Back to Journals » Neuropsychiatric Disease and Treatment » Volume 19
Oligodendrocyte Progenitor Cell Transplantation Ameliorates Preterm Infant Cerebral White Matter Injury in Rats Model
Authors Wang Z, Zhang L, Yang Y, Wang Q, Qu S, Wang X, He Z, Luan Z
Received 27 March 2023
Accepted for publication 24 August 2023
Published 11 September 2023 Volume 2023:19 Pages 1935—1947
DOI https://doi.org/10.2147/NDT.S414493
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Roger Pinder
Zhaoyan Wang,1,* Leping Zhang,1,2,* Yinxiang Yang,1 Qian Wang,1 Suqing Qu,1 Xiaohua Wang,1 Zhixu He,2,3 Zuo Luan1
1Laboratory of Pediatrics, The Sixth Medical Center of PLA General Hospital, Beijing, 100048, People’s Republic of China; 2Guizhou Medical University, Guiyang, 550004, People’s Republic of China; 3Department of Pediatrics, Affiliated Hospital of Zunyi Medical University, Zunyi, 563100, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Zuo Luan; Zhixu He, Email [email protected]; [email protected]
Background: Cerebral white matter injury (WMI) is the most common brain injury in preterm infants, leading to motor and developmental deficits often accompanied by cognitive impairment. However, there is no effective treatment. One promising approach for treating preterm WMI is cell replacement therapy, in which lost cells can be replaced by exogenous oligodendrocyte progenitor cells (OPCs).
Methods: This study developed a method to differentiate human neural stem cells (hNSCs) into human OPCs (hOPCs). The preterm WMI animal model was established in rats on postnatal day 3, and OLIG2+/NG2+/PDGFRα+/O4+ hOPCs were enriched and transplanted into the corpus callosum on postnatal day 10. Then, histological analysis and electron microscopy were used to detect lesion structure; behavioral assays were performed to detect cognitive function.
Results: Transplanted hOPCs survived and migrated throughout the major white matter tracts. Morphological differentiation of transplanted hOPCs was observed. Histological analysis revealed structural repair of lesioned areas. Re-myelination of the axons in the corpus callosum was confirmed by electron microscopy. The Morris water maze test revealed cognitive function recovery.
Conclusion: Our study showed that exogenous hOPCs could differentiate into CC1+ OLS in the brain of WMI rats, improving their cognitive functions.
Keywords: preterm infant, cerebral white matter injury, oligodendrocyte progenitor cells, cell replacement therapy, rat model
Introduction
Cerebral white matter injury (WMI) is the most common pathological alteration-related brain injury in preterm infants.1,2 WMI can lead to pediatric neurodevelopmental disorders, resulting in motor and developmental defects, and is accompanied by cognitive deficits, which persist to adulthood.3 In traumatic brain injury, inflammation, ischemia and hypoxia, and demyelinating lesions, oligodendrocytes change from a resting to an activated state. Preterm WMI is characterized by disturbed maturation of oligodendrocyte (OL) myelination, usually occurring at 23–32 gestational weeks.4 Clinical evidence indicates that maternal-fetal infection, inflammation, and hypoxia-ischemia are primary causes of WMI.5
There is no effective treatment for white matter damage in premature infants. The combination of cerebral hypothermia therapy and drugs to prevent brain death is a potential treatment for WMI in preterm infants. However, there is insufficient clinical information on the safety of hypothermia treatment in preterm infants.6 A previous study has shown that cell transplantation,7,8 an approach to transplant neural cells to replenish or replace damaged or dysfunctional tissue, is a promising WMI therapeutic strategy.9 Because the main target cell type of WMI in premature infants is pre-OLs, selecting cells from the early stage of the OL lineage can achieve better treatment results.10 Human oligodendrocyte progenitor cells (OPCs; hOPCs) are considered potential candidates for cellular transplantation.11 In the periventricular leukomalacia (PVL) rat model, transplanted hOPCs survived longer than 5 weeks and differentiated into oligodendrocytes, expressing myelin basic protein (MBP). Moreover, motor and cognitive recovery were also observed in the rat model.11 Also, several studies have shown that transplantation of fetal brain-derived hOPCs, human induced pluripotent stem cells, or embryonic stem cells (ESCs) leads to myelination in animal models.12–16
In this study, a rat model of preterm WMI that mirrors the characteristics of WMI in preterm infants17 was established to observe the fate of hOPCs transplanted into the injured corpus callosum and determine whether hOPCs transplantation improves myelination and cognitive functions.
Materials and Methods
Human Neural Stem Cell Culture
Aborted human embryos, aged 9 weeks post-conception, were obtained from the Department of Obstetrics and Gynecology of the Sixth Medical Center of PLA General Hospital, Beijing, China, from patients who had requested to terminate gestation. According to the guidelines approved by the hospital’s ethics committee, the donors provided written consent to donate the aborted fetuses after being fully informed about the study.
Human neural stem cells (hNSCs) were isolated from the cortex, ganglionic eminences, and thalamus of human fetuses as previously described.18 hNSCs were amplified in spheres in NSC medium (consisting of a 3:1 mixture of Dulbecco’s modified Eagle’s medium (DMEM)-F12 medium supplemented with 15 mM N-2-hydroxyethylpiperazine-N-ethane-sulphonic acid, 0.15% D-glucose, 100 g/mL transferrin, 20 nM progesterone, 60 M putrescine, 30 nM sodium selenite, 5 g/mL insulin, 5 g/mL heparin, 1% L-glutamine, 20 ng/mL basic fibroblast growth factor (bFGF), 20 ng/mL epidermal growth factor, 10 ng/mL leukemia inhibitory factor, and 100 U/mL penicillin and streptomycin). All reagents were purchased from Sigma (St. Louis, MO, USA).
OPC Differentiation
To generate hOPCs, hNSCs were cultured for 10 days to form neurospheres, which were dissociated into single cells and induced to differentiate in differentiation medium (DMEM-F12 medium [3:1] supplemented with 2% B27, 5 g/mL transferrin, 10 nM progesterone, 30 M putrescine, 15 nM sodium selenite, 5 g/mL insulin, 5 g/mL heparin, 5 mM lactate, 5 ng/mL bFGF, 10 ng/mL platelet-derived growth factor, 10 ng/mL neurotrophic factor-3, and 100 U/mL penicillin and streptomycin). The medium was changed every 2–3 days.19
Animal Models
This study was conducted per the National Institutes of Health Guide for the Care and Use of Laboratory Animals. The Clinical Pharmacology Ethics Committee of the Sixth Medical Center of PLA General Hospital, Pediatrics Ethics Committee in Beijing, approved all protocols (SCXK20170005). Sprague-Dawley rats (8–9 g) of either sex at postnatal day (P) 3 were randomly grouped according to the method and principles of the random number table (n = 9 in each group): normal control group, preterm WMI group (model), and graft group (graft). We started daily cyclosporine administration in each group of rats from 3 d prior to cell transplantation until perfusion. Hypoxic ischemia (HI) was induced in model and graft rats, as previously described.20–22 Briefly, the pups were cryoanesthetized by incubation on ice for 7 min, the right common carotid artery was severed from the accompanying vagus and sympathetic nerves and permanently ligated using a microelectrocauterizer, and the pups were returned to the dam for 1 h and then exposed to 6% O2 for 90 min. Litter-matched sham controls were not subjected to carotid ligation or hypoxia. For temperature management, the infant rats were placed on ice for 7 minutes for cryoanesthesia. The modelled pups were placed back to the mother to recover for 1 hour and then placed in a constant temperature hypoxic chamber (6% oxygen, 94% nitrogen) for 90 minutes at 37°C in hypoxia. After model preparation, pups were placed back to their mothers for further rearing. We blinded to treatment when assessing outcomes.
Hematoxylin and Eosin Staining
Three rats were randomly selected from the model and control groups seven days after the HI induction. After anesthetization, the rat brain was perfusion-fixed with 4% paraformaldehyde. The brain was dehydrated with gradient ethanol (70% ethanol for 2 h, 80% ethanol overnight, 90% ethanol for 2 h, and 100% ethanol for 1 h), vitrified with xylene, waxed, embedded in paraffin, and conventionally sliced into 5 µm tissue sections. Subsequently, the sections were stained with hematoxylin for 10 min, washed with water for 15 min, stained with eosin for 1 min, and washed with water for 10s.
OPC Transplantation
Seven days after HI induction, OPCs were harvested from the flask and resuspended in 18 μL of phosphate balanced solution (PBS) at a density of 1×105 cells/μL. The pups were anesthetized with ketamine (75 mg/kg, ip) and xylazine (12 mg/kg, ip) and placed in the stereotactic frame. Then, using a 5 mL syringe, 3 μL (3 × 105 cells) of the OPCs suspension was injected into the right corpus callosum of each rat in the graft group at the following stereotaxic coordinates from bregma: anteroposterior, −1.0 mm; mediolateral, 1.5 mm; deep level, −1.5 mm. The procedure lasted more than 10 min. The syringe was left in place for an additional 5 min, then gradually withdrawn, and the scalp was sutured and disinfected with 75% ethanol. After surgery, pups were placed on heating pads in individual cages until they recovered from anesthesia and returned to their mother until weaning on the 21st day. At that point, animals were weaned into individual cages, where they remained until euthanized. The immunosuppressant cyclosporine (Sandimmun, 10 mg/kg) was administered to all rats intraperitoneally once daily. The administration began 3 days before transplantation and continued throughout the study.
Immunocytochemistry
Cells were fixed with 4% paraformaldehyde (PFA) for 10 min at 25 °C, followed by three washes with PBS, blocked for 1 h with 3% bovine serum albumin (Sigma), and permeabilized with 0.3% Triton X-100 (Calbiochem). Immunolabeled cells were incubated with primary antibodies overnight at 4 °C and secondary antibodies for 1 h at 25 °C. Primary antibodies included rabbit anti-OLIG2 (1:200, Millipore, AB9610), mouse anti-NG2 (1:200, Abcam, AB83178), rabbit anti-PDGFR (1:400, Cell Signaling Technology, 5421S), mouse anti-GFAP (1:400, Abcam, ab10062), and mouse anti-O4 (1:100, R&D) and rat anti-MBP (1:100, Abcam). Appropriate Cy3-, Alexa Fluor 594-, and Alexa Fluor 488-conjugated secondary antibodies (Goat anti-mouse (1:500, Invitrogen, A-21145), goat anti-mouse (1:500, Invitrogen, A-21121), donkey anti-mouse (1:500, Abcam, ab150105), donkey anti-rat (1:200, Abcam, ab150153), rabbit anti-goat (1:500, Abcam, ab150144)) were used to detect primary antibodies. 4,6-diamino-2-phenyl indole (DAPI) was used as the nuclear counterstain.
Immunohistochemistry and Imaging of Tissue Sections
Three months after cell transplantation, all rats were perfused with 0.9% NaCl and 4% PFA after anesthetization. The brains were removed, post-fixed in 4% PFA overnight, and cryoprotected in 30% sucrose. Frozen coronal brain sections were cut at a thickness of 14 μm using a Leica CM 1950 cryostat (Leica Microsystems; Leica CM, 1850). The immunostained slides were washed with PBS three times; brain sections were incubated in blocking solution (PBS + 10% normal goat serum + 0.3% Triton X-100) for 1 h at 37 °C and then incubated with a primary antibody diluted in blocking solution overnight at 4 °C. Goat anti-GFAP (1:500, Abcam, ab53554), rabbit anti-OLIG2 (1:200, Millipore, AB9610), mouse anti-CC1 (Millipore, OP80), rabbit anti-Ki67 (Abcam, ab16667). Human cells were identified using mouse anti-human nuclear antigen (HNA) and clone 235–1 (1:500, Millipore, MAB1281), and MBP was detected with rat anti-MBP (1:100, Abcam, Ab7349). The next day, brain sections were washed three times in PBS, incubated with secondary antibodies (Alexa Fluor 488-, Alexa Fluor 594-, or Cy3-tagged secondary antibodies; 1:500) in PBS for 2 h at room temperature, and counterstained with DAPI. Secondary antibodies conjugated with Alexa Fluor-488 or −594 were used at 1:500 (Invitrogen, Carlsbad, CA, USA; Abcam, Cambridge, MA, USA). Pictures were taken under an Olympus DP71 microscope (Olympus Microsystems). The brightness and contrast were optimized using Adobe Photoshop CS5 (Adobe, USA).
Mapping of human cell engraftment-The positions of anti-human nuclei+ cells were mapped on 14 μm coronal sections from −5.04 to 2.04 bregma (random selection of a representative case of a graft rat; a total of seven sites and six slices were cut at each site) with image Pro Plus 6.0 software.
Cell counting-Six rats in the graft group (six sections per rat, 14 μm coronal section samples of the corpus callosum, from −3.5 to 1.5 bregma) were examined for cells co-expressing HNA and either MBP, GFAP, OLIG2, or Ki67.
Electron Microscopy
Brains were removed and fixed in 4% PFA overnight. Then, a piece of corpus callosum tissue near the transplantation site was resected and fixed in 2% PFA with 2.5% glutaraldehyde in sucrose-PBS. Tissue samples were osmicated, dehydrated in ethanol, and embedded in Epon. Ultrathin sectioning was performed using a PowerTome X Ultramicrotome (RMC Products, Boeckeler, Tucson, AZ, USA). The ultrathin sections were collected on formvar-coated copper one-hole grids, contrasted with lead citrate and uranyl acetate, and then examined using a FEIG2 Spirit BioTwin transmission electron microscope (FEI, USA). Six samples from each group were analyzed using a quantitative evaluation method (G-ratio and ultrastructural grading scores). One hundred myelinated axons from each sample and 600 myelinated axons from each group were calculated.
Morris Water Maze Test
The water maze testing took place in a 1.7 m (diameter) × 50 cm (height) hard black plastic tub that was filled with water (25 ± 1 °C), with the bottom 45 cm above ground level. The black platform (12 cm diameter × 30 cm height) was submerged 1.5–2.0 cm below the water surface. The test was performed 3 months after transplantation (n = 6 per group). For the navigation trial, rats underwent two trials per day, and there were four different starting points for each trial for a total of 5 days. Each trial ended when the rat rested on the platform for 3s. If a rat could not find the platform within 60s, it was led to the platform and allowed to rest for 10s. The space probe trial, with the platform removed, took place on the sixth day over 60s. With the platform removed, the space probe trial was conducted on the 6th day over 60s.22–24 The escape latency, number of platform crossings, percentage of time spent in the platform quadrant, and percent distance traveled in the platform quadrant were recorded. Data were analyzed using a tracking program (ZS-001moriss Morris Water Maze Video Analysis System, ZS Dichuang, www.zslab1.com). All protocols were approved by the Clinical Pharmacology Ethics Committee of the Sixth Medical Center of PLA General Hospital, Pediatrics Ethics Committee in Beijing (SCXK- 2017-0001).
Statistics
Data analysis and statistical inference were performed using SPSS version 25.0 (IBM, Armonk, NY, USA). Data are expressed as ± SEM. Continuous variables of the Morris water maze escape latency test were analyzed using repeated measures two-way analysis of variance (ANOVA), followed by Fisher’s least significant difference post-hoc test when appropriate. Continuous variables of the probe trials and myelin ultrastructure were compared using one-way ANOVA, followed by Scheffe’s multiple comparison test when the results were significant. The MBP immunoreactivity intensity was compared using the Student’s t-test. P values < 0.05 were considered statistically significant. The authors were blinded to treatment when assessing outcomes.
Results
Histological Analysis of Preterm WMI Brains
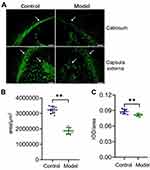
Brains were harvested and prepare for analysis 7 days after preterm WMI was established. Compared with the control group, the ipsilateral hemisphere of the model group was slightly atrophied. The white matter in corpus callosum and capsula externa of preterm WMI rats was thinner compared with the control group, and abnormal arrangements of neural fibers were observed, indicating net necrosis. Glial cell karyopyknosis and necrosis were obvious in the lesioned area (Figure 1).
MBP is mainly located in the myelin sheath, and its expression can reflect the integrity of the myelin sheath. Immunohistochemical staining showed that MBP expression in the corpus callosum and capsula externa in the hemisphere subjected to HI injury was significantly lower than in the control rats (Figure 2A). Quantitative analysis showed that MBP+ area and MBP intensity decreased significantly in the ipsilateral hemisphere, indicating a severe myelin loss in the ipsilateral white matter of the model rats (P<0.01, Figure 2B and C).
In vitro Induction of hNSCs to Obtain hOPCs
hNSCs were isolated from the human fetal brain and cultured in suspension, allowing neurosphere formation (Figure 3A). hNSCs differentiation into hOPCs was induced, and OPC-specific markers were detected to assess induction efficacy. The morphology of hOPCs derived from hNSCs showed a “bipolar” structure (Figure 3B). Positive staining of OLIG2 was detected, representing efficient induction.25 Besides OLIG2, other OPC-specific markers, including NG2, PDGFR-α, and O4, were detected. Immunofluorescence analysis showed positive staining for NG2, OLIG2, PDGFR-α, and O4 (Figure 3C–F), indicating appropriate differentiation of hNSCs to hOPCs.26
Survival, Migration, and Morphological Differentiation of hOPCs in the Preterm WMI Brain
hOPCs transplantation in preterm WMI rats was conducted via stereotaxic injection in the right corpus callosum, and immunosuppression agents were administered to control the rejection reaction. The brains of preterm WMI rats were harvested 3 months after transplantation. Because of our study’s relatively high rate of O4+ cells, we did not purify the induced hOPCs with anti-O4 antibodies, as usually done in prior studies before transplantation.15,27
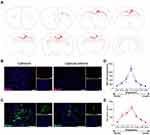
The brains were prepared for immunohistochemistry analysis using the human-specific markers stem121 (cytosolic) and human nuclear antigen (hNA). Transplanted hOPCs migrated from the injection site to white matter tracts in the corpus callosum, external capsule, striatopallidal striae, and cortical layer 6 of both hemispheres (Figure 4A–C). Furthermore, the engrafted cells morphology developed from bipolar to multipolar, similar to mature oligodendrocytes (Figure 4C). Quantitative analysis showed that the density of hNA+ cells in the corpus callosum near the transplant site (bregma −3.12) was 3986 ± 255 cells/mm2, and the number of hNA+ cells decreased in the rostral and caudal sections (Figure 4D and E).
The immunofluorescence assay showed positive expression of OLIG2, CCI, and MBP in the transplanted hOPCs. OLIG2 was expressed in over 80% (81 ± 2.5%) of hNA+ cells in the corpus callosum (Figure 5A). Over 50% (54.9 ± 8.9%) of hNA+ cells expressed CC1 protein (Figure 5B), indicating features of oligodendrocytes, as previously reported.28 Furthermore, few transplanted hOPCs differentiated into astrocytes (Figure 5C). The proliferation rate of engrafted hNA+ cells was less than 7% (5.6 ± 0.6%) (Figure 5D), suggesting a low mitotic capacity of hOPCs at 3 months post-transplant. 24.6±0.79% of Stem121+ cells expressed MBP protein (Figure 5E), suggesting that transplanted cells matured into MBP-producing OLs in injured white matter. Cell clusters and overt tumorigenesis were not observed.
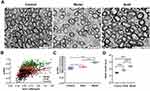
We further explored whether hOPC transplantation leads to myelination in preterm WMI rats. Transmission electron microscopy was used to analyze axonal ensheathment and myelin compaction. Compared with axons in normal rats, demyelination and deformed myelin sheaths were observed in most axons of the model rats (Figure 6A Model). In the graft group, demyelination was less common, although most myelin sheaths presented multilayers (Figure 6A Graft). In addition, the G-ratio between different groups was compared. G-ratio was defined as the ratio of axonal diameter to total myelin-ensheathed fiber diameter, and indicated the severity of myelin sheath damage; the higher the score, the worse the myelin injury.29 The results showed limited damage in the hOPC-transplanted group, as the G-ratio was significantly lower than that in the model group (Figure 6B and P<0.01, Figure 6C).
A grading system30 was implemented for the quantitative evaluation of myelin damage (Table 1). A high score indicates severe damage. Consistent with the G-ratio, the scores of the graft group were significantly lower than those of the model group without hOPC transplantation (P < 0.001, Figure 6D). Taken together, hOPC transplantation led to re-myelination in preterm WMI rats.
 |
Table 1 Ultrastructural Grading Scores System |
Effects of hOPCs on Spatial Learning and Memory in Preterm WMI Rats
To determine whether hOPC transplantation rescues neurobehavioral deficits in preterm WMI rats, we performed the Morris water maze test to compare spatial learning and memory capabilities between the control, model, and graft groups. The graft group showed a decreased escape latency in the navigation trials compared with the model group (Figure 7A and P<0.05, Figure 7B). In the probe trials, preterm WMI rats showed fewer platform crossings and less time spent in the target quadrant than the control and graft groups, but there was no significant difference between the control and graft groups (P<0.05, Figure 7C and P<0.05, Figure 7D). The results above showed that hOPC transplantation rescued spatial learning and memory deficits of preterm WMI rats.
Discussion
In the present study, we investigated the fate and therapeutic potential of hOPC transplanted into the injured corpus callosum of preterm WMI rats. We demonstrated that hOPC transplantation could lead to re-myelination and cognitive recovery.
Preterm WMI, which seriously affects premature infants quality of life, is the leading cause of neural deficits. However, there is no effective treatment for preterm WMI, and it is necessary to explore treatments by proving its efficacy in animal models. Rodents are the most commonly used species in the study of nervous system diseases due to their anatomical similarity to the human nervous system. However, there are some differences in the development of the nervous system between human and rodents; for example, rodents do not mature until after birth. The high-risk period of human preterm WMI is 23–32 weeks of gestational age which corresponds to 2–5 days after birth in rodents about the development of oligodendrocyte lineage in white matter. We established an animal model of hypoxic-ischemic injury in premature infants on the third day after birth in rodents. Seven days after establishing the model, the pathology was observed by HE and MBP staining, showing that the brain tissue was atrophied, the corpus callosum and external capsule were significantly thinned, and the myelin sheath was lost. Transmission electron microscopy showed that the ultramicrostructure of myelin sheath changes in preterm WMI rats was characterized by a decreased myelin sheath, disordered arrangement, thinning of the myelin sheath, loose structure, and even disintegration of some myelin sheath.
The hypoxic-ischemic injury model of rats at 3 days after birth blocked the oligodendrocyte lineage maturation and led to the disturbance of myelin formation. The model rats had cognitive impairment in long-term neu WMI brain, the cells survived and were successfully integrated into the host brain, showing strong migration ability along the white matter tracts. The cells were mainly distributed in the corpus callosum, cingulate, external capsule of the injured hemisphere, and the cortex near the corpus callosum. In contrast, some cells migrated to the corresponding parts of the contralateral cerebral hemisphere, and the trajectory of migration was consistent with the lesions of preterm WMI. However, Kim et al and Kuai et al showed that ESC-derived OPCs exhibit a poor survival rate and limited migration ability in EAE and twitcher mice, which lead to the failure of remyelination.31,32 Compared to previous studies, the long-term survival and extensive migration ability of hOPCs in our study can be attributed to three aspects. First, hOPCs identification strategy is a reliable strategy. hOPCs derived using our protocol expressed OLIG2, NG2, PDGFRα, and O4, close to the current definition of oligodendrocyte progenitors.33,34 Cell viability largely determines the effect of transplantation, and our cells have not undergone a long-term cell sorting operation process, which can ensure that the viability of transplanted cells is over 95%. Second, immunosuppressants can reduce the immune rejection of transplanted cells and prolong the survival time of transplanted cells,35 thereby exerting the cell substitution and paracrine effects, reducing myelin loss by promoting the formation of endogenous myelin.36 Third, it should be noted that the transplantation time window is also important. In the first 3 days after HI injury, many inflammatory factors were released, and brain edema did not subside,37,38 which is detrimental to the survival of hOPCs transplanted into cerebral parenchyma. Research has shown that transplantation attenuates brain injury at 3–10 days.39,40 In the present study, hOPC transplantation was performed 7 days after HI induction when inflammatory factors and toxic products subsided significantly. Based on the injury sites responsible for critical symptoms, hOPCs were injected into the injured corpus callosum using a stereotactic apparatus, and transplantation was performed in situ to achieve accurate repair of the damaged site.
Three months after hOPC transplantation, Ki67 and HNA immunofluorescence staining showed that the proportion of the double-positive cells was less than 6%. Therefore, we speculated that only a few cells were in the division and proliferation stages after transplantation. Most hOPCs showed multi-branched complex morphology, indicating that most hOPCs developed to a more mature oligodendrocyte stage. Immunofluorescence staining showed that the transplanted cells expressed more myelin marker CC1, and only 24.6±0.79% of cells expressed MBP. Based on this, we speculated that two different proteins might appear in different stages of myelin formation. Ultrastructural electron microscopy showed a compact myelin sheath wrapping the axon in the graft group. At the same time, some cells remained in the oligoprogenitor stage and expressed OLIG2, hardly differentiating into astrocytes, which corroborates the results of previous studies.41,42
A study has shown that improved neurological function in rats is related to repairing myelin and white matter structure because the latter can restore jumping conduction and brain functional connectivity.43 Previously it was suggested that after intracerebroventricular transplantation, genetically modified hOPCs survive for more than 5 weeks in vivo and ameliorate neurobehavioral and cognitive deficits in PVL rats,12 suggesting that hOPC replacement has the best therapeutic prospects for preterm WMI. Whether hOPCs can survive for a long time and improve neuropathological and neurobehavioral without immunosuppressant remains to be studied. In addition, it has been shown that impaired M2 microglia function leads to impaired OPC differentiation, further inhibiting the myelin regeneration process, suggesting that we need to pay more attention to microglia and oligodendrocyte interactions and the relationship to neuroinflammation and myelin regeneration in future studies. These were not addressed in the present study.44 Data from such studies should provide important insights that will help the development of novel cell therapeutic strategies for WMI patients.
The present study has limitations. The neurological abnormalities in PWMI rats are mainly due to abnormal brain connections caused by white matter damage, in addition to gray matter damage, such as damage to cortical motor areas and sensorimotor areas.12,22 Therefore, after modeling and cell transplantation, we should not only focus on the pathological changes of corpus callosum, cingulate, and external capsule in the white matter area on the injured side, but also on the extent of hippocampal damage in the gray matter area and the distribution and differentiation of transplanted cells.
Conclusions
This study confirmed that hOPCs transplanted through corpus callosum could migrate and distribute widely in preterm WMI rats, survive for at least 3 months, and gradually differentiate into OLS in the brain. At the same time, hOPCs can promote the repair of myelin ultrastructure and improve the cognitive function of rats. It is suggested that hOPCs transplantation restores cognitive deficits of preterm WMI animals by decrease demyelination in periventricular white matter. Our findings will further contribute to the improvement of cellular therapeutic strategies.
Highlights
- A method to differentiate human neural stem cells (hNSCs) into human OPCs (hOPCs) was developed.
- Transplanted hOPCs were morphological differentiation.
- Exogenous hOPCs could differentiate into CC1+ OLS in the brain of WMI rats improving their cognitive functions.
Statement of Human and Animal Rights
All protocols were conducted according to the World Medical Association Declaration of Helsinki and according to the ethical guidelines of the Ethics Committee of the Sixth Medical Center of PLA General Hospital, Pediatrics Ethics Committee in Beijing.
Data Sharing Statement
The data supporting this study’s findings are available from the corresponding author upon reasonable request.
Statement of Informed Consent
Aborted human embryos, aged 9 weeks post-conception, were obtained from the Department of Obstetrics and Gynecology of the Sixth Medical Center of PLA General Hospital, Beijing, China, from patients who had requested to terminate gestation. According to the guidelines approved by the hospital’s ethics committee, the donors provided written consent to donate the aborted fetuses after being fully informed about the study.
Funding
The National Key R&D Program of China supported this work [2018YFA0108601]. The funding source had no role in the study’s design, collection, analysis, interpretation of data, or writing of the manuscript.
Disclosure
The authors declared no potential conflicts of interest concerning this article’s research, authorship, and publication.
References
1. Cerisola A, Baltar F, Ferrán C, Turcatti E. Mechanisms of brain injury of the premature baby. Medicina. 2019;79:10–14.
2. Rantakari K, Rinta-Koski OP, Metsäranta M, et al. Early oxygen levels contribute to brain injury in extremely preterm infants. Pediatr Res. 2021;90(1):131–139. doi:10.1038/s41390-021-01460-3
3. Back SA. Brain injury in the preterm infant: new horizons for pathogenesis and prevention. Pediatr Neurol. 2015;53:185–192. doi:10.1016/j.pediatrneurol.2015.04.006
4. Bennet L, Dhillon S, Lear CA, et al. Chronic inflammation and impaired development of the preterm brain. J Reprod Immunol. 2018;125:45–55. doi:10.1016/j.jri.2017.11.003
5. van Tilborg E, Heijnen CJ, Benders MJ, et al. Impaired oligodendrocyte maturation in preterm infants: potential therapeutic targets. Prog Neurobiol. 2016;136:28–49. doi:10.1016/j.pneurobio.2015.11.002
6. Gunn AJ, Bennet L. Brain cooling for preterm infants. Clin Perinatol. 2008;35(4):735–748. doi:10.1016/j.clp.2008.07.012
7. Wang Y. Mesenchymal stem cells (MSC) delays the occurrence of graft-versus-host disease (GVHD) in the inhibition of hematopoietic stem cells in major histocompatibility complex semi-consistent mice by regulating the expression of IFN-γ/IL-6. Bioengineered. 2021;12(1):4500–4507. doi:10.1080/21655979.2021.1955549
8. Li L, Shi W, Zhou J. Effect of CMNa combined with radiotherapy on the tumor immune microenvironment of mouse cervical cancer cell transplantation tumor model. Bioengineered. 2021;12(1):1066–1077. doi:10.1080/21655979.2021.1899532
9. van der Knaap MS, Wolf NI, Heine VM. Leukodystrophies: five new things. Neurol Clin Pract. 2016;6:506–514. doi:10.1212/CPJ.0000000000000289
10. Nishiyama A, Boshans L, Goncalves CM, Wegrzyn J, Patel KD. Lineage, fate, and fate potential of NG2-glia. Brain Res. 2016;1638:116–128. doi:10.1016/j.brainres.2015.08.013
11. Dietz KC, Polanco JJ, Pol SU, Sim FJ. Targeting human oligodendrocyte progenitors for myelin repair. Exp Neurol. 2016;283:489–500. doi:10.1016/j.expneurol.2016.03.017
12. Kim TK, Park D, Ban YH, et al. Improvement by human oligodendrocyte progenitor cells of neurobehavioral disorders in an experimental model of neonatal periventricular leukomalacia. Cell Transplant. 2018;27:1168–1177. doi:10.1177/0963689718781330
13. Windrem MS, Nunes MC, Rashbaum WK, et al. Fetal and adult human oligodendrocyte progenitor cell isolates myelinate the congenitally dysmyelinated brain. Nat Med. 2004;10:93–97. doi:10.1038/nm974
14. Sim FJ, McClain CR, Schanz SJ, Protack TL, Windrem MS, Goldman SA. CD140a identifies a population of highly myelinogenic, migration-competent and efficiently engrafting human oligodendrocyte progenitor cells. Nat Biotechnol. 2011;29:934–941. doi:10.1038/nbt.1972
15. Wang S, Bates J, Li X, et al. Human iPSC-derived oligodendrocyte progenitor cells can myelinate and rescue a mouse model of congenital hypomyelination. Cell Stem Cell. 2013;12:252–264. doi:10.1016/j.stem.2012.12.002
16. Xu L, Ryu J, Hiel H, et al. Transplantation of human oligodendrocyte progenitor cells in an animal model of diffuse traumatic axonal injury: survival and differentiation. Stem Cell Res Ther. 2015;6(1):93. doi:10.1186/s13287-015-0087-0
17. Stadlin A, James A, Fiscus R, Wong YF, Rogers M, Haines C. Development of a postnatal 3-day-old rat model of mild hypoxic-ischemic brain injury. Brain Res. 2003;993:101–110. doi:10.1016/j.brainres.2003.08.058
18. Buchet D, Garcia C, Deboux C, Nait-Oumesmar B, Baron-Van Evercooren A. Human neural progenitors from different foetal forebrain regions remyelinate the adult mouse spinal cord. Brain. 2011;134:1168–1183. doi:10.1093/brain/awr030
19. Wang C, Luan Z, Yang Y, et al. High purity of human oligodendrocyte progenitor cells obtained from neural stem cells: suitable for clinical application. J Neurosci Methods. 2015;240:61–66. doi:10.1016/j.jneumeth.2014.10.017
20. Sheldon RA, Chuai J, Ferriero DM. A rat model for hypoxic-ischemic brain damage in very premature infants. Biol Neonate. 1996;69:327–341. doi:10.1159/000244327
21. Zhu LH, Bai X, Zhang N, Wang SY, Li W, Jiang L. Improvement of human umbilical cord mesenchymal stem cell transplantation on glial cell and behavioral function in a neonatal model of periventricular white matter damage. Brain Res. 2014;1563:13–21. doi:10.1016/j.brainres.2014.03.030
22. Chen LX, Ma SM, Zhang P, et al. Neuroprotective effects of oligodendrocyte progenitor cell transplantation in premature rat brain following hypoxic-ischemic injury. PLoS One. 2015;10:e0115997. doi:10.1371/journal.pone.0115997
23. Lin S, Fan LW, Pang Y, Rhodes PG, Mitchell HJ, Cai Z. IGF-1 protects oligodendrocyte progenitor cells and improves neurological functions following cerebral hypoxia-ischemia in the neonatal rat. Brain Res. 2005;1063(1):15–26. doi:10.1016/j.brainres.2005.09.042
24. Park D, Lee SH, Bae DK, et al. Transplantation of human adipose tissue-derived mesenchymal stem cells restores the neurobehavioral disorders of rats with neonatal hypoxic-ischemic encephalopathy. Cell Med. 2013;5(1):17–28. doi:10.3727/215517913X658936
25. Zhou Q, Choi G, Anderson DJ. The bHLH transcription factor Olig2 promotes oligodendrocyte differentiation in collaboration with Nkx2.2. Neuron. 2001;31:791–807. doi:10.1016/S0896-6273(01)00414-7
26. Goldman SA, Kuypers NJ. How to make an oligodendrocyte. Development. 2015;142:3983–3995. doi:10.1242/dev.126409
27. Douvaras P, Wang J, Zimmer M, et al. Efficient generation of myelinating oligodendrocytes from primary progressive multiple sclerosis patients by induced pluripotent stem cells. Stem Cell Rep. 2014;3:250–259. doi:10.1016/j.stemcr.2014.06.012
28. Mohammadi S, Carey D, Dick F, et al. Whole-brain in-vivo measurements of the axonal g-ratio in a group of 37 healthy volunteers. Front Neurosci. 2015;9:441. doi:10.3389/fnins.2015.00441
29. Hagiwara A, Hori M, Yokoyama K, et al. Analysis of white matter damage in patients with multiple sclerosis via a novel in vivo MR method for measuring myelin, axons, and g-ratio. AJNR Am J Neuroradiol. 2017;38:1934–1940. doi:10.3174/ajnr.A5312
30. Aktas S, Comelekoglu U, Yilmaz SN, et al. Electrophysiological, biochemical and ultrastructural effects of radiotherapy on normal rat sciatic nerve. Int J Radiat Biol. 2013;89:155–161. doi:10.3109/09553002.2013.734941
31. Kim H, Walczak P, Kerr C, et al. Immunomodulation by transplanted human embryonic stem cell-derived oligodendroglial progenitors in experimental autoimmune encephalomyelitis. Stem Cells. 2012;30:2820–2829. doi:10.1002/stem.1218
32. Kuai XL, Ni RZ, Zhou GX, et al. Transplantation of mouse embryonic stem cell-derived oligodendrocytes in the murine model of globoid cell leukodystrophy. Stem Cell Res Ther. 2015;6:30. doi:10.1186/s13287-015-0024-2
33. Alsanie WF, Niclis JC, Petratos S. Human embryonic stem cell-derived oligodendrocytes: protocols and perspectives. Stem Cells Dev. 2013;22:2459–2476. doi:10.1089/scd.2012.0520
34. Wilson HC, Scolding NJ, Raine CS. Co-expression of PDGF alpha receptor and NG2 by oligodendrocyte precursors in human CNS and multiple sclerosis lesions. J Neuroimmunol. 2006;176:162–173. doi:10.1016/j.jneuroim.2006.04.014
35. Wennersten A, Holmin S, Al Nimer F, Meijer X, Wahlberg LU, Mathiesen T. Sustained survival of xenografted human neural stem/progenitor cells in experimental brain trauma despite discontinuation of immunosuppression. Exp Neurol. 2006;199:339–347. doi:10.1016/j.expneurol.2005.12.035
36. Sypecka J, Sarnowska A. The neuroprotective effect exerted by oligodendroglial progenitors on ischemically impaired hippocampal cells. Mol Neurobiol. 2014;49:685–701. doi:10.1007/s12035-013-8549-9
37. Al Mamun A, Yu H, Romana S, Liu F. Inflammatory responses are sex specific in chronic hypoxic-ischemic encephalopathy. Cell Transplant. 2018;27:1328–1339. doi:10.1177/0963689718766362
38. Saliba E, Marret S. Cerebral white matter damage in the preterm infant: pathophysiology and risk factors. Semin Neonatol. 2001;6:121–133. doi:10.1053/siny.2001.0043
39. Donega V, van Velthoven CT, Nijboer CH, et al. Intranasal mesenchymal stem cell treatment for neonatal brain damage: long-term cognitive and sensorimotor improvement. PLoS One. 2013;8:e51253. doi:10.1371/journal.pone.0051253
40. Pimentel-Coelho PM, Magalhães ES, Lopes LM, deAzevedo LC, Santiago MF, Mendez-Otero R. Human cord blood transplantation in a neonatal rat model of hypoxic-ischemic brain damage: functional outcome related to neuroprotection in the striatum. Stem Cells Dev. 2010;19:351–358. doi:10.1089/scd.2009.0049
41. Webber DJ, van Blitterswijk M, Chandran S. Neuroprotective effect of oligodendrocyte precursor cell transplantation in a long-term model of periventricular leukomalacia. Am J Pathol. 2009;175:2332–2342. doi:10.2353/ajpath.2009.090051
42. Windrem MS, Schanz SJ, Guo M, et al. Neonatal chimerization with human glial progenitor cells can both remyelinate and rescue the otherwise lethally hypomyelinated shiverer mouse. Cell Stem Cell. 2008;2:553–565. doi:10.1016/j.stem.2008.03.020
43. Utzschneider DA, Archer DR, Kocsis JD, Waxman SG, Duncan ID. Transplantation of glial cells enhances action potential conduction of amyelinated spinal cord axons in the myelin-deficient rat. Proc Natl Acad Sci USA. 1994;91:53–57. doi:10.1073/pnas.91.1.53
44. Miron VE, Franklin RJ. Macrophages and CNS remyelination. J Neurochem. 2014;130(2):165–171. doi:10.1111/jnc.12705
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.