as IC50 Values (μM) ± Standard Deviation from Three Replicates |
Moreover, the activity of the most active compounds (4c, 4d and 8c) was also performed on another human colorectal cancer cell line like HT-29, which is a more resistant cell line, to explore the efficiency of the synthesized compounds (Table 3). Interestingly, compound 8c exerted higher potency (IC50 = 3.47± 0.79 μM) compared with harmine and cisplatin reference drugs with IC50 values of 4.59 and 11.68 μM, respectively. Also, compounds 4c and 4d showed promising IC50 values of 7.24 and 4.13 μM, respectively.
On the other hand, the order of inhibitory activity of the tested compounds toward the hepatocellular carcinoma (HepG2) cells was as follows: 4d > 4c > 8c > 11c > 11b > 11d > 8b > 4b >11a > 4a > 8a > 4e > 8d > 11e > 5 as indicated in Table 2. Compounds 4d, 4c, 8c and 11c were the most active (IC50 values of 2.31, 2.94, 4.57 and 9.86 μM, respectively) against the HepG2 cell line compared with harmine and cisplatin reference drugs with IC50 values of 2.54 and 9.41 μM.
To explore the mechanism of the anticancer action exerted by most active compounds (4c, 4d and 8c) inside the HCT-116 cancer cell line, apoptotic cell marker analysis was also investigated in this study. The Bcl-2 family, the best-characterized protein family involved in the regulation of apoptosis, consists of anti-apoptotic and pro-apoptotic members that modulate this programmed process.38 The anti-apoptotic members, such as Bcl-2, attenuate apoptosis either by preventing the release of mitochondrial apoptogenic factors like cytochrome C into the cytoplasm or by sequestering pro-forms of the caspases. On the other hand, pro-apoptotic members of the Bcl-2 family, such as Bax, trigger the release of caspases.5 Caspase-3 is a cysteine-containing aspartic acid-specific protease that provides crucial roles in cell regulatory systems directing cell death pathways.39
When HCT-116 cells were treated with compounds 4c, 4d and 8c there was a significant increase in the levels of the pro-apoptotic molecule Bax by 1.5, 1.9 and 2.28 folds, respectively, compared to control (Figure 1A). In contrast, exposure of HCT-116 cells to compounds 4c, 4d and 8c resulted in a significant decrease in the protein expression levels of the anti-apoptotic protein Bcl-2 by about 11.5, 25.6 and 39.7%, respectively, compared to control (Figure 1B). Furthermore, the Bax/Bcl2 ratio was calculated to give more profound insight into the pro-apoptotic activity. Analyzing the results revealed that compounds 4c, 4d and 8c increased the Bax/Bcl-2 ratio about 2, 2.5 and 4 folds in comparison to the control. These results agreed with the previous reports where the bax/bcl-2 ratio is the major influential value to determine cell susceptibility to apoptosis.35
However, treatment of HCT-116 cells by compounds 4c, 4d and 8c resulted in a significant elevation in the protein expression levels of active caspase-3 by about 2.59, 3.75 and 5 folds, respectively, compared to the non-treated HCT-116 control (Figure 1C).
 |
Figure 2 The anticancer activity of tested compounds (ordered) against HCT-116, HT-29 and HepG2 cell lines. |
The Anticancer Activity of Tested Compounds Against HCT-116 Cell Lines
For arylazothiazoles 4a–e: thiazole 4d(has two Cl atoms, electron-withdrawing group which increases activity) > 4c(has one Cl atom) > 4b (has CH3 group, electron-donating group which decreases activity). For arylhydrazothiazolones 8a–d: thiazole 4c(has Cl group, electron-withdrawing group which increases activity) > 4b (has CH3 group, electron-donating group that decreases activity). For arylthiazoles 11a–e: thiazole 4c and 4d (have Cl or Br atoms, electron-withdrawing groups which increases activity) > 4b and 4a (have OCH3 and CH3 groups, electron-donating group decreases activity).
Moreover, chlorophenyl-hydrazothiazolone 8c (IC50 = 3.16 ± 0.90 μM) has greater activity than chlorophenyl-azothiazole 4c (IC50 = 3.80 ± 0.80 μM) and than chlorophenyl-thiazole 11c.
Generally, in the three thiazole series 4a–e, 8a–d and 11a–e, the Cl atom increases activity while the NO2 group decreases the activity (Figure 2).
Conclusion
In our present work, we herein present an efficient synthesis of novel 1-(2-(2-benzylidenehydrazinyl)-4-methylthiazol-5-yl)ethanone. The latter compound was used as a building block for constructing novel three series of 5-(1-(2-(thiazol-2-yl)hydrazono)ethyl) thiazole derivatives in a one-pot three-component reaction. The structures of the newly synthesized compounds were established on the basis of spectroscopic evidence and their synthesis by alternative methods. The in vitro growth inhibitory activity of the synthesized compounds against three tumor cells (HCT-116, HT-29 and HepG2) was investigated in comparison with harmine and cisplatin reference drugs using an MTT assay and the results revealed promising activities of three compounds. The study also suggested that the mechanism of the anticancer action exerted by the most active compounds (4c, 4d and 8c) inside HCT-116 cells was apoptosis through the Bcl-2 family.
Acknowledgment
The financial support by the Deanship of Scientific Research (Project Number 1811008) King Faisal University, Saudi Arabia is gratefully acknowledged.
Author Contributions
All authors contributed to data analysis, drafting and revising the article, gave final approval of the version to be published, and agree to be accountable for all aspects of the work.
Disclosure
The authors declare that they have no conflicts of interest regarding this paper.
References
1. Torre LA, Bray F, Siegel RL, Ferlay J. Lortet‑Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65:87–108.
2. Coghlin C, Murray GI. Biomarkers of colorectal cancer: recent advances and future challenges. Proteomics Clin Appl. 2015;9:
3. Meier P, Finch A, Evan G. Apoptosis in development. Nature. 2000;407:796–801. doi:10.1038/35037734
4. Willis SN, Adams JM. Life in the balance: how BH3-only proteins induce apoptosis. Curr Opin Cell Biol. 2005;17:617–625. doi:10.1016/j.ceb.2005.10.001
5. Youle RJ, Strasser A. The BCL-2 protein family: opposing activities that mediate cell death. Nat Rev Mol Cell Biol. 2008;9(1):47–59. doi:10.1038/nrm2308
6. Dix MM, Simon GM, Cravatt BF. Global mapping of the topography and magnitude of proteolytic events in apoptosis. Cell. 2008;134(4):679–691. doi:10.1016/j.cell.2008.06.038
7. Kotschy A, Szlavik Z, Murray J, et al. The MCL1 inhibitor S63845 is tolerable and effective in diverse cancer models. Nature. 2016;538(7626):477–482. doi:10.1038/nature19830
8. da Silva EB, Silva DAO, Oliveira AR, et al. Desing and synthesis of potent anti-Trypanosoma cruzi agents new thiazoles derivatives which induce apoptotic parasite death. Eur J Med Chem. 2017;130:39–50. doi:10.1016/j.ejmech.2017.02.026
9. Espíndola JWP, de Oliveira Cardoso MV, de Oliveira Filho GB, et al. Synthesis and structure–activity relationship study of a new series of antiparasitic aryloxyl thiosemicarbazones inhibiting Trypanosoma Cruzi Cruzain. Eur J Med Chem. 2015;101:818–835. doi:10.1016/j.ejmech.2015.06.048
10. Netalkar PP, Netalkar SP, Revankar VK. Transition metal complexes of thiosemicarbazone: synthesis, structures and in vitro antimicrobial studies. Polyhedron. 2015;100:215–222. doi:10.1016/j.poly.2015.07.075
11. Hu WX, Zhou W, Xia CN, Wen X. Synthesis and anticancer activity of thiosemicarbazones. Bioorg Med Chem Lett. 2006;16:2213–2218. doi:10.1016/j.bmcl.2006.01.048
12. Wang Y, Wang Z, Kuang H, et al. Synthesis and antitumor activity of 2‐isocamphanyl thiosemicarbazone derivatives via ROS‐enhanced mitochondria damage. Chem Biol Drug Des. 2019. doi:10.1111/cbdd.13492
13. Sujatha K, Vedula RR. Novel one-pot expeditious synthesis of 2, 4-disubstituted thiazoles through a three-component reaction under solvent free conditions. Synth Commun. 2018;48:302–308. doi:10.1080/00397911.2017.1399422
14. Abdalla MA, Gomha SM, Abdelaziz M, Serag N. Synthesis and antiviral evaluation of some novel thiazoles and 1,3-thiazines substituted with pyrazole moiety against rabies virus. Turk J Chem. 2016;40:441–453. doi:10.3906/kim-1506-13
15. S M G, K D K. A convenient ultrasound-promoted synthesis and cytotoxic activity of some new thiazole derivatives bearing a coumarin nucleus. Molecules. 2012;17:9335–9347. doi:10.3390/molecules17089335
16. Nayak S, Gaonkar SL. A Review on recent synthetic strategies and pharmacological importance of 1, 3-thiazole derivatives. Mini Rev Med Chem. 2019;19:215–238. doi:10.2174/1389557518666180816112151
17. Kumar S, Aggarwal R. Thiazole: a privileged motif in marine natural products. Mini Rev Org Chem. 2019;16:26–34. doi:10.2174/1570193X15666180412152743
18. Gomha S, Edrees M, Altalbawy F. Synthesis and characterization of some new bis-pyrazolyl-thiazoles incorporating the thiophene moiety as potent anti-tumor agents. Int J Mol Sci. 2016;17:1499. doi:10.3390/ijms17091499
19. Gomha SM, Salah TA, Abdelhamid AO. Synthesis, characterization, and pharmacological evaluation of some novel thiadiazoles and thiazoles incorporating pyrazole moiety as anticancer agents. Monatsh Chem. 2015;146:149–158. doi:10.1007/s00706-014-1303-9
20. Dos Santos Silva TD, Bomfim LM, da Cruz Rodrigues ACB, et al. Anti-liver cancer activity in vitro and in vivo induced by 2-pyridyl 2, 3-thiazole derivatives. Toxicol Appl Pharmacol. 2017;329:212–223. doi:10.1016/j.taap.2017.06.003
21. Morigi R, Locatelli A, Leoni A, Rambaldi M. Recent patents on thiazole derivatives endowed with antitumor activity. Recent Pat Anticancer Drug Discov. 2015;10:280–297. doi:10.2174/1574892810666150708110432
22. Bremner WS, Organ MG. Multicomponent reactions to form heterocycles by microwave-assisted continuous flow organic synthesis. J Comb Chem. 2007;9:14–16. doi:10.1021/cc060130p
23. Murata T, Murai M, Ikeda Y, Miki K, Ohe K. Pd-and Cu-catalyzed one-pot multicomponent synthesis of hetero α, α′-dimers of heterocycles. Org Lett. 2012;14:2296–2299. doi:10.1021/ol300718x
24. Abdelrazek FM, Gomha SM, Shaaban ME, et al. One-pot three-component synthesis and molecular docking of some novel 2-thiazolyl pyridines as potent antimicrobial agents. Mini Rev Med Chem. 2019;19:527–538. doi:10.2174/1389557518666181019124104
25. Gomha SM, Muhammad ZA, Abdel‐aziz MR, Abdel‐aziz HM, Gaber HM, Elaasser MM. One‐pot synthesis of new thiadiazolyl‐pyridines as anticancer and antioxidant agents. J Heterocycl Chem. 2018;55:530–536. doi:10.1002/jhet.3088
26. Gomha SM, Edrees MM, Faty RA, Muhammad ZA, Mabkhot YN. Microwave-assisted one pot three-component synthesis of some novel pyrazole scaffolds as potent anticancer agents. Chem Cent J. 2017;11:37. doi:10.1186/s13065-017-0266-4
27. Gomha S, Kheder N, Abdelhamid A, Mabkhot Y. One pot single step synthesis and biological evaluation of some novel bis(1,3,4-thiadiazole) derivatives as potential cytotoxic agents. Molecules. 2016;21:1532. doi:10.3390/molecules21111532
28. Gomha SM. A facile one-pot synthesis of 6,7,8,9-tetrahydrobenzo[4,5]thieno[2,3-d]-1,2,4- triazolo[4,5-a]pyrimidin-5-ones. Monatsh Chem. 2009;140:213–220. doi:10.1007/s00706-008-0060-z
29. Gomha SM, Abdelrazek FM. A Facile three component-one-pot synthesis of some novel tri-cyclic hetero-ring systems. J Heterocycl Chem. 2016;53:1892–1896. doi:10.1002/jhet.2503
30. Gomha SM, Riyadh SM. Synthesis of triazolo[4,3-b][1,2,4,5]tetrazines and triazolo[3,4-b][1,3,4]thiadiazines using chitosan as ecofriendly catalyst under microwave irradiation. Arkivoc. 2009;xi:58–68.
31. Gomha SM, Abdalla MA, Abdelaziz M, Serag N. Eco-friendly one-pot synthesis and antiviral evaluation of pyrazolyl chalcones and pyrazolines of medicinal interest. Turk J Chem. 2016;40:484–498. doi:10.3906/kim-1510-25
32. Abbas IM, Riyadh SM, Abdallah MA, Gomha SM. A novel route to tetracyclic fused tetrazines and thiadiazines. J Heterocycl Chem. 2006;43:935–942. doi:10.1002/jhet.5570430419
33. Abbas IM, Gomha SM, Elaasser MM, Bauomi MA. Synthesis and biological evaluation of new pyridines containing imidazole moiety as antimicrobial and anticancer agents. Turk J Chem. 2015;39:334–346. doi:10.3906/kim-1410-25
34. Gomha SM, Riyadh SM, Mahmmoud EA, Elaasser MM. Synthesis and anticancer activity of arylazothiazoles and 1,3,4-thiadiazoles using chitosan-grafted-poly(4-vinylpyridine) as a novel copolymer basic catalyst. Chem Heterocycl Compd. 2015;51:1030–1038. doi:10.1007/s10593-016-1815-9
35. Eldehna WM, Abo-Ashour MF, Nocentini A, et al. Novel 4/3-((4-oxo-5-(2-oxoindolin-3-ylidene) thiazolidin-2-ylidene) amino) benzene sulfonamides: synthesis, carbonic anhydrase inhibitory activity, anticancer activity and molecular modelling studies. Eur J Med Chem. 2017;139:250–262. doi:10.1016/j.ejmech.2017.07.073
36. Maccioni E, Cardia MC, Distinto S, Bonsignore L, De Logu A. An investigation of the biological effect of structural modifications of isothiosemicarbazones and their cyclic analogues. Il Farmaco. 2003;58:951–959. doi:10.1016/S0014-827X(03)00154-X
37. Shawali AS, Gomha SM. A new entry for short and regioselective synthesis of [1, 2, 4] triazolo [4, 3-b][1, 2, 4]-triazin-7(1H)-ones. J Prakt Chemie Chem. 2000;342:599–604. doi:10.1002/1521-3897(200006)342:6<599::AID-PRAC599>3.0.CO;2-7
38. Tsujimoto Y. Cell death regulation by the Bcl-2 protein family in the mitochondria. J Cell Physiol. 2003;195:158–167. doi:10.1002/jcp.10254
39. Wu Z-R, Liu J, Li J-Y, et al. Synthesis and biological evaluation of hydroxycinnamic acid hydrazide derivatives as inducer of caspase-3. Eur J Med Chem. 2014;85:78–783. doi:10.1016/j.ejmech.2014.08.040
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

