Back to Journals » HIV/AIDS - Research and Palliative Care » Volume 10
Ocular surface squamous neoplasia in HIV-infected patients: current perspectives
Authors Rathi SG, Ganguly Kapoor A, Kaliki S
Received 6 September 2017
Accepted for publication 19 January 2018
Published 14 March 2018 Volume 2018:10 Pages 33—45
DOI https://doi.org/10.2147/HIV.S120517
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Professor Bassel Sawaya

Shweta Gupta Rathi, Anasua Ganguly Kapoor, Swathi Kaliki
Operation Eyesight Universal Institute for Eye Cancer, LV Prasad Eye Institute, Hyderabad, India
Abstract: Ocular surface squamous neoplasia (OSSN) refers to a spectrum of conjunctival and corneal epithelial tumors including dysplasia, carcinoma in situ, and invasive carcinoma. In this article, we discuss the current perspectives of OSSN associated with HIV infection, focusing mainly on the epidemiology, pathophysiology, clinical manifestations, diagnosis, and treatment of these tumors in patients with HIV. Upsurge in the incidence of OSSN with the HIV pandemic most severely affected sub-Saharan Africa, due to associated risk factors, such as human papilloma virus and solar ultraviolet exposure. OSSN has been reported as the first presenting sign of HIV/AIDS in 26%–86% cases, and seropositivity is noted in 38%–92% OSSN patients. Mean age at presentation of OSSN has dropped to the third to fourth decade in HIV-positive patients in developing countries. HIV-infected patients reveal large aggressive tumors, higher-grade malignancy, higher incidence of corneal, scleral, and orbital invasion, advanced-stage T4 tumors, higher need for extended enucleation/exenteration, and increased risk of tumor recurrence. Current management of OSSN in HIV-positive individuals is based on standard treatment guidelines described for OSSN in the general population, as there is little information available about various treatment modalities or their outcomes in patients with HIV. OSSN can occur at any time in the disease course of HIV/AIDS, and no significant trend has been discovered between CD4 count and grade of OSSN. Furthermore, the effect of highly active antiretroviral therapy on OSSN is controversial. The current recommendation is to conduct HIV screening in all cases presenting with OSSN to rule out undiagnosed HIV infection. Patient counseling is crucial, with emphasis on regular follow-up to address high recurrence rates and early presentation to an ophthalmologist for of any symptoms in the unaffected eye. Effective evidence-based interventions are needed to allow early diagnosis and treatment, as well as prevention of the disease.
Keywords: eye, conjunctiva, OSSN, ocular surface squamous neoplasia, HIV, human immunodeficiency virus
Introduction
Ocular surface squamous neoplasia (OSSN) refers to a spectrum of conjunctival and corneal epithelial tumors including dysplasia, carcinoma-in-situ and invasive carcinoma (squamous cell carcinoma) which may or may not be associated with intraocular or orbital extension.1,2 OS malignancies, such as OSSN, Kaposi’s sarcoma, and non-Hodgkin’s lymphoma, are notably expressed in people with HIV/AIDS, among which OSSN is known to occur in 4%–8% of patients.3,4
Although there has been a decline in the incidence of HIV in recent years, there has been a surge in the incidence of OSSN in the population with HIV, due to its linear relationship with the HIV pandemic in the last few decades.5–18 This dramatic rise in HIV infection during the pandemic has resulted in gradual drift in the age of presentation, clinical course, and management of patients with OSSN.5,19 HIV not only increases risk of OSSN but also influences the severity of disease and its prognosis.
Incidence and risk of OSSN with HIV infection
According to a World Health Organization report, the prevalence of HIV/AIDS worldwide was 36.7 million at the end of 2016. While the burden of the epidemic continues to vary substantially between countries and regions, sub-Saharan Africa remains most severely affected and accounts for almost two-thirds of the total new HIV infections globally.20 The geographical distribution of OSSN all over the world is also affected by the HIV pandemic.10,11,21
HIV infection is recognized as a risk factor for the development of OSSN in various studies from sub-Saharan Africa.13,22–26 The Kampala Cancer Registry in Uganda recorded a sixfold increase in the incidence of conjunctival squamous-cell carcinoma: from an average of six per million per year in 1988 to 35 per million per year in 1992.10 Various studies have observed a three- to 30-fold increased risk of OSSN developing in HIV infected individuals.7,27–29
Although a strong association between HIV and OSSN has been established by numerous studies, some patients with OSSN are not aware of their HIV status until HIV screening confirms the seropositivity. In studies from sub-Saharan Africa where HIV screening was done in all patients with OSSN, seropositivity was detected in 49%–92% cases, indicating a high association between OSSN and HIV status in African countries.5,7–12,14,15,30 In Africa, OSSN has been reported as the first presenting sign of HIV/AIDS in 50%–86% cases.8–12,31 This trend is now well documented in many African countries, and with increasing migration it is appreciated in other developing and developed countries as well. In studies from India by Kaliki et al and Kamal et al, HIV positivity was noticed in 38%–41% of diagnosed OSSN patients, of which 70% were unaware of their status prior to screening.32,33 In 26% of patients, OSSN was the first and only evident manifestation of HIV.32
There are limited data from the US, which shows a higher incidence of OSSN among people with HIV than the general population. Guech-Ongey et al noted a significant 12-fold risk of OSSN in people with HIV/AIDS.6 Frish et al also noticed similar results while studying relative risk of human papilloma virus (HPV)-associated cancers in HIV-infected individuals.34 Goedert and Coté observed that the risk of OSSN among people with HIV changed with duration from diagnosis of AIDS, with an exponential rise in squamous-cell carcinoma in HIV patients 2 years after the diagnosis of AIDS.35 In a study by Karp et al among younger patients (<50 years) with OSSN, 50% were HIV-positive, 33% of whom were unaware of their HIV infection.16 In a systematic review and meta-analysis of 12 studies, it was revealed that HIV infection augmented the risk of OSSN, with an overall relative risk estimate of 8.06 (95% CI 5.29–12.3).18
Epidemiology
Mean age at presentation
The epidemiology of OSSN has changed over the last few decades, especially in developing countries. Although once considered an uncommon tumor occurring in the elderly,36 it is becoming more common and more likely to affect young populations.12,14,32,37–40 It has been observed that the mean age at presentation of OSSN has dropped to the third to fourth decade in HIV-positive patients from the sixth decade in HIV-negative patients.7,11,12,39,41,42 The mean age at presentation of OSSN in people with HIV/AIDS in various studies is approximately 35–41 years.7,8,11,12,14,15,32,39–41 In developed countries, patients with OSSN have a different disease profile and continue to present in elderly in both the general population36,38,43,44 and in HIV-infected individuals.6
Sex predisposition
Studies from Africa have reported a striking feature of either a female preponderance7,10,14,39,41,45 or no sex difference3 in HIV-positive OSSN patients, while others have noted dominance by elderly males.8,15,45 This predilection may result from the presence of the HIV/AIDS pandemic, high HPV exposure, and solar radiation in the region.45 In developed countries, males are more commonly affected than females in the general population,36,38,43,44 as well as in people with HIV.6 Studies from Australia, Britain, and San Francisco have found that 70%–80% of patients with OSSN are males,36,38,46 with rare occurrence of HIV seropositivity.
Etiology
The cause of OSSN is multifactorial, but the precise etiopathogenesis is unknown. HIV,10,11 HPV,11,13,47 and solar ultraviolet exposure14,48 are postulated risk factors for the development of OSSN.6,17 An association between OSSN and cigarette smoking, vitamin A deficiency, and allergic eye disease has also been described. It is suggested that the increase in incidence of OSSN in sub-Saharan Africa is related to the coexistence of the HIV/AIDS pandemic, infection with HPV, and exposure to solar radiation in the region.45,49 The overlap of these factors in parts of the world with rapidly rising incidence of OSSN indicates that they possibly interact with one another. Therefore, it is difficult to describe a single one as the causative factor.50
It is considered that HIV infection causes the breakdown of immunosurveillance of malignant cells as a result of suppression of cell-mediated immunoresponse.51 The relationship between HPV and OSSN is controversial, with variable results about the two types of HPV.52 Studies have established no association between mucosal HPV types and OSSN, while others found an uncertain role of cutaneous HPV type in OSSN.53,54 A systematic review by Carreira et al suggested that only the cutaneous HPV subtypes were associated with an increased risk of OSSN.18 Studies suggest that HPV alone may be a contributing factor to OSSN, but is unlikely to cause it.11,27 Karp et al hypothesized that HIV predisposes to OSSN by creating a “permissive environment” for activation of oncogenic HPV, which subsequently act as a cofactor in the development of neoplasia.16,55 This also explains the increased prevalence of HPV in immunosuppressed individuals with HIV disease.11,56–58
Clinical features
Symptoms
Symptoms in patients with OSSN may range from none at all to severe pain and/or visual loss.1 The most common presentations are a red eye, ocular irritation,46 or appearance of a mass lesion in the eye.14,32 OSSN presents as a slowly growing lesion in the general population,38,59 while it behaves aggressively in HIV-infected individuals in both developing10,16,33 and developed countries.55 It often presents with large and unsightly disfiguring lesions in late disease.45 In HIV-infected individuals, longer duration between onset of symptoms and diagnosis of tumor is noted in a few studies from Africa,7,33,39 with a mean history of 3 months at presentation.28,37 Such longer duration and largeness of the lesion imply that many patients either do not seek medical care early or receive long-term conservative treatment for other ocular conditions due to misdiagnosis.60 Satisfactory training of eye-health care professionals is necessary for active participation to rule out malignancy clinically, to consider incisional biopsy for histopathological confirmation in doubtful cases, and to follow up closely.3
Signs
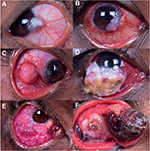
OSSN commonly affects the interpalpebral conjunctiva, and frequently arises from the nasal limbus.1,16 It can present either as a solitary growth or with diffuse involvement of the OS. Solitary tumors can be nodular, noduloulcerative, gelatinous, leukoplakic, placoid, or papillary in morphology61 (Figure 1). Makupa et al noted higher incidence of leukoplakia and feeder vessels,7 while Kabra et al mentioned large lesions with fornicial extension62 at the time of presentation in HIV-infected patients.
The majority of cases are unilateral;3 however, 15% of cases present with bilateral involvement, and multifocal lesions are noted in 3% of cases.32 Bilateral presentation can be simultaneous or sequential, as highlighted in a series of four patients in seropositive patients by Masanganise et al.63 Finances and fear of losing the only eye were the possible reasons for delayed follow-up and advanced disease requiring enucleation or orbital exenteration.
Studies from the sub-Saharan region have established that HIV-infected patients have larger tumors, higher-grade malignancy, and increased risk of tumor recurrence.7,12 Kaliki et al found significant differences between HIV-positive and HIV-negative patients, including larger tumors, higher incidence of corneal, scleral, and orbital invasion, advanced stage T4 American Joint Committee on Cancer tumors, and higher need for extended enucleation/orbital exenteration in HIV-positive cases.32,33 Similar findings of aggressive and invasive OSSN in individuals with HIV were described in a study by Shields et al.55 The invasive nature of the tumor may be correlated with longer duration of symptoms.33
Rarely, this tumor can present as necrotizing scleritis with scleral perforation and prolapse of uveal tissue as a manifestation of intraocular extension.64 Early identification of corneal, scleral invasion, intraocular or orbital extension clinically or with ancillary investigations is crucial. This will reduce morbidity and will result in better anatomical outcomes in terms of globe salvage and cosmesis.65 An unusual case in a seropositive male has been described who presented with widespread metastatic squamous-cell carcinoma after the primary OSSN in the lower palpebral conjunctiva had been misdiagnosed and treated conservatively as chalazion.66 OSSN can often extend to regional lymph nodes, surrounding paranasal sinuses and the brain in HIV-positive individuals. Death may result from regional or distant metastases, as well as intracranial spread.55,67 Therefore, antiretroviral therapy (ART)-center referral is mandatory in every single OSSN patient with positive serology for a complete systemic examination, counseling, and management. The published literature on OSSN and HIV is summarized in Table 1.
Diagnosis
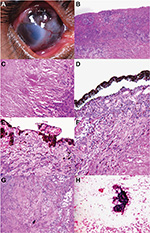
Although OSSN is predominantly a clinical diagnosis, overlap between the clinical features of OSSN and benign lesions sometimes causes difficulty in differentiating between the two.14 Additionally, the occurrence of malignant features on histopathology in pterygia and other benign lesions68,69 shows that histopathological confirmation is indispensable for a definitive diagnosis of OSSN46 (Figure 2). Investigative modalities, such as ultrasound biomicroscopy, anterior-segment optical coherence tomography, computed tomography, or magnetic resonance imaging, of the orbit are necessary to rule out stromal invasion, corneal/scleral involvement, and intraocular/orbital extension of the tumor.
Management
Although the management of OSSN in the general population has been well elaborated in numerous studies, current clinical practice in treatment of OSSN in HIV-positive individuals is based on limited medical literature. There is little information about various treatment modalities currently used against this tumor or their outcomes in patients with HIV. The treatment of OSSN in patients with HIV/AIDS depends on the tumor laterality, extent, invasion into adjacent structures, and overall systemic status. The most commonly performed treatment modality for resectable tumors (fewer than two quadrants) with well-defined lesions is surgical management by wide excision. The main objective of surgical excision is complete removal of the tumor to minimize recurrence.70 Wide excision biopsy is executed by a “no-touch” technique with 4 mm tumor-free margins for conjunctival component and alcohol keratoepitheliectomy with 2 mm tumor-free margins for corneal component, followed by adjunct double-freeze–thaw cryotherapy to the surrounding resected conjunctival margins and OS reconstruction by direct closure or amniotic membrane graft, based on the size of the surgical defect.32,70,71
Partial lamellar sclerectomy is needed in cases with clinical evidence of scleral invasion. Adjunctive cryotherapy is applied to the tumor bed after tumor excision in cases with episcleral fixity, scleral invasion on imaging, or adherence of lesion to the base during the surgery.32 Concomitant primary simple limbal epithelial transplantation after wide excisional biopsy for OSSN involving >3 clock hours prevents LSCD in cases requiring extensive corneoscleral limbal dissection ≥6 clock hours during surgery.72
Diffuse tumors (≥three quadrants of the OS) and large annular lesions can be managed by chemoreduction/chemotherapy with topical agents, such as mitomycin-C, cidofovir, and 5-fluorouracil,74–77 or immunoreduction/immunotherapy with topical and perilesional injection IFNα2b.73,77,78 Chemoprevention with topical chemotherapeutic agents or immunoprevention with topical IFNα2b can be considered in patients with microscopic tumor residue.72,74
Extended enucleation with cryotherapy to the resected conjunctival margins is ideal for tumors with intraocular extension or those with entire OS involvement, including fornices. Orbital exenteration is needed for tumors with orbital extension based on computed-tomography scan of the orbit.33,45,72,79 Secondary treatment with plaque radiotherapy is considered in cases with microscopic residual tumors with positive tumor base on histopathology.22,32,40,80,81 External-beam radiotherapy can be used as an alternative for diffuse lesions.1,44,67 Proton-beam therapy is also suggested as a substitute to enucleation for the treatment of OSSN with intraocular invasion.82
Immunotherapy in OSSN and related concerns in HIV patients
IFNα2b is used in medical treatment of OSSN due to its antiviral and oncostatic effects, as well as its property to activate natural killer cells, which further recognize and destroy tumor cells.83 An intact immune system could be an important connection between IFNα2b therapy and tumor resolution. Therefore, topical IFNα2b may not be an ideal choice for patients with underlying immunosuppression, and it is preferred to switch the treatment to a nonimmunomodulating agent, such as 5-fluorouracil or mitomycin C in this patient population.84 Moreover, in patients with HIV, treatment response could be paradoxical, and lesions may increase in size following the use of IFNα2b. Therefore, serology for HIV is recommended in patients with OSSN before commencing treatment with topical IFNα2b.85
Histopathology
Higher-grade malignancy in the form of stromal, corneal, and scleral invasion has been noted in HIV-positive OSSN patients.7,33 Invasive squamous-cell carcinoma has been noted in 55%–80% of patients with OSSN and HIV, and is associated with reduced prognosis.3,11,32 It is noted that OSSN in HIV-positive patients has higher-grade malignancy with increased tumor invasion, irrespective of the size of lesion or age at presentation.7
Recurrence of OSSN in HIV population
Recurrence rates after complete surgical excision of OSSN in the general population vary between 5% and 33% in various studies from Australia, the US, and Canada.36,38,44,45,65,86,87 In a study from the US, a recurrence rate of 28.5% was noted with simple excisional biopsy, which decreased to 7.7% when combined with cryotherapy.87 About 43% of patients experienced recurrence after treatment with topical medications.75,86
Furthermore, HIV-positive OSSN patients have also shown high recurrence after surgical excision.1,6–15,17,19,31,33,42,49,50,55,88 Recurrence rates of 3%–43% were noted postsurgery in several studies from Africa,3,4,7,11,21,39,45,56 frequently occurring during 6 months of follow-up. High recurrence rates in Africa may be due to late presentation, poor surgery with incomplete or simple excision, and unavailability of adjunctive therapies, such as cryotherapy and chemotherapy, and amniotic membranes.45 However, a study from India also documented high recurrence of 30% in HIV-positive patients compared to 20% in HIV-negative patients, despite adjunctive cryotherapy following wide surgical excision of OSSN.33
The standardized surgical procedure for conjunctival tumors described by Shields et al is fundamental in minimizing recurrences, irrespective of HIV status.55,70 Combined treatment with surgical excision and topical IFNα2b might reduce the risk of recurrence or new tumors in eyes with advanced disease.55,89 It is not clear whether these interventions have different efficacy in people with HIV infection. However, postoperative topical 5-FU drops have been shown to reduce the recurrence of OSSN in HIV patients.52,90
Moreover, accurate documentation of histopathology reports, including interpretations about the base and margins of the tumor, is mandatory. Tabin et al validated the importance of excision margins at the time of surgery in predicting recurrence. They observed that recurrence was 33% in completely excised tumors, but 56% in incompletely excised lesions with mild–severe dysplasia in surgical margins.65 They described that in intraepithelial lesions, the most important factor for tumor recurrence was incomplete tumor excision, not histologic depth of the lesion.60 It has been noted that disease prognosis depends on the grades and types of OSSN, with worse prognosis in mucoepidermoid and spindle-cell carcinoma, but recurrence rates have not been compared with them.91
Cases with poor response or tumor recurrence after topical mitomycin C treatment and residual/recurrent tumors after excisional biopsy require appropriate secondary treatment. Protocol for positive margins includes repeated surgical resection or topical IFNα2b (1 million IU/mL).71 Despite aggressive treatment, patients with advanced disease or multiple recurrences are prone to develop intraocular invasion, with subsequent need for enucleation or orbital exenteration.71,92
Posttreatment follow-up
As the majority of tumors recur within 6 months of treatment, frequent follow-up is recommended post-operatively at 1 week, 6 weeks, 3 months, and 6 months, and subsequently at 6-monthly intervals till 2 years after treatment.45 Recurrent lesions have slow growth and malignant potential, and thus lifelong annual follow-up is mandatory for all patients with a history of OSSN.65 Patient counseling is crucial, with an emphasis on regular follow-up and early presentation to an ophthalmologist for any symptoms in the unaffected eye.
Highly active antiretroviral therapy and OSSN
The effect of highly active ART (HAART) on OSSN is controversial.45 According to a study by Guech-Ongey et al, HAART does not reduce the incidence of OSSN.93 However, complete regression of invasive OSSN with HAART alone has been reported in a patient 6 months after initiation of treatment,94 though anecdotal evidence suggests that in patients who develop the malignancy prior to commencing HAART, HAART does not interrupt tumor growth or recurrence.63
With the introduction of HAART, HIV/AIDS has gradually transformed from an acute and fatal disease into a treatable chronic condition with increased survival. This may lead to a possible increase in the disease burden of OSSN, as well as prevalence of bilateral OSSN.63 Strategies for the long-term management of ophthalmic disorders for HAART responders are important. In developed regions, especially the US and Europe, most patients experience a rise in CD4 lymphocytes during the first few months of HAART. This is followed by reduction in opportunistic infections involving the posterior segment, which frequently occur in advanced HIV/AIDS.95,96 On the other hand, the majority of HIV-infected people have no access to HAART in developing countries, and hence develop other HIV-related ocular complications before reaching levels of immunosuppression so as to cause Cytomegalovirus retinitis.37 Therefore, anterior-segment involvement by HIV appears to predominate in the HAART era, and thus attention should be focused on these disorders for early diagnosis and treatment.97
Association with CD4 counts
OSSN can occur at any time in the disease course of HIV/AIDS. It is hypothesized that altered tumor surveillance on the OS in addition to decreased circulating CD4 T cells can aid in the development of conjunctival and corneal lesions. However, no significant trend has been discovered between CD4 count and grade of OSSN.5,7,20 It is speculated that immunosuppression resulting from HIV plays a role in the etiopathogenesis of OSSN. Therefore, CD4 monitoring is recommended in all OSSN patients affected by HIV. A CD4 lymphocyte count <200 cells/mm3 has been noted in 85%–100% patients at the time of OSSN detection.7,11,42 These cell counts indicate that a majority of HIV-positive patients with OSSN are significantly immunosuppressed at presentation. However, a linear relationship between OSSN presentation and CD4 lymphocyte count or initiation of HAART has not been confirmed.5,6 It is suggested that OSSN be considered a criterion to commence AR treatment in HIV-positive patients in African countries with financial and technological restraints when CD4 counts are not accessible.7,29
Serotesting for HIV in OSSN patients
Previously, HIV screening was advised in patients presenting with OSSN at a younger age (<40 years) and with a history of rapid tumor growth (<6 months). The current recommendation is to conduct HIV screening in all cases presenting with OSSN to rule out undiagnosed HIV infection, especially in those <60 years, atypical conjunctival lesions at presentation, large lesions, bilateral or multifocal tumors, and history of aggressive tumor growth.32 In an era of increasing global travel, young patients from countries with high HIV prevalence and high ultraviolet B-radiation exposure should be given a low threshold for serotesting and excision biopsy.66
Comments and recommendations
Africa is the global epicenter of the HIV/AIDS pandemic,98 a region with the highest prevalence of HPV in the world and high solar ultraviolet exposure year-round.99 In the presence of this triad of risk factors, the number of cases of OSSN is expected to stay high. After the introduction of HAART, HIV/AIDS has progressively changed into a nonfatal chronic disease with increased life expectancy. This may lead to a possible increase in the burden of patients living with HIV and OSSN prevalence subsequently in countries with limited resources.5,19,63,100
Large unsightly lesions in the eye expose affected persons to the stigma and discrimination associated with HIV infection, and untreated tumors threaten survival. Without an orbital prosthesis, the cosmetic outcome of advanced disease after orbital exenteration is very poor, which leads to facial disfigurement and limited social interaction subsequently. Orbital prosthesis and facilities for periocular reconstruction are often unavailable in low-income countries.
Treatment of opportunistic tumors in HIV/AIDS is a very important component in HIV care. HIV/AIDS research should focus on treatment of this tumor. Further understanding of the correlation between HIV and OSSN would be of great significance, because of the potential to reduce the spread of HIV with early diagnosis and to reduce morbidity in already immunosuppressed individuals. It is imperative to improve our understanding of this condition so that we can identify and manage it better as it becomes more common.
Effective evidence-based interventions are needed to allow early diagnosis and treatment. Active search for early manifestations of OSSN, early and complete surgical excision, and close follow-up to address current high recurrence rates is recommended. No randomized controlled trials on the effectiveness of interventions against OSSN in HIV-infected individuals were identified in this review. The majority of studies from the developed world have small samples, due to the rarity of the condition. More studies need to be conducted in regions with high disease prevalence so that results can be extrapolated. There is a need to explore the alternative methods like the use of topical antimetabolites alone or in combination with surgery in early OSSN. Efficacy of new techniques used in the developed world, such as amniotic membrane transplant and photodynamic therapy, need to be assessed.
Practical and inexpensive ways of preventing OSSN in the HIV population should be recognized in view of the gradual rise in incidence of the disease. Interventions to reduce the mutagenic effects of solar ultraviolet B radiation, such as protective sunglasses, and the scope for development of HPV vaccination for oncogenic viruses associated with this disease need to be identified.
Acknowledgments
Support for this work was provided by the Operation Eyesight Universal Institute for Eye Cancer (SK) and Hyderabad Eye Research Foundation (SK), Hyderabad, India.
Disclosure
The authors report no conflicts of interest in this work.
References
Lee GA, Hirst LW. Ocular surface squamous neoplasia. Surv Ophthalmol. 1995;39(6):429–450. | ||
Basti S, Macsai MS. Ocular surface squamous neoplasia: a review. Cornea. 2003;22(7):687–704. | ||
Chisi SK, Kollmann MK, Karimurio J. Conjunctival squamous cell carcinoma in patients with human immunodeficiency virus infection seen at two hospitals in Kenya. East Afr Med J. 2006;83(5):267–270. | ||
Nkomazana O, Tshitswana D. Ocular complications of HIV infection in sub-Sahara Africa. Curr HIV/AIDS Rep. 2008;5(3):120–125. | ||
Gichuhi S, Sagoo MS, Weiss HA, Burton MJ. Epidemiology of ocular surface squamous neoplasia in Africa. Trop Med Int Health. 2013;18(12):1424–1443. | ||
Guech-Ongey M, Engels E, Goedert J, Biggar R, Mbulaiteye S. Elevated risk for squamous cell carcinoma of the conjunctiva among adults with AIDS in the United States. Int J Cancer. 2008; 122 (11):2590–2593. | ||
Makupa II, Swai B, Makupa WU, White VA, Lewallen S. Clinical factors associated with malignancy and HIV status in patients with ocular surface squamous neoplasia at Kilimanjaro Christian Medical Centre, Tanzania. Br J Ophthalmol. 2012;96(4):482–484. | ||
Osahon AI, Ukponmwan CU, Uhunmwangho OM. Prevalence of HIV seropositivity among patients with squamous cell carcinoma of the conjunctiva. Asian Pac J Trop Biomed. 2011;1(2):150–153. | ||
Mahomed A, Chetty R. Human immunodeficiency virus infection, Bcl-2, p53 protein, and Ki-67 analysis in ocular surface squamous neoplasia. Arch Ophthalmol. 2002;120(5):554–558. | ||
Ateenyi-Agaba C. Conjunctival squamous-cell carcinoma associated with HIV infection in Kampala, Uganda. Lancet. 1995;345(8951):695–696. | ||
Waddell KM, Lewallen S, Lucas SB, Atenyi-Agaba C, Herrington CS, Liomba G. Carcinoma of the conjunctiva and HIV infection in Uganda and Malawi. Br J Ophthalmol. 1996;80(6):503–508. | ||
Porges Y, Groisman GM. Prevalence of HIV with conjunctival squamous cell neoplasia in an African provincial hospital. Cornea. 2003;22(1):1–4. | ||
Newton R, Ziegler J, Ateenyi-Agaba C, et al. The epidemiology of conjunctival squamous cell carcinoma in Uganda. Br J Cancer. 2002;87(3):301. | ||
Gichuhi S, Macharia E, Kabiru J, et al. Clinical presentation of ocular surface squamous neoplasia in Kenya. JAMA Ophthalmol. 2015;133(11):1305–1313. | ||
Steele KT, Steenhoff AP, Bisson GP, Nkomazana O. Ocular surface squamous neoplasia among HIV-infected patients in Botswana. S Afr Med J. 2015;105(5):379–383. | ||
Karp CL, Scott IU, Chang TS, Pflugfelder SC. Conjunctival intraepithelial neoplasia: a possible marker for human immunodeficiency virus infection? Arch Ophthalmol. 1996;114(3):257–261. | ||
Karcioglu ZA, Wagoner MD. Demographics, etiology, and behavior of conjunctival squamous cell carcinoma in the 21st century. Ophthalmology. 2009;116(11):2045–2046. | ||
Carreira H, Coutinho F, Carrilho C, Lunet N. HIV and HPV infections and ocular surface squamous neoplasia: systematic review and meta-analysis. Br J Cancer. 2013;109(7):1981–1988. | ||
Kheir WJ, Tetzlaff MT, Pfeiffer ML, et al. Epithelial, non-melanocytic and melanocytic proliferations of the ocular surface. Semin Diagn Pathol. 2016;33(3):122–132. | ||
World Health Organization. HIV/AIDS. 2017. Available from: http://www.who.int/mediacentre/factsheets/fs360/en. Accessed October 12, 2017. | ||
Masanganise R, Rusakaniko S, Makunike R, et al. A historical perspective of registered cases of malignant ocular tumors in Zimbabwe (1990 to 1999): is HIV infection a factor? Cent Afr J Med. 2008;54(5–8):28–32. | ||
Belfort R Jr. The ophthalmologist and the global impact of the AIDS epidemic: LV Edward Jackson Memorial Lecture. Am J Ophthalmol. 2000;129(1):1–8. | ||
Wabinga HR, Parkin DM, Wabwire-Mangen F, Nambooze S. Trends in cancer incidence in Kyadondo County, Uganda, 1960–1997. Br J Cancer. 2000;82(9):1585–1592. | ||
Parkin DM, Wabinga H, Nambooze S, Wabwire-Mangen F. AIDS-related cancers in Africa: maturation of the epidemic in Uganda. AIDS. 1999;13(18):2563–2570. | ||
Orem J, Otieno MW, Remick SC. AIDS-associated cancer in developing nations. Curr Opin Oncol. 2004;16(5):468–476. | ||
Chokunonga E, Levy LM, Bassett MT, et al. AIDS and cancer in Africa: the evolving epidemic in Zimbabwe. AIDS. 1999;13(18):2583–2588. | ||
Newton R. A review of the aetiology of squamous cell carcinoma of the conjunctiva. Br J Cancer. 1996;74(10):1511–1513. | ||
Tornesello ML, Duraturo ML, Waddell KM, et al. Evaluating the role of human papillomaviruses in conjunctival neoplasia. Br J Cancer. 2006;94(3):446–449. | ||
Chinogurei TS, Masanganise R, Rusakaniko S, Sibanda E. Ocular surface squamous neoplasia (OSSN) and human immunodeficiency virus at Sekuru Kaguvi Eye Unit in Zimbabwe: the role of operational research studies in a resource poor environment? Cent Afr J Med. 2006;52(5–6):56–58. | ||
Kestelyn PH, Stevens AM, Ndayambaje A, Hanssens M, van de Perre PH. HIV and conjunctival malignancies. Lancet. 1990;336(8706):51–52. | ||
Spitzer MS, Batumba NH, Chirambo T, et al. Ocular surface squamous neoplasia as the first apparent manifestation of HIV infection in Malawi. Clin Exp Ophthalmol. 2008;36(5):422–425. | ||
Kaliki S, Kamal S, Fatima S. Ocular surface squamous neoplasia as the initial presenting sign of human immunodeficiency virus infection in 60 Asian Indian patients. Int Ophthalmol. Epub 2016 Nov 8. | ||
Kamal S, Kaliki S, Mishra DK, Batra J, Naik MN. Ocular surface squamous neoplasia in 200 patients: a case-control study of immunosuppression resulting from human immunodeficiency virus versus immunocompetency. Ophthalmology. 2015;122(8):1688–1694. | ||
Frisch M, Biggar RJ, Goedert JJ. Human papillomavirus-associated cancers in patients with human immunodeficiency virus infection and acquired immunodeficiency syndrome. J Natl Cancer Inst. 2000;92(18):1500–1510. | ||
Goedert J, Coté T. Conjunctival malignant disease with AIDS in USA. Lancet. 1995;346(8969):257–258. | ||
Lee GA, Hirst LW. Retrospective study of ocular surface squamous neoplasia. Aust N Z J Ophthalmol. 1997;25(3):269–276. | ||
Poole TR. Conjunctival squamous cell carcinoma in Tanzania. Br J Ophthalmol. 1999;83(2):177–179. | ||
McKelvie PA, Daniell M, McNab A, Loughnan M, Santamaria JD. Squamous cell carcinoma of the conjunctiva: a series of 26 cases. Br J Ophthalmol. 2002;86(2):168–173. | ||
Kaimbo DW, Parys-van Ginderdeuren R, Missotten L. Conjunctival squamous cell carcinoma and intraepithelial neoplasia in AIDS patients in Congo Kinshasa. Bull Soc Belge Ophtalmol. 1998;268:135–141. | ||
Timm A, Stropahl G, Schittkowski M, Sinzidi C, Kayembe D, Guthoff R. [Association of malignant tumors of the conjunctiva and HIV infection in Kinshasa (DR Congo): first results]. Ophthalmologe. 2004;101(10):1011–1016. German. | ||
Pola EC, Masanganise R, Rusakaniko S. The trend of ocular surface squamous neoplasia among ocular surface tumour biopsies submitted for histology from Sekuru Kaguvi Eye Unit, Harare between 1996 and 2000. Cent Afr J Med. 2003;49(1–2):1–4. | ||
Pradeep TG, Gangasagara SB, Subbaramaiah GB, Suresh MB, Gangashettappa N, Durgappa R. Prevalence of undiagnosed HIV infection in patients with ocular surface squamous neoplasia in a tertiary center in Karnataka, south India. Cornea. 2012;31(11):1282–1284. | ||
Sun EC, Fears TR, Goedert JJ. Epidemiology of squamous cell conjunctival cancer. Cancer Epidemiol Biomarkers Prev. 1997;6(2):73–77. | ||
Cervantes G, Rodríguez AA, Leal AG. Squamous cell carcinoma of the conjunctiva: clinicopathological features in 287 cases. Can J Ophthalmol. 2002;37(1):14–20. | ||
Gichuhi S, Irlam JJ. Interventions for squamous cell carcinoma of the conjunctiva in HIV-infected individuals. Cochrane Database Syst Rev. 2007;(2):CD005643. | ||
Tunc M, Char DH, Crawford B, Miller T. Intraepithelial and invasive squamous cell carcinoma of the conjunctiva: analysis of 60 cases. Br J Ophthalmol. 1999;83(1):98–103. | ||
Nakamura Y, Mashima Y, Kameyama K, Mukai M, Oguchi Y. Detection of human papillomavirus infection in squamous tumours of the conjunctiva and lacrimal sac by immunohistochemistry, in situ hybridisation, and polymerase chain reaction. Br J Ophthalmol. 1997;81(4):308–313. | ||
Ateenyi-Agaba C, Dai M, le Calvez F, et al. TP53 mutations in squamous-cell carcinomas of the conjunctiva: evidence for UV-induced mutagenesis. Mutagenesis. 2004;19(5):399–401. | ||
Gichuhi S, Macharia E, Kabiru J, et al. Risk factors for ocular surface squamous neoplasia in Kenya: a case-control study. Trop Med Int Health. 2016;21(12):1522–1530. | ||
Kiire CA, Dhillon B. The aetiology and associations of conjunctival intraepithelial neoplasia. Br J Ophthalmol. 2006;90(1):109–113. | ||
Winward KE, Curtin VT. Conjunctival squamous cell carcinoma in a patient with human immunodeficiency virus infection. Am J Ophthalmol. 1989;107(5):554–555. | ||
Gichuhi S. Epidemiology and Management of Ocular Surface Squamous Neoplasia in Kenya [doctoral thesis]. London: London School of Hygiene and Tropical Medicine; 2016. | ||
Ateenyi-Agaba C, Franceschi S, Wabwire-Mangen F, et al. Human papillomavirus infection and squamous cell carcinoma of the conjunctiva. Br J Cancer. 2010;102(2):262–267. | ||
de Koning MN, Waddell K, Magyezi J, et al. Genital and cutaneous human papillomavirus (HPV) types in relation to conjunctival squamous cell neoplasia: a case-control study in Uganda. Infect Agent Cancer. 2008;3:12. | ||
Shields CL, Ramasubramanian A, Mellen PL, Shields JA. Conjunctival squamous cell carcinoma arising in immunosuppressed patients (organ transplant, human immunodeficiency virus infection). Ophthalmology. 2011;118(11):2133–2137. | ||
Lewallen S, Shroyer KR, Keyser RB, Liomba G. Aggressive conjunctival squamous cell carcinoma in three young Africans. Arch Ophthalmol. 1996;114(2):215–218. | ||
Maclean H, Dhillon B, Ironside J. Squamous cell carcinoma of the eyelid and the acquired immunodeficiency syndrome. Am J Ophthalmol. 1996;121(2):219–221. | ||
Safai B, Lynfield R, Lowenthal DA, Koziner B. Cancers-associated with HIV infection. Anticancer Res. 1987;7(5B):1055–1067. | ||
Lee GA, Hirst LW. Incidence of ocular surface epithelial dysplasia in metropolitan Brisbane: a 10-year survey. Arch Ophthalmol. 1992;110(4):525–527. | ||
Cackett P, Gillies M, Leen C, Dhillon B. Conjunctival intraepithelial neoplasia in association with HIV infection. AIDS. 2005;19(3):351–352. | ||
Kaliki S, Freitag SK, Chodosh J. Nodulo-ulcerative ocular surface squamous neoplasia in 6 patients: a rare presentation. Cornea. 2017;36(3):322–326. | ||
Kabra RC, Khaitan IA. Comparative analysis of clinical factors associated with ocular surface squamous neoplasia in HIV infected and non HIV patients. J Clin Diagn Res. 2015;9(5):NC01–NC03. | ||
Masanganise R, Mukome A, Dari J, Makunike-Mutasa R. Bilateral HIV related ocular surface squamous neoplasia: a paradigm shift. Cent Afr J Med. 2010;56(5–8):23–26. | ||
Kim RY, Seiff SR, Howes EL, O’Donnell JJ. Necrotizing scleritis secondary to conjunctival squamous cell carcinoma in acquired immunodeficiency syndrome. Am J Ophthalmol. 1990;109(2):231–233. | ||
Tabin G, Levin S, Snibson G, Loughnan M, Taylor H. Late recurrences and the necessity for long-term follow-up in corneal and conjunctival intraepithelial neoplasia. Ophthalmology. 1997;104(3):485–492. | ||
De Silva DJ, Tumuluri K, Joshi N. Conjunctival squamous cell carcinoma: atypical presentation of HIV. Clin Exp Ophthalmol. 2005;33(4):419–420. | ||
Tabbara KF, Kersten R, Daouk N, Blodi FC. Metastatic squamous cell carcinoma of the conjunctiva. Ophthalmology. 1988;95(3):318–321. | ||
Oellers P, Karp CL, Sheth A, et al. Prevalence, treatment, and outcomes of coexistent ocular surface squamous neoplasia and pterygium. Ophthalmology. 2013;120(3):445–450. | ||
Hirst LW, Axelsen RA, Schwab I. Pterygium and associated ocular surface squamous neoplasia. Arch Ophthalmol. 2009;127(1):31–32. | ||
Shields JA, Shields CL, de Potter P. Surgical management of conjunctival tumors: the 1994 Lynn B McMahan Lecture. Arch Ophthalmol. 1997;115(6):808–815. | ||
Tsatsos M, Karp CL. Modern management of ocular surface squamous neoplasia. Expert Rev Ophthalmol. 2013;8(3):287–295. | ||
Kaliki S, Mohammad FA, Tahiliani P, Sangwan VS. Concomitant simple limbal epithelial transplantation after surgical excision of ocular surface squamous neoplasia. Am J Ophthalmol. 2017;174 (Suppl C):68–75. | ||
Shields CL, Kaliki S, Kim HJ, et al. Interferon for ocular surface squamous neoplasia in 81 cases: outcomes based on the American Joint Committee on Cancer classification. Cornea. 2013;32(3):248–256. | ||
Shields CL, Naseripour M, Shields JA. Topical mitomycin C for extensive, recurrent conjunctival-corneal squamous cell carcinoma. Am J Ophthalmol. 2002;133(5):601–606. | ||
Midena E, Angeli CD, Valenti M, de Belvis V, Boccato P. Treatment of conjunctival squamous cell carcinoma with topical 5-fluorouracil. Br J Ophthalmol. 2000;84(3):268–272. | ||
Sherman MD, Feldman KA, Farahmand SM, Margolis TP. Treatment of conjunctival squamous cell carcinoma with topical cidofovir. Am J Ophthalmol. 2002;134(3):432–433. | ||
Besley J, Pappalardo J, Lee GA, Hirst LW, Vincent SJ. Risk factors for ocular surface squamous neoplasia recurrence after treatment with topical mitomycin C and interferon α2B. Am J Ophthalmol. 2014;157(2):287–293. | ||
Karp CL, Galor A, Chhabra S, Barnes SD, Alfonso EC. Subconjunctival/perilesional recombinant interferon α2B for ocular surface squamous neoplasia: a 10-year review. Ophthalmology. 2010;117(12):2241–2246. | ||
Shields JA, Shields CL, Suvarnamani C, Tantisira M, Shah P. Orbital exenteration with eyelid sparing: indications, technique, and results. Ophthalmic Surg. 1991;22(5):292–297. | ||
Walsh-Conway N, Conway RM. Plaque brachytherapy for the management of ocular surface malignancies with corneoscleral invasion. Clin Exp Ophthalmol. 2009;37(6):577–83. | ||
Arepalli S, Kaliki S, Shields CL, Emrich J, Komarnicky L, Shields JA. Plaque radiotherapy in the management of scleral-invasive conjunctival squamous cell carcinoma: an analysis of 15 eyes. JAMA Ophthalmol. 2014;132(6):691–696. | ||
Ramonas KM, Conway RM, Daftari IK, Crawford JB, O’Brien JM. Successful treatment of intraocularly invasive conjunctival squamous cell carcinoma with proton beam therapy. Arch Ophthalmol. 2006;124(1):126–128. | ||
Lake DF, Briggs AD, Akporiaye ET. Immunopharmacology. In: Katzung BG, editor. Basic and Clinical Pharmacology. 9th ed. Boston: McGraw-Hill; 2004:931–957. | ||
Ashkenazy N, Karp CL, Wang G, Acosta CM, Galor A. Immunosuppression as a possible risk factor for interferon nonresponse in ocular surface squamous neoplasia. Cornea. 2017;36(4):506–510. | ||
Mata E, Conesa E, Castro M, Martínez L, de Pablo C, González ML. [Conjunctival squamous cell carcinoma: paradoxical response to interferon eyedrops]. Arch Soc Esp Oftalmol. 2014;89(7):293–296. Spanish. | ||
Frucht-Pery J, Sugar J, Baum J, et al. Mitomycin C treatment for conjunctival-corneal intraepithelial neoplasia: a multicenter experience. Ophthalmology. 1997;104(12):2085–2093. | ||
Sudesh S, Rapuano CJ, Cohen EJ, Eagle RC Jr, Laibson PR. Surgical management of ocular surface squamous neoplasms: the experience from a cornea center. Cornea. 2000;19(3):278–283. | ||
Fogla R, Biswas J, Kumar SK, Kumarasamy N, Madhavan HN, Solomon S. Squamous cell carcinoma of the conjunctiva as initial presenting sign in a patient with acquired immunodeficiency syndrome (AIDS) due to human immunodeficiency virus type-2. Eye (Lond). 2000;14(2):246–247. | ||
Siedlecki AN, Tapp S, Tosteson AN, et al. Surgery versus interferon α2B treatment strategies for ocular surface squamous neoplasia: a literature-based decision analysis. Cornea. 2016;35(5):613–618. | ||
Gichuhi S, Macharia E, Kabiru J, et al. Topical fluorouracil after surgery for ocular surface squamous neoplasia in Kenya: a randomised, double-blind, placebo-controlled trial. Lancet Glob Health. 2016;4(6):e378–e385. | ||
Mittal R, Rath S, Vemuganti GK. Ocular surface squamous neoplasia: review of etio-pathogenesis and an update on clinico-pathological diagnosis. Saudi J Ophthalmol. 2013;27(3):177–186. | ||
Shields JA, Shields CL, Gunduz K, Eagle RC. The 1998 Pan American Lecture: Intraocular invasion of conjunctival squamous cell carcinoma in five patients. Ophthal Plast Reconstr Surg. 1999;15(3):153–160. | ||
Guech-Ongey M, Engels EA, Goedert JJ, Biggar RJ, Mbulaiteye SM. Elevated risk for squamous cell carcinoma of the conjunctiva among adults with AIDS in the United States. Int J Cancer. 2008;122(11):2590–2593. | ||
Holkar S, Mudhar HS, Jain A, et al. Regression of invasive conjunctival squamous carcinoma in an HIV-positive patient on antiretroviral therapy. Int J STD AIDS. 2005;16(12):782–783. | ||
Forrest DM, Seminari E, Hogg RS, et al. The incidence and spectrum of AIDS-defining illnesses in persons treated with antiretroviral drugs. Clin Infect Dis. 1998;27(6):1379–1385. | ||
Palella FJ, Delaney KM, Moorman AC, et al. Declining morbidity and mortality among patients with advanced human immunodeficiency virus infection. N Engl J Med. 1998;338(13):853–860. | ||
Jeng BH, Holland GN, Lowder CY, Deegan WF, Raizman MB, Meisler DM. Anterior segment and external ocular disorders associated with human immunodeficiency virus disease. Surv Ophthalmol. 2007;52(4):329–368. | ||
Joint United Nations Programme on HIV/AIDS. Global report: UNAIDS report on the global AIDS epidemic — 2010. Available from: http://www.unaids.org/globalreport/global report.htm 2010. Accessed October 12, 2017. | ||
Clifford GM, Gallus S, Herrero R, et al. Worldwide distribution of human papillomavirus types in cytologically normal women in the International Agency for Research on Cancer HPV prevalence surveys: a pooled analysis. Lancet. 2005;366(9490):991–998. | ||
Reynolds JW, Pfeiffer ML, Ozgur O, Esmaeli B. Prevalence and severity of ocular surface neoplasia in African nations and need for early interventions. J Ophthalmic Vis Res. 2016;11(4):415–421. |
 © 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.