Back to Journals » Infection and Drug Resistance » Volume 14
One Health Approach of Melioidosis and Gastrointestinal Parasitic Infections from Macaca fascicularis to Human at Kosumpee Forest Park, Maha Sarakham, Thailand
Authors Damrongsukij P, Doemlim P, Kusolsongkhrokul R, Tanee T, Petcharat P, Siriporn B , Piratae S, Pumipuntu N
Received 9 April 2021
Accepted for publication 28 May 2021
Published 15 June 2021 Volume 2021:14 Pages 2213—2223
DOI https://doi.org/10.2147/IDR.S299797
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Sahil Khanna
Panitporn Damrongsukij, 1 Papichchaya Doemlim, 1 Ratchanon Kusolsongkhrokul, 1 Tawatchai Tanee, 2 Pitchakorn Petcharat, 3 Bunnada Siriporn, 4, 5 Supawadee Piratae, 1, 5 Natapol Pumipuntu 1, 5
1One Health Research Unit, Faculty of Veterinary Sciences, Mahasarakham University, Maha Sarakham, 44000, Thailand; 2Faculty of Environment and Resource Studies, Mahasarakham University, Maha Sarakham, 44000, Thailand; 3Farm Animal and Wildlife Clinic, Pak Chong Animal Hospital, Nakhon Ratchasima, 30130, Thailand; 4Stress and Oxidative Stress Research Unit, Faculty of Veterinary Sciences, Mahasarakham University, Maha Sarakham, 44000, Thailand; 5Faculty of Veterinary Sciences, Mahasarakham University, Maha Sarakham, 44000, Thailand
Correspondence: Natapol Pumipuntu
One Health Research Unit, Faculty of Veterinary Sciences, Mahasarakham University, Maha Sarakham, 44000, Thailand
Email [email protected]
Background and Objectives: Gastrointestinal parasitic and melioidosis infections are major causes of morbidity and mortality from infectious disease in rural areas, especially in northeastern Thailand. Both diseases are zoonotic giving rise to health problems in both long-tailed macaques and in humans. In Thailand, macaques have adapted to live and share space with humans and can spread some zoonoses to humans. Therefore, this research aimed to measure the prevalence of gastrointestinal parasitic infections and melioidosis in long-tailed macaques at Kosumpee Forest Park and measure associated risk factors of their diseases among people in this area.
Methods: This study was conducted at Kosumpee Forest Park, Maha Sarakham, Thailand. Twenty-eight blood samples and 135 fecal samples were collected from free-ranging long-tailed macaques. Blood samples were tested by indirect hemagglutination test and fecal samples were analyzed by formalin–ethyl acetate concentration technique. A cross-sectional study was conducted among 350 respondents who were involved with the Forest Park using a multi-stage stratified random sampling method and performed to measure knowledge, attitude, and practice toward the zoonoses among the respondents.
Results: It was found that seroprevalence of melioidosis was 57.1% from macaque samples. The prevalence of gastrointestinal parasites infection was 35.11% from fecces samples, including Strongyloides spp. (15.27%), Trichuris spp. (22.9%), hookworm (4.58%) and Ascarid spp. (1.53%). KAP study indicated that the level of knowledge related to melioidosis and gastrointestinal parasites of people in the area was very low and moderate, respectively. The attitude of respondents who were aware of the diseases was at a moderate level for melioidosis and a high level for parasitic infection.
Conclusion: The study therefore emphasizes the importance of one health approach for diagnosis, surveillance and management of zoonotic diseases to promote the development of hygiene measures and to educate people in the community around Kosumpee Forest Park.
Keywords: long-tailed macaques, prevalence, melioidosis, gastrointestinal parasites, risk factors
Corrigendum for this paper has been published
Introduction
The long-tailed macaque (Macaca fascicularis) is one of the most geographically widely distributed primates. They occur mostly in Africa and the mainland or islands of Southeast Asia. The macaques have a variety of habitats such as rainforest along rivers and mangroves.1,2 The macaques are the most common monkey in Thailand. However, they are threatened due to inbreeding and loss of habitat, from expansion of residential areas and encroaching deforestation for agriculture or communication which destroys natural habitats as a result. The macaques have adapted to live and share space with humans.3 According to some religious beliefs, monkeys are believed to represent gods or sacred things and lived in this area before humans, so the macaques are found in many religious sites. At such places, people in the area have the patience to coexist with the monkeys and take measures to protect them in temples or sacred sites.4 Due to macaques and people sharing the same living space, there is sometimes conflict between them, such as damage of agricultural areas, property and housing, including local people or tourists who may be harmed or contract disease caused by macaques.
At present, 75% of the emerging infectious diseases are caused by zoonotic organisms.5 Zoonotic diseases such as gastrointestinal (GI) parasitic infection from macaques to humans are important in Thailand and this remains a current health problem, especially in the Northeast, where rural areas lack good sanitation and hygiene practices. In both humans and animals, the infections can be obtained through eating food contaminated via the fecal-oral route, such as by eating vegetables grown with manure, eating meat that has been contaminated with bacteria and drinking water from natural sources that are not sterile.6 Symptoms of acute GI parasitic infections may include ground itch, cutaneous larva migrans, bronchitis and pneumonia. Adult parasites may cause gastrointestinal symptoms such as diarrhea, nausea, vomiting, loss of appetite and chronic parasitic infections cause anorexia, reducing intestinal absorption of nutrients leading to lack of essential nutrients or anemia from blood loss in the intestines.7 Clinical signs in macaques are similar to those in humans with anemia, tissue damage, abortion, malnutrition and death if symptoms are severe.8
Another important disease in the northeastern Thailand is melioidosis. This is caused by the Gram-negative bacteria, Burkholderia pseudomallei that can infect both humans and animals. Burkholderia pseudomallei is found in the soil. It is contagious through oral or aerosol routes and wounds contaminated with soil or water polluted with bacteria. They are often found in farmers because they have natural contact with soil and water. It is often not communicated from person to person or animal to person but it can arise bites from infected animal or injury causing infection through the wound. In addition, there are risk factors that that increase the likelihood of developing the disease in humans such as diabetes and alcoholism.9 Approximately 40–60% of people have systemic symptoms such as septicemia, pericarditis and respiratory tract symptoms such as pulmonary edema and lung abscess. About 10–33% of symptoms are in the gastrointestinal tract, liver and spleen abscesses. Urinary tract infection occurs in 14–28% causing liver abscess, acute nephritis and various symptoms such as purulent abscess in soft tissue, and arthritis.10 Clinical signs in monkeys are similar to humans, such as fever, dyspnea, neutrophilia, lung abscess and bone marrow inflammation.9
Both these zoonotic pathogens can survive as free-living organisms in environmental niches such as water or soil and they can be transmitted to susceptible hosts including human and animal by direct and indirect contact. They are important as endemic zoonoses in the tropical area especially in the Northeast and rural areas of Thailand due to risky behaviors associated with daily activities of the people.11,12 Effective control, prevention or eradication of zoonotic diseases, nowadays uses a one health approach, which is a synergistic approach, with experts from all sectors involved with human health, animal health and environmental health (the One Health Triads). This approach can solve problems by designing long-term preventive measures to reduce contagious zoonotic diseases in the future.13,14 We studied GI parasitic infections and melioidosis and measured their prevalence in long-tailed macaques at Kosumpee Forest Park. The macaques are known wildlife reservoirs of these infections that closely interact with humans in Thailand. In addition, this study analyzed the risk factors of people in that community as the human aspect, which is considered important according to the One Health concept. The assessment of knowledge, attitude, and practice (KAP) is crucial to the prevention of GI parasites and melioidosis infection. As the prevention and control of these diseases is challenging, human behavioral approaches with proper understanding of social background and health beliefs have become a crucial measure in decreasing morbidity and mortality due to these zoonotic diseases.
Materials and Methods
Ethics Statement
All procedures were approved by Mahasarakham University for human and animal subjects research (protocol numbers 037/2016 and 0009/2016, respectively). The questionnaire participants provided informed consent, and that this was conducted in accordance with the Declaration of Helsinki.
Fecal Sample Collection
Fecal sample collection was performed without contact with animals. Each macaques at Kosumpee Forest Park, Maha Sarakham, Northeastern Thailand (Figure 1) was assessed individually by the following method15 and using completely randomized design (CRD) to obtain representative samples from the population. Fresh stool samples were collected using gloves from 135 macaque fecal samples. Approximately 2 g of each sample was collected into a clean plastic bag, stored at 4°C, and transported to the laboratory for GI parasites detection.
 |
Figure 1 Geographical location of study area; Kosumpee Forest Park in Kosum Phisai district, Maha Sarakham province, Northeast Thailand (from Google Earth). |
Blood Sample Collection
The investigation of melioidosis seroprevalence in the wild animals was determined via antibody titer of macaques by using blood collection. Twenty-eight macaques were temporarily caught in a soft mesh cage (4x4x1.5 m) and Tiletamine-zolazepam was administered for sedation the monkeys through the intramuscular route by the blowpipe as show in Figure 2. The blood samples were collected from the femoral vein. Samples were collected aseptically using sterile a 5 mL syringe. All protocols were controlled by wildlife veterinary specialists. All blood samples were stored at 4°C and taken to the Veterinary Public Health laboratory, Faculty of Veterinary Sciences, Mahasarakham University to separate and collect serum. Each serum sample was separated by centrifugation of blood at 3000 g for 10 minutes at room temperature. The serum samples were transferred into 1.5 mL sterile micro tube (Eppendorf) and were kept at −20 °C until further testing.
 |
Figure 2 Long-tailed macaques were sedated by the blowpipe. |
Indirect Hemagglutination Assay (IHA)
Monkey serum samples were tested for specific antibody to B. pseudomallei by indirect hemagglutination assay (IHA) following the IHA protocol of Mahidol-Oxford Tropical Medicine Research Unit.16 It was performed using antigen pooled from clinical B. pseudomallei isolates from a Springbok positive case at Khon Kaen Zoo, Thailand, following standard US CDC laboratory protocols. The optimal concentration of antigen was pooled before sensitizing with sheep red blood cells for one hour. Monkey serum samples which diluted with 2-fold serial dilutions and starting from of 1:10 dilution and heat-inactivated and then incubated with nonsensitized sheep red cells for 2 hours at room temperature and then incubated overnight at 4°C. Titers for monkey serum samples in microwell plates were analyzed, and antigen-sensitized red cells were added. The appearance of antibody was confirmed by the agglutination of red blood cells. A titer of ≥1:80 was considered positive for melioidosis infection.
Formalin–Ethyl Acetate Concentration Technique
Fecal samples were processed for detecting the presence of GI parasitic eggs or larva with the formalin–ethyl acetate concentration technique (FECT), then the identification of those parasitic eggs or larva was examined under microscope. The occurrence of GI parasitic eggs or larva which related to the infection of monkeys was calculated using the descriptive statistics on MS Excel version 2013 (Microsoft, USA).
Risk Factors Assessment in Humans and Data Analysis
The KAP (knowledge, attitude, and practice) questionnaire was used as a tool in this study for assessing the risk factors in humans. The study questionnaire was administered orally in area around Kosumpee Forest Park by divided the sample into 6 groups; Forest Park staffs, students, teachers, temple dwellers, inhabitants (living around the park) and tourists. Questionnaires included close-ended and free-response questions covering the following topics: personal information about respondents, basic knowledge about the diseases (such as knowledge of signs and symptoms of these diseases in humans and animals, transmission, testing and treatment), attitude towards the diseases, behaviors that are risk factors for the disease and personal hygiene for prevention and treatment practices. The respondents received explanations and recommendations from the interviewer and all personal information was confidential.
Data Analysis
The melioidosis seroprevalence and GI parasitic infection in long-tailed macaques were analyzed by using descriptive statistics in the SPSS statistics program version 16.0 (IBM, Armonk, NY, USA). Questionnaire data was also entered and analyzed using the IBM Statistics for Social Sciences (SPSS) version 16 software for Windows (IBM, Armonk, NY, USA). Data were checked and cleaned. Chi square tests and the Fisher tests were performed to determine the associated factors for good KAP toward melioidosis and GI parasitic infection among the respondents. P-values ≤ 0.05 was considered to be statistically significant. Frequency and percentage were calculated for all variables.
Results
Prevalence of Melioidosis in Long-Tailed Macaques
A total of 28 serum samples of free ranging long-tailed macaques were collected and tested by IHA. Sixteen (57.14%) samples were positive by using a cut-off value of 1:80 as suggested by Mekaprateep and team,17 while, 12 samples (42.86%) were negative as shown in Table 1. Estimation of the population prevalence at 95% CI was calculated from the formula of p ± 1.96 * SE resulting in a prevalence of 56.97% to 57.31%.
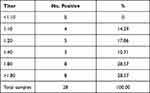 |
Table 1 Frequencies of Melioidosis IHA Serum Titers of Long-Tailed Macaques at Kosumpee Forest Park, Maha Sarakham Province, Thailand |
Long-Tailed Macaque Fecal Analysis
Individual fecal samples from 131 macaques were tested by FECT. Overall, 35.11% (46 of 131) of macaques were infected with one or more gastrointestinal parasites including Strongyloides spp., Trichuris spp., Ascaris spp., and hookworm as shown in Table 2. The result revealed the highest prevalence of Trichuris infection in long-tailed macaques followed by Strongyloides infection. Ascaris spp. was the least prevalent of GI parasitic infections in long-tailed macaques. Interestingly, rhabditiform larvae were identified under microscope from 8 fecal samples which detected as positive samples of Strongyloides sp. infection.
 |
Table 2 Prevalence of GI Parasites in Long-Tailed Macaques in Kosumpee Forest Park, Maha Sarakham Province, Thailand |
Human Survey
A total of 370 and 362 respondents participated in the questionnaire regarding KAP analysis for melioidosis and gastrointestinal parasitic infections, respectively. According to the sociodemographic characteristics of the respondents in the questionnaire of melioidosis infection, all respondents were Thai, and females (59.46%) outnumbered the males (40.54%). The age range with the greatest number of respondents was 7 to 14 years (38.65% preliminary students) while ages ranged from 35 to 44 years (4.86%) is the least respondent population. For the gastrointestinal parasitic infections questionnaire, all respondents were Thai, and females (58.29%) outnumbered the males (47.71%). The age range with the greatest number of respondents was 7 to 14 years (38.12% preliminary students) while ages ranged up to 65 years (3.59%) is the least respondent population. The mean scores for respondent’s knowledge, attitudes, and practices of melioidosis and gastrointestinal parasitic infections were compared among the different groups as shown in Tables 3 and 4, respectively. The knowledge, attitudes, and practices scores varied significantly by the group of people which relevant to those macaques. It is very interesting that all group of people had very low knowledge about melioidosis (<30% of all groups), while, they had a good knowledge about GI parasitic infection (>50% of all groups). Forest Park staff, students and tourists tended to have a better attitude with concern about melioidosis and all groups were greatly concerned about GI parasitic infection. This research revealed that basic self-protection practices to protect them from melioidosis infection were minimal, while, they had a good level of self-protection practices from GI parasitic infection except in the case of temple dwellers.
 |
Table 3 Respondents’ knowledge, Attitude and Practices of melioidosis |
 |
Table 4 Respondents’ knowledge, Attitude and Practices of Gastrointestinal Parasitic Infections |
Discussion
Based on the IHA results, 56.97% to 57.31% melioidosis seroprevalence was determined for the studied free ranging population of long-tailed macaques. There have been no other investigations in macaques in Thailand. Previous study of melioidosis seroprevalence from livestock in Thailand reported a seroprevalence of 8135 animals in 2005–2006 consisting of cattle (2.56%), goats (0.33%), sheep (7.23%), swine (7.23%) and deer (1.61%).18 The seroprevalence of melioidosis in macaques in Kosumpee Forest Park in this study was higher than in all other animals studied. This is due to significantly higher environmental contamination in soils19 such as paddy fields, open fields, plantations, forests and ponds.20–22 Characteristics of the environment within and around Kosumpee Forest Park resulted in the high risk and incidence of melioidosis in the macaque population.
The prevalence of GI parasitic infection in macaque from this study was 35.11%. Overall, 35.11% (36 of 131) of macaques were infected with one or more GI parasites; Strongyloides spp. (15.27%, 20 of 131) Trichuris spp. (22.90%, 30 of 131), Hookworm (4.58%, 6 of 131) and Ascarid spp. (1.53, 2 of 131). The prevalence of all parasitic infections in macaques at Kosumpee Forest Park was lower than previous report by Pumipuntu in 2018 (62.6%)23 but was approximately identical to that reported by Schurer and team in 2019 (44%).24 Trichuris spp. tended to increase while Strongyloides spp. decreased in 2019 and increased in this study. Hookworm tended to decrease and Ascarid spp. was not found in 2018, but it was detected in 2019 and during this study. Interestingly, this research found rhabditiform larvae of Strongyloides sp. in 8 out of 20 fecal samples from the monkeys which amounts to 40% of all Strongyloides infection. The rhabditiform larvae can be excreted from the feces of long-tailed macaques, survive within the environment such as soil or water, and act as soil-transmitted helminths which can cause reinfection to animal and human via the environmental sources as their reservoirs.23 This finding implies the significance of this GI pathogen in the perspective of “One Health” that needs to be emphasized. Recording the ongoing prevalence and the diversity of parasitic species in detail will help to understand the relationship between the parasite and human knowledge, awareness and social behavior.23,25 The prevalence of both melioidosis and GI parasitic infection in this study emphasizes and highlights the presence of zoonotic potential diseases which can transmit infection between wildlife to humans as animal reservoirs. It implies the current dynamics and consequences of their infections in wildlife, especially in free ranging long-tailed macaques which have coexistence with humans thus bringing them in closer proximity to one another.
More than that, this study indicated the knowledge level associated with melioidosis among the local population sample was at low level (12.97%), consistent with another study in Thailand (7%)26 and GI parasitic infections were at moderate level (68.78%), consistent with previous study in Thailand (68.50%).27 Awareness of melioidosis, accounted for 49.72% of the sample and was at moderate level and GI parasitic infections was at high level (87.56%).
Practices that are risk factors for melioidosis that were statistically significant were (i) cleaning the body after visiting the Forest Park (P < 0.001), (ii) walking barefoot in stables and agricultural areas (P < 0.001), (iii) having ever been into a natural water resource (P = 0.002) and (iv) having ever touched the body or stools of animals (P < 0.001). More than 95.14% of the sample had visited the Forest Park. If people touched animals or their manure without cleaning and protective contact, there was a risk of infections. Because the pathogens may be transmitted from the animal through breathing, feces, urine and secretions into the environment, when people enter an environment in which the animal lives, they can easily become infected.28
Practices that are statistically significant risk factors for GI parasitic infections were consisting of (i) level of personal hygiene (P < 0.001), (ii) eating uncooked vegetables (P < 0.001), (iii) eating uncooked fish (P = 0.003), (iv) wearing shoes when leaving the house (P = 0.001) and (v) housing near the community littering area (P = 0.031). An environment where people and humans live together provides opportunity for contact with the monkey’s body or feces and was a risk factor for human parasite infection.24 The result is related to a previous study which reported that there was a statistically significant association between eating uncooked food as food culture and GI parasitic infections of Thai people in rural areas.27 More than that, lack of systematic waste management, knowledge and understanding of animal reservoirs and their role of the people, especially in old ages, are the important factors which promotes infection.29 In addition, not wearing shoes while leaving the house had a statistically significant association with GI parasitic infections.27,30
This finding revealed the significance of melioidosis and GI parasitic infection which are some of the most sylvatic infectious disease in wildlife. In addition, they could be potentially important zoonotic pathogens, with macaques playing an important role as animal reservoirs of zoonotic infections in this area. Interestingly, those zoonotic diseases are the essential problem of “One Health” issues which at present which involve human, animal and environmental health. Melioidosis and GI parasitic infection are potential zoonotic diseases in which animals are reservoirs that can contaminate the environment and cause re-infections.23,24,31,32 Although transmission of infection from animals to humans is difficult and needs suitable factors for spillover, it cannot be ignored.33,34 Animal excretion or secretions can carry various pathogens and shed them into the environment around human communities as natural reservoirs that harbor infectious zoonotic pathogens outside the bodies of animal reservoirs. Climate change, such as rainfall in the rainy season, will increase the risk of exposure or infection from natural reservoirs to human.28
Kosumpee Forest Park is an obvious coexistence model of humans and wildlife in the same environment. It is an eco-tourism destination, thus increasing the opportunity for infection. There should be surveillance measures and reduction of the risk factors for infections through interdisciplinary cooperation to share knowledge, attitudes and tools between the government, the private sector and the people. This is a study of knowledge, attitudes and practices related to zoonotic infection to assist in directing policy in the provision of unique resources that meet local needs, which is necessary for operating suitable preventive and control programs, improving personal sanitation, educating about zoonotic diseases to increase risk awareness of people in this area.
Conclusion
This study provides an obvious evidence that there is a high prevalence of melioidosis and GI parasitic infections in long-tailed macaques at Kosumpee Forest Park. The long-tailed macaque seems to be the important reservoir hosts for zoonotic melioidosis and GI parasites found in the study area including Strongyloides spp., Ascaris spp., Trichuris spp. and hookworms. These all have sylvatic cycles among free-ranging wildlife hosts and lead a potential risk to health from zoonotic diseases of people living and working near the Forest Park. People’s occupation had an effect on risk factors of knowledge, attitudes and practices of both infectious diseases and can help identify the cause and design measures or solutions for future problems in areas of wildlife and human community coexistence. There also needs to be a continuous assessment of knowledge, attitudes and practices of people in vulnerable areas and to educate and understand effects on behavioral modification to reduce risk factors and prevent infections to promote human, animal and environmental health.
Acknowledgments
This research project was financially supported by Mahasarakham University. We would like to thank Prof. Dr. Randall Kyes, Departments of Psychology and Global Health, Center for Global Field Study, Washington National Primate Research Center, University of Washington, Seattle, Washington, USA and all staff at Kosumpee Forest Park for guidance and assistance at the sampling field. We really feel thankful to Dr. Panyupha Thammawat, Faculty of Humanities and Social Sciences, Khon Kaen University for her valuable advice in the questionnaire design. We are grateful to Faculty of Veterinary Sciences, Mahasarakham University for diagnostic laboratory and Wildlife hospital, Khon Kaen Zoo for melioidosis diagnostic laboratory. We also thank the Wildlife and Exotic Friends Club, MSU and students from Faculty of Environmental and Resource Studies for the assistance with animal and human sampling. Finally, we really feel thank to Department of National Parks Wildlife and Plant Conservation (DNP), Thailand for permission to work in the area.
Disclosure
The authors declare that there is no conflicts of interest in this work.
References
1. Fooden J. Comparative review of Fascicularis-group species of macaque (primate: macaca). Fieldiana Zool. 2006;107:1–43. doi:10.3158/0015-0754(2006)107[1:CROFSM]2.0.CO;2
2. Fuentes A. Monkeys on the Edge: Ecology and Management of Long-Tailed Macaques and Their Interface with Humans (Cambridge Studies in Biological and Evolutionary Anthropology). Gumert M, Jones-Engel L, editors. Cambridge: Cambridge University Press; 2011. doi:10.1017/CBO9780511974434
3. Malaivijitnond S, Hamada Y. Current situation and status of long-tailed macaques (Macaca fascicularis) in Thailand. Nat Hist J Chulalongkorn. 2008;8(2):185–204.
4. Radhakrishna S, Huffman MA, Sinha A. The Macaque Connection: Cooperation and Conflict Between Humans and Macaques. New York: Springer; 2013. doi:10.1007/978-1-4614-3967-7
5. Cantas L, Suer K. The important bacterial zoonoses in “one health” concept. 2014. Front Public Health. 2014;2(144). doi:10.3389/fpubh.2014.00144
6. Boonjaraspinyo S, Boonmars T, Kaewsamut B, et al. A cross-sectional study on intestinal parasitic infections in rural communities, northeast Thailand. Korean J Parasitol. 2013;51(6):727–734. doi:10.3347/kjp.2013.51.6.727
7. Hamer DH, Griffiths JK, Marguire JH, et al. Public Health and Infectious Diseases. Amsterdam: Elsevier; 2010.
8. Wang T, Yang GY, Yan HJ, et al. Comparison of efficacy of selamectin, ivermectin and mebendazole for the control of gastrointestinal nematodes in rhesus macaques, China. Vet Parasitol. 2008;153(1–2):121–125. doi:10.1016/j.vetpar.2008.01.012
9. Yeager JJ, Facemire P, Dabisch PA, et al. Natural history of inhalation melioidosis in rhesus macaques (Macaca mulatta) and African green monkeys (Chlorocebus aethiops). Infect Immun. 2012;80(9):3332–3340. doi:10.1128/IAI.00675-12
10. Wiersinga WJ, Virk HS, Torres AG, et al. Melioidosis. Nat Rev Dis Primers. 2018;4(1):1–22. doi:10.1038/nrdp.2017.107
11. Muangjaiphet P, Suggaravetsiri P. Prevalence and factors associated with melioidosis in Ubon Ratchathani province. J Office DPC7 Khon Kaen. 2019;26(2):1–13.
12. Lausatianragit W, Jaroenprasert S, Lausatianragit K, et al. Factors related to the consumption of raw fish among the people in Sisaket province. J Health Sci. 2019;28(6):974–985.
13. Thompson RA. Parasite zoonoses and wildlife: one health, spillover and human activity. Int J Parasitol. 2013;43(12–13):1079–1088. doi:10.1016/j.ijpara.2013.06.007
14. Vorou RM, Papavassiliou VG, Tsiodras S. Emerging zoonoses and vector-borne infections affecting humans in Europe. Epidemiol Infect. 2007;135(8):1231–1247. doi:10.1017/S0950268807008527
15. Kyes R, Tanee T, Thamsenanupap P, et al. Population status of the long-tailed macaques (Macaca fascicularis) at Kosumpee Forest Park, Maha Sarakham, Thailand. In:
16. Mahidol-Oxford Tropical Medicine Research Unit. Standard Operating Procedure (SOP) of Indirect Haemagglutination Assay (IHA) for melioidosis. Available from: http://www.melioidosis.info/download/MICRO_SOP_IHA_ENG_v1%203_8Dec11_SDB.pdf.
17. Mekaprateep M, Jiwakanon N, Jongkajornpong L, et al. Use of indirect hemagglutination (IHA) test for serodiagnosis of melioidosis in dairy cow. J Med Assoc Thai. 1998;49:35–44.
18. Srikawkheaw N, Lawhavinit OA. Detection of antibodies against melioidosis from animal sera in Thailand by indirect haemagglutination test. Kasetsart J. 2007;41:81–85.
19. Vuddhakul V, Tharavichitkul P, Na-Ngam N, et al. Epidemiology of Burkholderia pseudomallei in Thailand. Am J Trop Med Hyg. 1999;60(3):458–461. doi:10.4269/ajtmh.1999.60.458
20. Strauss JM, Groves MG, Mariappan M, et al. Melioidosis in Malaysia. II. Distribution of Pseudomonas pseudomallei in soil and surface water. Am J Trop Med Hyg. 1969;18(5):698–702. doi:10.4269/ajtmh.1969.18.698
21. Thin RN, Groves M, Rapmund G, et al. Pseudomonas pseudomallei in the surface water of Singapore. Singap Med J. 1971;12:181–182.
22. Nachiangmai NP, Patamasucon B, Tipayamonthein A, et al. Pseudomonas pseudomallei in southern Thailand. Southeast Asian. J Trop Med Public Health. 1985;16:83–87.
23. Pumipuntu N. Detection for potentially zoonotic gastrointestinal parasites in long-tailed macaques, dogs and cattle at Kosamphi forest park, Maha Sarakham. Vet Integr Sci. 2018;16(2):69–77.
24. Schurer JM, Ramirez V, Kyes P, et al. Long-tailed macaques (Macaca fascicularis) in urban landscapes: gastrointestinal parasitism and barriers for healthy coexistence in northeast Thailand. Am J Trop Med Hyg. 2019;100(2):357–364. doi:10.4269/ajtmh.18-0241
25. Kouassi RY, McGraw SW, Yao PK, et al. Diversity and prevalence of gastrointestinal parasites in seven non-human primates of the Taï National Park, Côte d’Ivoire. Parasite. 2015;22:1. doi:10.1051/parasite/2015001
26. Chansrichavala P, Wongsuwan N, Suddee S, et al. Public awareness of melioidosis in Thailand and potential use of video clips as educational tools. PLoS One. 2015;10(3):e0121311. doi:10.1371/journal.pone.0121311
27. Kaset P. Prevalence and factors associated with helminthiasis among people in Chaloem Phra Kiat district, Nan Province. Prim Health Care Div J. 2016;12(4):36–37.
28. Estrada-Peña A, Ostfeld RS, Peterson AT, et al. Effects of environmental change on zoonotic disease risk: an ecological primer. Trends Parasitol. 2014;30(4):205–214. doi:10.1016/j.pt.2014.02.003
29. Division of Innovation and Research. Research Program: Protection, Disease Control and Health Hazards 2019–2021. Bangkok;2019:122
30. Jia F, Song H, Li Z, Guo Y. Prevalence and predictors of food-borne parasites among residents in Tianhe district, Guangzhou City, China. Biomed Res. 2019;30(4):1–5.
31. Dedie K, Bockemuehl J, Kuehn H, et al. Melioidose. In: Bakterielle Zoonosen bei Tier und Mensch. Ferdinand Enke; 1993:169–181.
32. Mbora DN, Munene E. Gastrointestinal parasites of critically endangered primates endemic to Tana River, Kenya: Tana River red colobus (Procolobus rufomitratus) and crested mangabey (Cercocebus galeritus). J Parasitol. 2006;92(5):928–932. doi:10.1645/GE-798R1.1
33. Lo Iacono G, Cunningham AA, Fichet-Calvet E, et al. A unified framework for the infection dynamics of zoonotic spillover and spread. PLOS Negl Trop Dis. 2016;10(9):e0004957. doi:10.1371/journal.pntd.0004957
34. Choy JL, Mayo M, Janmaat A, Currie BJ. Animal melioidosis in Australia. Acta Trop. 2000;74(2–3):153–158. doi:10.1016/s0001-706x(99)00065-0
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
