Back to Journals » Infection and Drug Resistance » Volume 15
Old Age is an Independent Risk Factor for Pneumonia Development in Patients with SARS-CoV-2 Omicron Variant Infection and a History of Inactivated Vaccine Injection
Authors Tong X , Huang Z, Zhang X, Si G, Lu H, Zhang W, Xue Y , Xie W
Received 25 June 2022
Accepted for publication 15 September 2022
Published 21 September 2022 Volume 2022:15 Pages 5567—5573
DOI https://doi.org/10.2147/IDR.S380005
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Prof. Dr. Héctor Mora-Montes
Xuecheng Tong,1,2 Zeyu Huang,1 Xiujun Zhang,1,2 Guocan Si,2 Huifen Lu,2 Wei Zhang,2 Yuan Xue,1,2 Weibin Xie3
1Institute of Hepatology, the Third People’s Hospital of Changzhou, Changzhou, People’s Republic of China; 2Department of Infectious Diseases, the Third People’s Hospital of Changzhou, Changzhou, People’s Republic of China; 3Department of Anesthesiology, the Third People’s Hospital of Changzhou, Changzhou, People’s Republic of China
Correspondence: Yuan Xue; Weibin Xie, Institute of Hepatology, the Third People’s Hospital of Changzhou, No. 300 Lanling North Road, Changzhou, Jiangsu, 213000, People’s Republic of China, Tel +86 519 82009059, Email [email protected]; [email protected]
Objective: Analyzing the risk factors for pneumonia development in breakthrough cases with a history of inactivated vaccine injection is important. The present study aimed to investigate the risk factors for pneumonia development during Omicron variant infection.
Design and Methods: The clinical data were retrospectively collected from 187 patients who previously received inactivated vaccine and were infected by the Omicron variant.
Results: Among the 187 patients, 73 had 2 doses of inactivated vaccine injection and the remaining 114 had 3 doses; 19 patients had pneumonia at admission. The univariate logistic analysis showed that age, baseline platelet count, D-dimer level, and CD8+ T lymphocyte count were associated with pneumonia development at admission. The multivariate analysis showed that only age was the independent risk factor for pneumonia development (odds ratio = 1.046, 95% confidence interval: 1.003– 1.091, P = 0.04). With an optimal cutoff value of 46, 4.4% (4/91) patients in the age < 46 years group and 15.63% (15/96) patients in the age ≥ 46 years group had pneumonia (χ2 = 6.454, P = 0.01). Moreover, age negatively correlated with CD8+ T cell count, B cell count, and albumin and uric acid levels (all P < 0.01), while age positively correlated with the glucose level (P < 0.01).
Conclusion: Old age was the only independent risk factor for pneumonia development in patients with Omicron variant infection and a history of inactivated vaccine injection.
Keywords: COVID-19, glucose, Omicron, pneumonia, SARS-CoV-2
Introduction
Coronavirus disease 2019 (COVID-19) is a global public health concern. It is caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). The Omicron variant, possessing at least 35 mutations in the spike protein, has become the dominant variant, and its reduced sensitivity to neutralizing antibodies elicited by vaccination is a major concern.1–3
Antiviral therapy against Omicron variant remains limited, and specific monoclonal antibodies show less effective due to the high mutation rate of the S-protein.4,5 Intranasal variant-specific vaccine which can induce IgA-mediated mucosal immune responses, is still in continuous improvement.6
The virulence of the Omicron variant is milder than that of other variants of concern, and hence critically ill patients are not common in Omicron variant waves. Omicron is associated with a lower risk of hospitalization and a lower prevalence of pneumonia.7,8 Nevertheless, the risk factors for stratifying patients at high risk of pneumonia development are of significance for early intervention. Data from a retrospective study showed that 4 in 14 children developed pneumonia and 2 required mechanical ventilation.9 Another retrospective study showed that male sex, increasing age, and smoking were associated with pneumonia development.10 Also, patients with chronic comorbidities, including pulmonary obstructive disease, diabetes, hypertension, kidney disease, and immunosuppression, were more likely to develop pneumonia.10 Despite a reduction in severity and mortality, patients with persistent high fever were recommended to undergo chest computed tomography (CT).11
Concerns about the immune evasion of the Omicron variant have increased.1,12 Moreover, the possibility of full-vaccination coverage and a booster vaccination was relatively lower in older individuals or in patients with chronic comorbidities.3 Full vaccination and a booster vaccination can significantly protect old patients from pneumonia and serious COVID-19.13 Considering that pneumonia is the most common manifestation of serious COVID-19,14 analyzing the risk factors for pneumonia development in breakthrough cases with a history of inactivated vaccine injection is crucial.
To date, the incidence of pneumonia and its severity remains largely unknown in patients with breakthrough cases caused by the Omicron variant. This study investigated the risk factors for pneumonia development during Omicron variant infection in patients with a history of inactivated vaccine injection.
Methods
Patients
The data from 187 adult patients with laboratory-confirmed COVID-19 were retrospectively analyzed. These patients were diagnosed according to the Chinese guidelines for the diagnosis and treatment of COVID-19,14 and were admitted to the Third People’s Hospital of Changzhou between March 13, 2022, and May 10, 2022. Pneumonia was confirmed based on the CT manifestations and positive SARS-CoV-2 RNA tests.
The study protocol was approved by the ethics committee of the Third People’s Hospital of Changzhou according to the Declaration of Helsinki 2013.
Reverse Transcription–Polymerase Chain Reaction Assay
Reverse transcription–polymerase chain reaction (RT-PCR) assay was performed in the Laboratory of the Third People’s Hospital of Changzhou using a commercial kit (Jiangsu Bioperfectus Technologies Co., Taizhou, China). Positive SARS-CoV-2RNA tests were judged based on the lower cycle threshold (Ct) values of ORF1ab and N genes (less than 35). For Ct ≥35, duplicate RT-PCR tests were performed more than once at 24-h intervals.
Statistical Analysis
The continuous data were expressed as median (interquartile range) and compared using the Kruskal–Wallis test. The categorical data were expressed as frequencies and compared using the chi-square test. The univariate and multivariate logistic analyses were performed to investigate the risk factors for pneumonia development. An optimal cutoff value was determined using MedCalc version 15.2.2 software for Windows (Medcalc Software, Mariakerke, Belgium). A comparison was performed using SPSS 25.0 (NY, USA), and a two-sided P value <0.05 indicated a statistically significant difference.
Results
Baseline Characteristics of Patients with COVID-19
Among the 187 patients, 73 had 2 doses of inactivated vaccine injection and the remaining 114 had 3 doses. The main kinds of vaccines were CoronaVac® (101/187, 54%) and COVILO® (55/187, 29.4%). Further, 97.3% (182/187) of the patients received the second dose of vaccine after 6 months prior to infection. No patient developed a critical illness.
The comorbidities were rare in these patients, including 8 with diabetes, 17 with hypertension, 1 with stable pulmonary tuberculosis, and 1 with chronic kidney disease. None of the patients were under treatment with glucocorticoids or antibiotics at admission.
The CT scan revealed 13 patients with unilateral pneumonia and 6 with bilateral pneumonia. The patchy ground-glass opacities (GGO) were the main characteristics, while consolidation with subpeural distribution was not seen in these patients. Just one patient had cluster-like GGO. Repeated CT scan was performed in 11 patients, and GGO had no obvious regress in the first week of hospitalization. For patients with and without pneumonia, no significant difference was found in the incidence of diabetes (1/19 and 7/168, χ2 = 0.050, P = 0.58) or hypertension (1/19 and 16/168, χ2 = 0.375, P > 0.99) (Table 1).
 |
Table 1 Characteristics of Patients with and without Pneumonia at Admission |
Characteristics of Patients With and Without Pneumonia at Admission
The median age of the patients with pneumonia was 50 year-old, while the median age was 45 year-old in non-pneumonia group. Table 1 presents that patients with pneumonia were significantly older than those without pneumonia (Z = 2.342, P = 0.02). Among the 19 patients with pneumonia, 7 received 2 doses of vaccine, and the remaining 12 patients received 3 doses. For patients without pneumonia, 66 patients received 2 doses of vaccine, and 102 patients received 3 doses. The doses of vaccine, as well as the duration from the first or second injection to infection, showed no significant difference between patients with and without pneumonia (all P > 0.05).
Most patients with and without pneumonia had fever (13/19 and 112/168, χ2 = 0.024, P > 0.99), and the duration of fever was not significantly different between the two groups (Z = 0.623, P = 0.53). Moreover, cough was not common with pneumonia or without pneumonia (2/19 and 28/168, χ2 = 0.478, P = 0.74).
For patients with and without pneumonia, the median duration of RNA positivity was 10 and 9 days, respectively, and the difference was not significant (Z = 1.653, P = 0.10). In addition, patients with pneumonia had higher D-dimer levels (Z = 2.401, P = 0.02).
Risk Factors for Pneumonia Development
As shown in Table 2, the univariate logistic analysis showed that age, baseline platelet count, D-dimer level, and CD8+ T lymphocyte count were associated with pneumonia development at admission. The multivariate analysis showed that only age was the independent risk factor for pneumonia development [odds ratio (OR) = 1.046, 95% confidence interval (CI):1.003–1.091, P = 0.04].
 |
Table 2 Risk Factors for Pneumonia at Admission |
With an optimal cutoff value of 46, 4.4% (4/91) patients in the age <46 years group and 15.63% (15/96) patients in the age ≥46 years group had pneumonia (χ2 = 6.454, P = 0.01); the sensitivity and specificity were 78.95 and 57.74, respectively. The patients were divided into two groups: low-risk group (age <46) and high-risk group (age ≥46), respectively.
Correlation Between Age, Glucose and Albumin Levels, and Immune Cell Count
Figure 1 demonstrates that age negatively correlated with CD8+ T cell count, B cell count, and albumin (all P < 0.01), while age and glucose level were positively correlated (P < 0.01). Moreover, age did not significantly correlate with CD4+ T cell count.
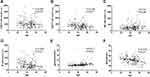 |
Figure 1 Correlation between age and CD4+ T cell count (A), CD8+ T cell count (B), NK cell count (C), B cell count (D), glucose (E) and albumin (F) in Omicron variant-infected patients. |
Discussion
The present study analyzed the risk factors for pneumonia development in patients with Omicron variant infection and a history of inactivated vaccine injection. Among the 187 breakthrough cases, 19 patients developed pneumonia. The patients with pneumonia were older than those without pneumonia. The univariate logistic analysis showed that age, platelet count, D-dimer level, and CD8+ T lymphocyte count were associated with pneumonia development. The multivariate analysis showed that age was the only independent risk factor.
The neutralizing activity of antibodies against the Omicron variant is limited.12,15,16 Also, the knowledge about the efficiency of inactivated vaccines during the Omicron wave is scarce. Recently, Zheng et al reported that full vaccination and a booster vaccination could protect old patients from pneumonia and serious COVID-19.13 The kinds of vaccine, the doses of injection, and the duration between vaccination to infection may affect the protections provided by the vaccine.10,17 Jassat et al reported that 37% of Omicron variant-infected patients had pneumonia in South Africa.18 In the present study, only 10.2% (19/187) of breakthrough cases had pneumonia. Although increasing evidence shows a decrease in the severity of COVID-19 during the Omicron variant wave, pneumonia development remains a concern in clinical practice.18 Compared with data from Africa,18 the decreased incidence of pneumonia among the breakthrough cases suggested that the inactivated vaccine might effectively reduce pneumonia development. In addition, intranasal variant-specific vaccine which can induce IgA-mediated mucosal immune responses, is a promising alternative.6 Knowledge about the breakthrough cases receiving intranasal vaccine remain largely unknown.
The data from the present study showed that age was the independent risk factor for pneumonia development. The correlation analysis showed that age negatively correlated with CD8+ T cell count, B cell count, and albumin and uric acid levels, while it positively correlated with the glucose level. These results suggested that both metabolic and immune factors might influence the outcome. The glucose level correlated with the poor outcome and prolonged SARS-CoV-2 shedding in Delta variant-infected patients.19–22 Patients with lower uric acid levels were more likely to have severe symptoms.23,24 It was speculated that the cellular and humoral immunity was impaired and the level of neutralizing antibodies reduced with age. Thus, it was anticipated that the regulation of metabolism and immunity might be a promising intervention in treating COVID-19.
There are several limitations to the present study. First, the neutralizing antibodies were not tested in the breakthrough cases. Consequently, the relationship between the titers of neutralizing antibodies and pneumonia development could not be revealed. Second, the number of patients was comparatively limited, and the comorbidities including diabetes and pulmonary tuberculosis were rare. Third, no patient was critically ill, or received glucocorticoids or antineoplastic agents, which may affect the viral shedding time. Thus, multicenter, lager-scale and prospective studies are warranted to confirm the current findings.
Conclusion
Old age was the only independent risk factor for pneumonia development in patients with Omicron variant infection and a history of inactivated vaccine injection.
Abbreviations
CI, Confidence interval; COVID-19, coronavirus disease 2019; Ct, cycle threshold; CT, computed tomography; IQR, interquartile range; OR, odds ratio; RT-PCR, reverse transcription–polymerase chain reaction; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2; VOCs, variants of concern.
Data Sharing Statement
The data are available from the corresponding author upon request.
Ethical Approval
The study was approved by the ethics committee of the Third People’s Hospital of Changzhou according to the Declaration of Helsinki 2013.
Consent for Publication
Written consent was exempted for this retrospective and anonymous study.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
This study was supported by the 333 High-level Talents Project of Jiangsu Province (LGY2020032), the Science and Technology Project of Changzhou (CJ20200057), Qingmiao Talents Cultivation Project of Changzhou Health Commission (CZQM2020089), Project of High-quality Education Resources of Nanjing Medical University (2021H049).
Disclosure
The authors declare that there are no conflicts of interest in this work.
References
1. Ai J, Wang X, He X, et al. Antibody evasion of SARS-CoV-2 Omicron BA.1, BA.1.1, BA.2, and BA.3 sub-lineages. Cell Host Microbe. 2022;30(8):1077–1083.e4. doi:10.1016/j.chom.2022.05.001
2. Yu X, Wei D, Xu W, et al. Reduced sensitivity of SARS-CoV-2 Omicron variant to antibody neutralization elicited by booster vaccination. Cell Discov. 2022;8(1):4. doi:10.1038/s41421-022-00375-5
3. Zhang X, Zhang W, Chen S. Shanghai’s life-saving efforts against the current omicron wave of the COVID-19 pandemic. Lancet. 2022;399(10340):2011–2012. doi:10.1016/S0140-6736(22)00838-8
4. Chavda VP, Hanuma Kumar Ghali EN, Yallapu MM, Apostolopoulos V. Therapeutics to tackle Omicron outbreak. Immunotherapy. 2022;14(11):833–838. doi:10.2217/imt-2022-0064
5. YA E-M YM, Saleh AK, Saleh AK, et al. A comprehensive insight into current control of COVID-19: immunogenicity, vaccination, and treatment. Biomed Pharmacother. 2022;153:113499. doi:10.1016/j.biopha.2022.113499
6. Fan Y, Li X, Zhang L, et al. SARS-CoV-2 Omicron variant: recent progress and future perspectives. Signal Transduct Target Ther. 2022;7(1):141. doi:10.1038/s41392-022-00997-x
7. Harrigan SP, Wilton J, Chong M, et al. Clinical severity of Omicron SARS-CoV-2 variant relative to Delta in British Columbia, Canada: a retrospective analysis of whole genome sequenced cases. Clin Infect Dis. 2022. doi:10.1093/cid/ciac705
8. Ito N, Kitahara Y, Miwata K, Okimoto M, Takafuta T. Comparison of COVID-19 pneumonia during the SARS-CoV-2 Omicron wave and the previous non-Omicron wave in a single facility. Respir Investig. 2022. doi:10.1016/j.resinv.2022.08.001
9. Kinikar AA, Vartak S, Dawre R, et al. Clinical profile and outcome of hospitalized confirmed cases of omicron variant of SARS-CoV-2 among children in Pune, India. Cureus. 2022;14(4):e24629. doi:10.7759/cureus.24629
10. Murillo-Zamora E, Trujillo X, Huerta M, et al. First-generation BNT162b2 and AZD1222 vaccines protect from COVID-19 pneumonia during the Omicron variant emergence. Public Health. 2022;207:105–107. doi:10.1016/j.puhe.2022.04.001
11. Ito N, Kitahara Y, Miwata K, Okimoto M, Takafuta T. Can the Omicron variant of COVID-19 cause pneumonia in young patients without risk factors? Clin Case Rep. 2022;10(5):e05684. doi:10.1002/ccr3.5684
12. Hoffmann M, Kruger N, Schulz S, et al. The Omicron variant is highly resistant against antibody-mediated neutralization: implications for control of the COVID-19 pandemic. Cell. 2022;185(3):447–456e411. doi:10.1016/j.cell.2021.12.032
13. Li M, Liu Q, Wu D, et al. Association of COVID-19 vaccination and clinical severity of patients infected with delta or Omicron variants - China, May 21, 2021-February 28, 2022. China CDC Wkly. 2022;4(14):293–297. doi:10.46234/ccdcw2022.074
14. China NHCotPsRo. Diagnosis and treatment plan for COVID-19(trial version 9). Int J Epidemiol Infect Dis. 2;2022. doi:10.3760/cma.j.cn331340-20220325-00065
15. Iketani S, Liu L, Guo Y, et al. Antibody evasion properties of SARS-CoV-2 Omicron sublineages. Nature. 2022;604(7906):553–556. doi:10.1038/s41586-022-04594-4
16. Planas D, Saunders N, Maes P, et al. Considerable escape of SARS-CoV-2 Omicron to antibody neutralization. Nature. 2022;602(7898):671–675. doi:10.1038/s41586-021-04389-z
17. Wang K, Jia Z, Bao L, et al. Memory B cell repertoire from triple vaccinees against diverse SARS-CoV-2 variants. Nature. 2022;603(7903):919–925. doi:10.1038/s41586-022-04466-x
18. Abdullah F, Myers J, Basu D, et al. Decreased severity of disease during the first global omicron variant covid-19 outbreak in a large hospital in Tshwane, South Africa. Int J Infect Dis. 2022;116:38–42. doi:10.1016/j.ijid.2021.12.357
19. Long H, Li J, Li R, et al. Plasma glucose levels and diabetes are independent predictors for mortality in patients with COVID-19. Epidemiol Infect. 2022;150:e106. doi:10.1017/S095026882200022X
20. Vargas-Rodriguez JR, Valdes Aguayo JJ, Garza-Veloz I, et al. Sustained hyperglycemia and its relationship with the outcome of hospitalized patients with severe COVID-19: potential role of ACE2 upregulation. J Pers Med. 2022;12(5):805. doi:10.3390/jpm12050805
21. Yang P, Wang N, Wang J, et al. Admission fasting plasma glucose is an independent risk factor for 28-day mortality in patients with COVID-19. J Med Virol. 2021;93(4):2168–2176. doi:10.1002/jmv.26608
22. Zhao Y, Xing H, Xu Y. Influence of fasting plasma glucose level on admission of COVID-19 patients: a retrospective study. J Diabetes Res. 2022;2022:7424748. doi:10.1155/2022/7424748
23. Chen B, Lu C, Gu HQ, et al. Serum uric acid concentrations and risk of adverse outcomes in patients with COVID-19. Front Endocrinol. 2021;12:633767. doi:10.3389/fendo.2021.633767
24. Hu F, Guo Y, Lin J, et al. Association of serum uric acid levels with COVID-19 severity. BMC Endocr Disord. 2021;21(1):97. doi:10.1186/s12902-021-00745-2
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
