Back to Journals » Nature and Science of Sleep » Volume 12
Obstructive Sleep Apnea Syndrome: A Preliminary Navigated Transcranial Magnetic Stimulation Study
Authors Rogić Vidaković M , Šoda J , Jerković A, Benzon B, Bakrač K, Dužević S, Vujović I , Mihalj M , Pecotić R , Valić M , Mastelić A, Hagelien MV, Zmajević Schőnwald M, Đogaš Z
Received 23 March 2020
Accepted for publication 25 June 2020
Published 6 August 2020 Volume 2020:12 Pages 563—574
DOI https://doi.org/10.2147/NSS.S253281
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Steven A Shea
Maja Rogić Vidaković,1 Joško Šoda,2 Ana Jerković,1 Benjamin Benzon,1 Karla Bakrač,1 Silvia Dužević,1 Igor Vujović,2 Mario Mihalj,3 Renata Pecotić,1,4 Maja Valić,1,4 Angela Mastelić,5 Maximilian Vincent Hagelien,1 Marina Zmajević Schőnwald,6 Zoran Đogaš1,4
1University of Split, School of Medicine, Department of Neuroscience, Laboratory for Human and Experimental Neurophysiology (LAHEN), Split, Croatia; 2University of Split, Faculty of Maritime Studies, Signal Processing, Analysis and Advanced Diagnostics Research and Education Laboratory (SPAADREL), Split, Croatia; 3University Hospital Split, Department of Neurology, Laboratory of Electromyoneurography, Split, Croatia; 4University of Split, Split Sleep Medical Center, Split 21000, Croatia; 5University of Split, School of Medicine, Department of Medical Chemistry and Biochemistry, Split, Croatia; 6Clinical Medical Centre “Sisters Of Mercy”, Department of Neurosurgery, Clinical Unit for Intraoperative Neurophysiologic Monitoring, Zagreb, Croatia
Correspondence: Maja Rogić Vidaković
University of Split, School of Medicine, Department of Neuroscience, Laboratory for Human and Experimental Neurophysiology (LAHEN), Split, Croatia
Tel +385 21 557 876
Fax +358 21 557 955
Email [email protected]
Purpose: An increase in resting motor threshold (RMT), prolonged cortical silent period duration (CSP), and reduced short-latency afferent inhibition (SAI), confirmed with previous transcranial magnetic stimulation (TMS), suggest decreased cortical excitability in obstructive sleep apnea syndrome (OSAS). The present study included MRI of OSAS patients for navigated TMS assessment of the RMT, as an index of the threshold for corticospinal activation at rest, and SAI as an index of cholinergic neurotransmission. We hypothesize to confirm findings on SAI and RMT with adding precision in the targeting of motor cortex in OSAS.
Subjects and Methods: After acquiring head MRIs for 17 severe right-handed OSAS and 12 healthy subjects, the motor cortex was mapped with nTMS to assess the RMT and SAI, with motor evoked potentials (MEPs) recorded from the abductor-pollicis brevis (APB) muscle. The 120%RMT intensity was used for the SAI by a paired-pulse paradigm in which the electrical stimulation to the median nerve is followed by magnetic stimulation of the motor cortex at inter-stimulus intervals (ISIs) of 18– 28 ms (ISIs18-28). The SAI control condition included a recording of MEPs without peripheral stimulation. Latency and amplitude of MEP at RMT at 120%RMT for eleven different at ISIs18-28 were analyzed.
Results: The study showed a significantly lower percentage deviation of MEP amplitude at ISIs(18-28ms) from the control condition between OSAS and healthy subjects (U=44.0, p=0.01). The intensity of stimulation at RMT was significantly higher in OSAS subjects (U=55.0, p=0.04*). Correlation analysis showed that BMI significantly negatively correlated (ρ=− 0.47) with MEP amplitude percentage deviation in OSAS patients.
Conclusion: The nTMS study results in increased RMT, and reduced cortical afferent inhibition in OSAS patients for SAI at ISIs18-28, confirming previous findings of impaired cortical afferent inhibition in OSAS. Future nTMS studies are desirable to elucidate the role of RMT and SAI in diagnostics and treatment of OSAS, and to elucidate the usefulness of nTMS in OSAS research.
Keywords: short-latency afferent inhibition, obstructive sleep apnea, transcranial magnetic stimulation, motor evoked potentials, primary motor cortex, transcutaneous electrical nerve stimulation
Plain Language Summary
Obstructive sleep apnea syndrome (OSAS) contributes to changes in brain functioning previously confirmed by neuropsychological and neuroimaging studies. For the past 30 years, transcranial magnetic stimulation (TMS) was used to investigate sleep to understand its physiology, and in OSAS research to investigate corticospinal excitability during apneas as well as in OSAS patients during the awake state. TMS studies to date indicate decreased cortical excitability in OSAS, confirmed by reduced cortical silent period duration (index of an inhibitory gamma-aminobutyric acid activity in the motor cortex), increased resting motor threshold (RMT) (index of the threshold for corticospinal activation at resting), and reduced short-latency afferent inhibition measures (SAI) (index of cholinergic neurotransmission). However, the major obstacle in relevant TMS investigations of OSAS was to locate precisely the magnetic coil over the motor cortex. It has been reported that the motor target cortical area is considerably widespread in traditional TMS, and when combined with MRI-based navigation, it permits more precise stimulation of structural areas of the motor cortex. The present study for the first time included MRI data of OSAS patients for the navigated-TMS (nTMS) assessment of the RMT and SAI phenomena. Our nTMS study provided findings on increased RMT, as well as the reduction of cortical afferent inhibition in OSAS tested with the SAI protocol. We believe the study contributed to the field of TMS research of OSAS by including MRI data and nTMS technology and confirmed the previous findings related to SAI and RMT in OSAS patients conducted with traditional TMS. It would be highly recommended to explore in future studies the role of the potential neurophysiological markers, such as RMT and SAI in early detection and therapy outcomes (ie, CPAP). Further nTMS studies are also needed to investigate the performance of OSAS patients on other experimental protocols investigating cortical inhibition, facilitation, and cortical plasticity (ie, MEP recruitment curve, paired associative stimulation (PAS)) with psychomotor and cognitive performance, to clarify the usefulness of nTMS in OSAS research.
Introduction
The obstructive sleep apnea syndrome (OSAS) is characterized by repetitive upper airway obstructions that occur during sleep and are associated with a reduction in blood oxygen saturation,1,2 resulting in intermittent hypoxemia, sympathetic excitation and sleep fragmentation.3–5 OSAS is associated with a range of medical conditions such as hypertension, obesity, type 2 diabetes, depression, peripheral neuropathy, as well as with psychological and cognitive deficits.4–10 However, the pathophysiologic mechanisms of OSAS are still challenging to disentangle. Transcranial magnetic stimulation (TMS) studies proposed an altered motor cortical excitability as part of the mechanisms underlying OSAS in terms of a dysfunctional cortico-motoneuronal system.10–20 It has been suggested that the pattern of cortical excitability seems to be disease-specific rather than reflect a generic consequence of the sleep architecture disruption or sleep fragmentation.19
In sleep physiology of OSAS disorder, TMS is used for exploring motor cortical and corticospinal excitability by evaluating resting motor threshold (RMT), amplitudes and latencies of motor evoked potentials (MEPs), cortical silent period, intracortical inhibition and facilitation, transcallosal inhibition.13–19 So far, one TMS study performed by Nardone et al20 investigated cortical excitability in OSAS using the short-latency afferent inhibition (SAI) technique without including the magnetic resonance imaging (MRI) data for the enrolled patient.
The SAI technique refers to an MEP inhibition produced by a conditioning afferent electrical stimulus applied to the median nerve at the wrist prior to TMS of the hand area of the contralateral motor cortex.21,22 Cortical afferent inhibition measured with the SAI protocol is thought to reflect the sensorimotor interaction and cholinergic activity of the cerebral cortex and is likely mediated by the GABAA receptor subtype bearing the α1-subunit.23–25 All TMS studies to date have indicated decreased cortical excitability in OSAS, confirmed by reduced cortical silent period duration (CSP), increased resting motor threshold (RMT), and reduced SAI.13,15-17,19,20
Nardone et al20 investigated SAI phenomenon in severe OSAS by determining median nerve-cortical TMS inter-stimulus intervals (ISIs) with the standard approach based on the latency of the N20 response of somatosensory evoked potentials (SEPs).21,26 According to our knowledge, the present study for the first time used MRI data of OSAS patients and healthy subjects with navigated TMS (nTMS) technology to systematically investigate the SAI phenomenon in severe OSAS patients by exploring an eleven nerve-cortical TMS ISIs ranging from 18 m to 28 ms (ISI(18-28)),25 control measurement (without peripheral stimulation) related to SAI protocol, and RMT investigation. With the hypothesis that OSAS is associated with impaired afferent cortical inhibition, we expected to contribute to the field of TMS research of OSAS by including MRI data and nTMS technology to confirm the previous findings related to SAI20 and RMT16,17,19 in OSAS patients conducted with traditional TMS.
Methods
General Procedures
Severe OSAS patients and healthy adult subjects were included in nTMS study. After a diagnosis of severe OSAS in Split Sleep Medicine Center (SMC), and if fulfilling all excluding and including criteria, the subjects (including recruited healthy subjects) were admitted to the University Hospital Split, Department of Diagnostic and Interventional Radiology for magnetic resonance imaging (MRI) of the head. The expert radiologist reviewed all the MRI findings and excluding one female subject in whom lesion was detected in the occipital region and was referred to continue further examinations at the Neurosurgical Department.
Participants
We studied seventeen adult OSAS patients (thirteen males and four females); mean age 55±12 years, with severe apnea (Apnea-hypopnea index – AHI ≥30 h−1) diagnosed at the Split SMC centre following whole-night polysomnography (PSG) or polygraphy (PG). Eleven OSAS patients were with High School Degree and six of them with Graduate Degree. A diagnosis of OSAS was defined in accordance with the guidelines of the American Academy of Sleep Medicine (AASM) and the European Sleep Research Society (ESRS)27 and was given by expert certified sleep medicine – physician specialist. The subjects with severe apnea were included in the nTMS study after being diagnosed and with no initiation of continuous positive airway pressure (CPAP) therapy. Exclusion criteria for present nTMS study were following: (1) age younger than 18; (2) contraindication to TMS (ie, presence of metal objects like a denture or cardiac pacemaker); (3) history of psychiatric, neurological, or respiratory disease; (4) abnormal findings during oto-rhino-laryngological examination; and (5) epileptic seizures, or a history of epileptic seizures. The comorbidity that was tolerated for OSAS patients to be included in the study was the prescription of antihypertensive medication (10 patients), analgetics (one patient), diabetes medication (three patients), immunosuppressive medication (one patient), and antihistaminics (one patient).
OSAS subjects included in this study underwent an initial medical history interview, physical examination with anthropometric measurements, and neuropsychological evaluation.28,29 Body weight and height were measured using a calibrated scale (Seca, Birmingham, UK) while subjects wore light clothes. Body mass index (BMI) was calculated as body weight (kg) divided by height squared (m2). Excessive daytime sleepiness was assessed using the self-administered Epworth Sleepiness Scale (ESS) for the OSAS diagnostics as well as before TMS procedure.30 The Edinburgh Handedness Inventory was used to evaluate the hand dominance.31
For the healthy subject group, 12 healthy subjects (six males and six females; mean age 46±10) were recruited from a pool of healthy subjects, which previously participated in nTMS studies at the Department of Neuroscience, University of Split School of Medicine; or were recruited by advertisement. All healthy subjects underwent screening with the STOP-BANG Questionnaire,32, and a Croatian version of the ESS and STOP questionnaire for evaluation of risk for OSAS.30 The results of the screening tools indicated a low risk of OSAS in the group of healthy subjects, according to STOP-BANG and STOP questionnaires and low daytime sleepiness (Epworth = 2 (0–24)).
Polysomnography (PSG) and Polygraphy (PG)
Whole-night in-laboratory PSG (Alice 5LE or Alice PDX, Philips Respironics, Eindhoven, Netherlands) or whole-night unattended polygraphy (PG) (PolyMesam, MAP, Martinsried, Germany) was performed in OSAS patients. PSG recordings included electroencephalography, electrooculography, mental and tibial electromyography, electrocardiography, nasal airflow, pulse oximetry, thoracic and abdominal movements, and snoring intensity (ALICE 5LE, Philips Respironics, Eindhoven, the Netherlands). PSG recordings included nasal airflow, pulse oximetry, thoracic and abdominal movements (Somnocheck, Weinmann, Hamburg, Germany; Alice PdX, Philips Respironics, Eindhoven, the Netherlands; Embleta, Natus Neuro, Middleton, WI, USA). Acquired data were evaluated in agreement with AASM and ESRS guidelines by a certified sleep physician.3,33 Sleep evaluations of less than 6-h duration were discarded, and in such cases, the PSG/PG was repeated on a later occasion. Apnea was defined as a complete cessation of respiratory airflow for a minimum duration of 10 s. In contrast, hypopnea was identified as a decrease in airflow by more than 50% from the baseline for at least 10 s, both combined with a reduction in hemoglobin oxygen saturation of at least 3%. The Apnea-hypopnea index (AHI) was defined as the average number of apneas and hypopneas per hour of sleep (Table 1). Oxygen desaturation index (ODI) was calculated as the number of significant oxygen saturation (SpO2) drops of 3% or more per hour of sleep.
nTMS Study Protocol
Electromyographic Recording
Subjects were first prepared by gently abrading the skin and cleaning it with acetone and alcohol. Electromyography (EMG) was recorded from the right abductor pollicis-brevis (APB) with a pair of self-adhesive surface electrodes (Ambu® Blue Sensor BR, BR-50-K/12, Ballerup, Denmark) in a belly-tendon montage (Figure 1). The reference electrode for the APB was placed on the metacarpophalangeal joint of the thumb (Figure 1). Electrodes were attached to the Nexstim EMG electrode cable with a 1.5 mm touch-proof female safety connector (DIN 42–802) and connected to 6-channel EMG and one common ground EMG Amplifier (external module) with TMS-artefact rejection circuitry. The following were characteristics of the EMG used in testing as a component of the NBS (Nexstim NBS System 4, Nexstim Oy, Helsinki, Finland) system: sampling rate equal to 3 kHz (per channel), resolution of 0.3 µV, the scale between −7.5 mV and 7.5 mV, common-mode rejection ratio (CMRR) > 90 dB, peak-to-peak noise <5 µV and frequency band in the range of 10–500 Hz.
Electrical Stimulation of the Median Nerve
Conditioning stimuli were single pulses (200 µs duration) of electrical stimulation applied with a stimulating bar electrode. The stimulating bar electrode, manufactured by ADInstruments (North America), had flat disks and a 30 mm spacing of 9 mm contacts, and the anode was positioned distally (Figure 1). The intensity of the conditioning stimuli was set at twofold the sensory threshold, which was frequently a threshold for evoking a visible twitch of the thenar muscles. The conditioning stimulus to the peripheral nerve preceded the test magnetic cortical stimulus. For cutaneous afferents stimulation, ISIS Neurostimulator of manufacturer Inomed Medizintechnik GmbH (Version 1.0.2.0., Inomed Medizintechnik GmbH, Emmendingen, Germany) enabling a constant current stimulation was used. The ISIS Neurostimulator is a USB-powered module used with stand-alone Windows-based PC software to deliver electrical stimulation. In the present study, the electrical stimulator as a stand-alone device was triggered by a script executed within Presentation software (Neurobehavioral Systems, Inc., Version 20.2) installed on an external personal computer. A standard BNC cable was customized to the needs of the study with a BNC connector on one end (connection to Trig in of the electrical stimulator) and a USB connector on the other (connection to the PC).
nTMS Assessment
Magnetic resonance imaging (MRI) of the head for all OSAS and healthy subjects was performed with a Magnetom Aera of 1.5 T strength (Siemens Healthcare GmbH, Erlangen, Germany). The recommended MRI protocol for nTMS brain reconstruction includes T1-weighted images, voxel size of approximately 1x1x1 mm, sagittal images recommended, axial and coronal supported, sequential scans of 1 mm thickness and 1 mm slice gap, with angulation less than ± 10 degrees. Navigated transcranial magnetic stimulation (nTMS) was delivered using a figure-of-eight coil connected to a Nexstim TMS II stimulator module (integrated with mobile NBS chart) (Nexstim NBS System 4, Nexstim Oy, Helsinki, Finland). MRI images were obtained to suit the TMS requirements and were integrated into the nTMS system and used for 3D reconstruction of individual brain anatomy (3D optical tracking unit; Polaris® Vicra). Sophisticated real-time data processing allows the accurate display of the induced electric field (E-field) within the brain tissue. Targeting tools available on-screen are the following: a grid for systematic brain mapping, a targeting tool for optimal coil placement, an aiming tool for precise repetition of a given stimulus, and automated stimulation (location controlled). The subject wears an optical head tracker and, by using a pointer, 12 points are registered on the subject scalp. A figure-of-eight coil with winding diameter ca. 50 mm, and an outer winding diameter of ca 70 mm was used. The maximum E-field was 172 V/m below the Nexstim Focal coil in the spherical conductor model representing the human head. The magnetic stimulation was externally triggered by the same Presentation software script responsible for triggering electrical stimulation (Figure 2).
Experimental Protocol
At the beginning of nTMS investigations, baseline cortical excitability (RMT)34 was measured by inducing MEPs in the APB muscle by placing the coil tangentially over the central sulcus and targeting the omega shape structure (determined on sagittal and axial MRIs) of the precentral gyrus (primary motor cortex, M1) on 3D reconstructed brain. The RMT was performed by introducing the 30% of the maximal stimulator output and slightly increasing the intensity until MEPs are elicited in the APB muscle. A total of 10 to 20 MEP responses were collected, and 50% of the trials had peak-to-peak amplitude MEP amplitude of >50µV.34
After determining RMT, an intensity of 120% of maximum stimulator output was used to map the M1 hotspot for the APB muscle following peripheral electric stimulation of the median nerve at the wrist (Figure 2). Figure 2 depicts the APB hotspot with visualization of the direction of the coil orientation over the precentral gyrus for one subject. The conditioning stimulation (electric) preceded the test TMS single pulse over M1 at ISIs of 18, 19, 20, 21, 22, 23, 24, 25, 26, 27, and 28 ms (Figure 2). The magnetic pulses were given in random order at these ISIs after the onset of electric stimulation in all subjects. Each session block consisted of 10 trials with an inter-trial interval of 5.5 s. The control condition referred to TMS over the M1 without peripheral electric stimulation and was included at the beginning of each session in all subjects (Figure 2) using the 120% RMT.
During nTMS, subjects were seated comfortably in a reclining, electronically controlled chair with their forearm in a semi-pronated, resting position. All subjects were tested in the afternoon, between 1 and 3 pm, to manage the potential differences in both drowsiness and vigilance fluctuations, which might affect the level of cortical excitability and related motor responses. The ceiling lights were always kept off during the experimental measures with nTMS. The head support was individually adjusted for providing a comfortable head position during the session. Participants were instructed to avoid napping throughout the experiment, to remain relaxed entirely with the eyes open. Muscle relaxation was visually inspected by an examiner (author of the study) and was continuously visually monitored also by the subject giving him/her instructions on how to relax.
Data Analysis and Statistics
The MEP responses were analyzed using a custom-made script in MATLAB 2018b. The script was programmed to automatically read off latency and amplitudes (peak-to-peak) of individual MEP response with custom-made a novel algorithm for detecting MEP latency (submitted elsewhere for publication). We have used the standard peak-to-peak amplitude approach defined as the difference between the maximum and minimum value of the MEP response representing an accurate indicator for estimating the MEP amplitude oscillation.
The statistical data analysis was conducted using STATISTICA 12 (StatSoft, Inc., Tulsa, USA). Kolmogorov–Smirnov test and Mauchly’s Test of Sphericity were used to test assumptions for ANOVA testing and showed no departs from the normal distribution of MEP-APB latency values (MEP latency) for all conditions (control and ISIs of 18, 19, 20, 21, 22, 23, 24, 25, 26, 27, 28 ms). Therefore, two-factorial repeated-measures ANOVA was performed to test whether ISIs as a within-subject factor and “group” (healthy subjects vs OSAS patients) as a between-subject measure had a significant influence on MEP latency.
Normality tests confirmed that MEP-APB amplitudes (MEP amplitudes) at several ISIs conditions were not well modelled by a normal distribution. Therefore, to identify the differences between healthy subjects and OSAS patients, the MEP amplitude data from all ISIs were treated as univariate. In both groups separately (OSAS and healthy subjects) we averaged MEP amplitude results for all ISIs(18-28ms), but first, we performed Friedman’s ANOVA for dependent samples to ensure there were no differences in MEP amplitude between ISIs(18-28ms). Also, using a percentage deviation equation,35 we calculated how much the averaged ISIs(18-28ms) differ from the control condition in each group of subjects separately. The statistical differences for MEP percentage deviation between OSAS patients and healthy were analyzed with the Mann–Whitney U-test for independent samples. Furthermore, Mann–Whitney U-test for independent samples was also conducted to test the statistical differences between OSAS patients and healthy subjects for other relevant clinical and demographic measures: age, BMI, ESS, RMT intensity, 120% RMT intensity, and electrical stimulation intensity. Spearman rank-order correlation (ρ)36 was used to measure dependence between MEP amplitude percentage deviation and age, gender and BM. The results were expressed as ρcoefficients. The differences were considered statistically significant when p was <0.05. The descriptive statistics were displayed as median value and interquartile range (IQR). The descriptive for MEP-APB amplitude and MEP-APB latency results were additionally displayed as the arithmetic mean and standard deviation (SD).
Results
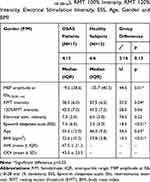
The demographic and clinical features for OSAS patients and healthy subjects are presented in Table 1. Table 1 also shows the results of Mann–Whitney U testing for group differences in MEP amplitudes at ISIs(18-28ms), RMT intensity, RMT 120% intensity, electrical stimulation intensity, ESS, age, and BMI. The intensity of stimulation at RMT was significantly higher in OSAS patients compared to control (U=55.0, p=0.04*) (Table 1). Statistical differences were also found for age (U=54.5, p=0.03*), BMI (U=10.5, p<0.01*) and ESS (U=18.5, p<0.01*) (Table 1). No significant differences were found for MEP latency between groups (OSAS vs healthy subjects) (F1=3.26, p=0.10), nor between MEP latency at ISIs (control, 18 −28 ms) in each group (F11=1.14, p=0.33).
Furthermore, the Friedman test showed no differences in MEP amplitude between ISIs(18-28ms) for healthy subjects (χ2=13.32, p=0.21) or OSAS patients (χ2=2.81, p=0.98). Since no significant differences were found between ISIs(18-28ms) in any group, the percentage deviation from the control condition was calculated for MEPs at averaged ISIs(18-28ms) in each group. The results show a significant difference in the MEP amplitude percentage deviation from the control condition between OSAS and healthy subjects (U=44.0, p=0.01*) (Table 1, Figure 3). The median values indicated lower MEP amplitude percentage deviation from the control condition at ISIs(18-28ms) for OSAS patients compared to healthy subjects (−9.5 < −35.7) (Tables 1 and 2, Figures 3 and 4).
 |
Table 2 Mean Values (±SD) of MEPs Amplitude for Control Condition and ISIs(18-28 Ms) in OSAS Patients and Healthy Subjects |
 |
Figure 4 Graphical presentation of mean and median MEP responses for control condition and ISIs(18-28ms) for one OSAS patient and healthy subject. |
Further, correlation analysis provided evidence that BMI significantly negatively correlated with MEP amplitude percentage deviation in OSAS patients (Table 3). A significant negative ρ coefficient (ρ=−0.47) suggests that the higher the BMI, the lower the MEP amplitude percentage deviations from the control condition in OSAS patients.
 |
Table 3 Summary of Spearman Rank-Order Correlations for MEP Amplitude Percentage Deviation with Age, Gender and BMI in OSAS Patients |
Discussion
In the present nTMS study, we were able to demonstrate, by application of SAI protocol, a significantly lower percentage deviation of MEP amplitude from the control condition between OSAS and healthy subjects confirming the results of Nardone et al.20 We have also shown increased RMT in OSAS, which is accordance with previous TMS findings.16,17,19 All obtained data suggest that chronic OSAS condition decreases motor cortical excitability in these patients.
Alterations in cortical afferent inhibition might indicate probable changes in cholinergic neurotransmission in OSAS patients.23,37-39 The cholinergic pathways play an important role in the activation of the motor cortex40,41 and provide control over circuit dynamics underlying cognitive processing42 and respiratory control.43,44 Ponto-mesencephalic cholinergic neurons play a role in sleep-wakefulness, locomotor behaviour, and memory.45,46 In addition, preliminary pharmacological studies have shown beneficial effects of cholinergic medication (donepezil, physostigmine) in OSAS patients by improving the AHI index, oxygen saturation, and sleepiness.47,48 The reduction of cholinergic pontine projection might contribute to OSAS in neurological diseases.49,50
Our data regarding SAI results complement the findings of Nardone et al20 and extend previous TMS findings mainly based on MRI data and nTMS technology, as well as the methodology for data analysis of MEP responses in SAI measurement. Nardone et al20 expressed MEP amplitudes obtained at several ISIs as a percentage of the MEP amplitudes of the control MEP condition. In contrast, in the present study, MEP amplitude differences between OSAS and healthy subjects were explored by the averaged percentage deviation from the control condition. Further, previous TMS studies suggested a minor decrease in motor cortical excitability in OSAS confirmed through measures such as CSP, RMT, amplitudes, and latencies of MEPs in awake state and during apneas. The most robust finding confirmed by several studies was prolonged CSP,13,15-17, pointing to an increase of inhibitory gamma-aminobutyric acid activity in the motor cortex of OSAS patients. Furthermore, increased RMT16,17,19 in the awake OSAS patients, as well as reduced amplitudes and prolonged latencies of MEPs during the second non-rapid eye movement (REM) sleep stage,13 also suggested depression of the motor cortical activity in OSAS. RMT reflects the excitability of corticomotor projections during muscle relaxation; therefore, several neural structures (cortical motor neurons, corticospinal pathways and the spinal structures) are excited by TMS.51 In the present nTMS study, we also confirmed increased RMT in OSAS, suggesting that chronic OSAS condition decreases motor cortical excitability in these patients. Lastly, BMI negatively correlated with MEP amplitude percentage deviation in OSAS patients. BMI is a well-established indicator of obesity and frequently used measure defining the severity of respiratory stress associated with OSAS.52
Our study has some limitations. The sample included severe OSAS patients (AHI>30/h), both males and females. We targeted only severe patients to have a convenient homogeneous sample and enrolled both sexes for several reasons: time restrictions for the recruitment of OSAS patients, a limited number of severe OSAS patients, and a high percentage of positive TMS excluding factors (denture, cardiac diseases, neurological disease). Further, the reason why we could not introduce several neurophysiological measures like F-wave or central motor conduction like in the study of Nardone et al20 was the real situation that we did not use EEG-EP method to obtain SEP, but rather used MRI data and mapping of the motor cortex with nTMS and performing the SAI protocol by including eleven ISIs intervals (18 ms, 19 ms, 20 ms, 21 ms, 22 ms, 23 ms, 24 ms, 25 ms, 26 ms, 27 ms, 28 ms) at 120% RMT which was time-consuming. The entire experimental protocol laster approximately 2 h, and the subjects often tend to be anxious after 1 h. Also, in the present study, we did not correlate the results of cognitive and psychomotor results of neuropsychological testing with the SAI data20 since we plan to conduct additional nTMS study of these OSAS patients 1 year or several years after CPAP therapy. It was previously reported that CPAP therapy improves cognition and psychomotor performance.53,54 We believe that the correlation of SAI results before and after CPAP therapy will yield more understanding of SAI and its role as a potential marker of the CPAP treatment. Lastly, the reliability of the results might have been affected by the fact that the PSG/PG was not performed in healthy subjects. PSG/PG is a costly and time-consuming procedure, and it was shown previously that the STOP questionnaire reliably and accurately identifies subjects in the general population with increased risk of OSA.30,55 When considering the high sensitivity of the STOP questionnaire,56,57 which was also established in the recent study of our group,58 we decided not to perform PSG/PG in healthy subjects, to minimize medical costs and unnecessary medical procedures in subjects not at risk. Accordingly, if asymptomatic undiagnosed OSAS patients with no risk according to the above-mentioned questionnaires were still included in the healthy subject group, which is possible if PSG is not performed, this may interfere with the results only in a way to diminish the differences between OSAS and healthy subjects.
Since the main objective of the present study was not solely to investigate predictors of MEP changes, future studies may investigate specific measures that influence the MEP amplitude changes in the SAI protocol (ie, BMI, cognitive, psychomotor) by considering the limiting factors of the present study. Furthermore, it would be curious to explore the role of potential neurophysiological markers such as SAI also in mild OSAS patients, which may have possibly significance even in the early detection of OSAS. Moreover, future nTMS studies might investigate neurophysiological measures of cortical motor excitability like SAI in OSAS before commencing CPAP therapy and after a particular therapy time (ie, after 1 year or 2) to detect potential biomarkers of therapy outcome. Further, it is important to elucidate the MRI findings of silent signs of cerebrovascular disease in OSAS patients,58 but unfortunately, our nTMS protocol for MRI scanning was not like the one for detecting silent lacunar infarction and periventricular hyperintensity consisting of T2-weighted images and fluid-attenuated inversion recovery (FLAIR) images. Even though our MRI protocol did not resemble MRI protocol needed for the detection of silent signs of cerebrovascular disease, we suggest future nTMS studies if possible to extend the traditional protocol for MRI scanning required for nTMS brain reconstruction to additional MRI protocols for detecting silent signs of cerebrovascular disease, especially if studies aim to investigate SAI and cognition.59–61
Even though our nTMS study confirmed previous findings related only to SAI and RMT, it would be highly recommended to use nTMS to investigate other neurophysiological protocols for assessment of cortical inhibition, cortical excitation, and cortical plasticity, to elucidate the exact precision and usefulness of nTMS technology in OSAS research.62
Conclusion
The present nTMS study results in decreased RMT, as well as in the reduction of cortical afferent inhibition in OSAS tested with the SAI protocol, confirming previous findings related to SAI and RMT protocols in OSAS patients.16,17,19,20 The results provide further evidence for the role of impaired cortical afferent inhibition in OSAS pathogenesis, with MRI data and nTMS technology contributing to more precise targeting of the motor cortex in OSAS patients compared to Nardone et al.20 The future nTMS studies investigating cortical inhibition, facilitation and plasticity, with the neuropsychological assessment will provide more understanding related to the precision of MRI-data combined with nTMS in OSAS research.
Abbreviations
AASM, American Academy of Sleep Medicine; AHI, apnea-hypopnea index; APB, abductor pollicis-brevis; BMI, body mass index; CPAP, continuous positive airway pressure; EDS, excessive daytime sleepiness; ESS, Epworth sleepiness scale; MRI, magnetic resonance imaging; MEP, motor evoked potential; ODI, oxygen index desaturation; OSAS, obstructive sleep apnea syndrome; PSG, polysomnography; PG, polygraphy; RMT, resting motor threshold; SAI, short-latency afferent inhibition; SEP, somatosensory evoked potentials; SMC, Sleep Medicine Center; TMS, transcranial magnetic stimulation; nTMS, navigated transcranial magnetic stimulation.
Ethics Approval
Ethical standards of the institutional research committee and the 1964 Helsinki declaration with its last amendments were applied to the protocol of the study. Ethics approval was obtained from the Ethics Committee for Biomedical Research at the University of Split School of Medicine (Approval date: May 30, 2014; Code: 003-08/14-03/0001).
Informed Consent
Following the Ethics Committee for Biomedical research and institutional approvals at the University of Split School of Medicine, all participants were informed about the procedures and the aims of the study and signed the informed consent.
Acknowledgments
We would like to thank our colleagues Professor Krešimir Dolić, Professor Ante Buća, and technical and engineering personnel from the University Hospital Split, Department of Diagnostic and Interventional Radiology (Split, Croatia), who provided expertise that significantly assisted the research. We are especially thankful to all the patients and healthy subjects who participated in the study as well as medical personal from the Department of Neuroscience and Split Sleep Medicine Center, University of Split School of Medicine (Natalija Ivković, Ivana Pavlinac Dodig, Linda Lušić).
Author Contributions
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; gave final approval of the version to be published; and agree to be accountable for all aspects of the work.
Disclosure
Part of the results of this paper was presented at the 7th Croatian Neuroscience Congress in Zadar, September 12th, 2019, to September 15th, 2019. The abstract is cited as: Maja Rogić Vidaković, Ana Jerković, Joško Šoda et al Short latency afferent cortical inhibition in obstructive sleep apnea syndrome: a TMS study (PP71 – Book of abstracts: http://www.hiim.unizg.hr/images/ostalo/VII_CNsC_Book_of_Abstracts.pdf).
The authors declare they have no conflict of interest. All authors have seen and approved the final version of the manuscript. No competing interests were reported by the authors for any financial interests or commercial associations held by the authors or their family members.
References
1. Grunstein RR, Phillips CL, Liu PY. Sleep apnea-past, present, future. Sleep Med Rev. 2008;12:1–4. doi:10.1016/j.smrv.2007.11.001
2. Nardone R, Höller Y, Brigo F, Tezzon F, Golaszewski S, Trinka E. Transcranial magnetic stimulation and sleep disorders: pathophysiologic insights. Sleep Med. 2013;14:1047–1058. doi:10.1016/j.sleep.2013.04.025
3. Fischer J, Dogas Z, Bassetti CL, et al. Standard procedures for adults in accredited sleep medicine centres in Europe. J Sleep Res. 2012;21:357–368. doi:10.1111/j.1365-2869.2011.00987.x
4. Jordan AS, McSharry DG, Malhotra A. Adult obstructive sleep apnoea. Lancet. 2014;22(9918):736–747. doi:10.1016/S0140-6736(13)60734-5
5. McNicholas WT. Sleep-related breathing disorders: nosological classification, definitions, epidemiology. In: Bassetti C, Dogas Z, Peigneux P, editors. Sleep Medicine Textbook. Regensburg, Germany: European Sleep Research Society; 2014:215–220.
6. Quan SF, Chan CS, Dement WC, et al. The association between obstructive sleep apnea and neurocognitive performance-the Apnea Positive Pressure Long-term Efficacy Study (APPLES). Sleep. 2011;34:303–314. doi:10.1093/sleep/34.3.303
7. Phillips CL, Grunstein RR, Darendeliler MA, et al. Health outcomes of continuous positive airway pressure versus oral appliance treatment for obstructive sleep apnea: a randomized controlled trial. Am J Respir Crit Care Med. 2013;187:879–887. doi:10.1164/rccm.201212-2223OC
8. Gagnon K, Baril AA, Gagnon JF, et al. Cognitive impairment in obstructive sleep apnea. Pathol Biol (Paris). 2014;62:233–240. doi:10.1016/j.patbio.2014.05.015
9. Pepin JL, Borel JC, Borel AL, Levy P, Tamisier R. Sleep-related breathing disorders: comorbidities and special populations. In: Bassetti C, Dogas Z, Peigneux P, editors. Sleep Medicine Textbook. Regensburg, Germany: European Sleep Research Society; 2014:251–258.
10. Mihalj M, Lušić L, Đogaš Z. Reduced evoked motor and sensory potential amplitudes in obstructive sleep apnoea patients. J Sleep Res. 2016;25:287–295. doi:10.1111/jsr.12368
11. Horner RL. Motor control of the pharyngeal musculature and implications for the pathogenesis of obstructive sleep apnea. Sleep. 1996;19:827–853. doi:10.1093/sleep/19.10.827
12. Horner RL. Impact of brainstem sleep mechanisms on pharyngeal motor control. Respir Physiol. 2000;119:113–121. doi:10.1016/S0034-5687(99)00106-1
13. Civardi C, Naldi P, Cantello R. Cortico-motoneurone excitability in patients with obstructive sleep apnoea. J Sleep Res. 2004;13:159–163. doi:10.1111/j.1365-2869.2004.00391.x
14. Bertini M, De Gennaro L, Ferrara M, et al. Reduction of transcallosal inhibition upon awaking from REM sleep in humans as assesses by transcranial magnetic stimulation. Sleep. 2004;27:875–882. doi:10.1093/sleep/27.5.875
15. Grippo A, Carrai R, Romagnoli I, et al. Cortical excitability in obstructive sleep apnea syndrome: transcranial magnetic stimulation study. Sleep. 2005;28:1547–1553.
16. Joo EY, Kim HJ, Lim YH, Koo DL, Hong SB. Altered cortical excitability in patients with untreated obstructive sleep apnea syndrome. Sleep Med. 2010;11:857–861. doi:10.1016/j.sleep.2010.02.015
17. Das A, Anupa AV, Radhakrishnan A. Reduced plastic brain responses to repetitive transcranial magnetic stimulation in severe obstructive sleep apnea syndrome. Sleep Med. 2013;14:636–640. doi:10.1016/j.sleep.2013.04.008
18. Opie GM, Catcheside PG, Usmani ZA, Ridding MC, Semmler JG. Motor cortex plasticity induced by theta burst stimulation is impaired in patients with obstructive sleep apnoea. Eur J Neurosci. 2013;37:1844–1852. doi:10.1111/ejn.12203
19. Lanza G, Lanuzza B, Aricò D, et al. Direct comparison of cortical excitability to transcranial magnetic stimulation in obstructive sleep apnea syndrome and restless legs syndrome. Sleep Med. 2015;16:138–142. doi:10.1016/j.sleep.2014.08.016
20. Nardone R, Bergmann J, Höller Y, et al. Cortical afferent inhibition reflects cognitive impairment in obstructive sleep apnea syndrome: a TMS study. Sleep Med. 2016;24:51–56. doi:10.1016/j.sleep.2016.08.003
21. Tokimura H, Di Lazzaro V, Tokimura Y, et al. Short latency inhibition of human hand motor cortex by somatosensory input from the hand. J Physiol. 2000;523(Pt 2):503–513. doi:10.1111/j.1469-7793.2000.t01-1-00503.x
22. Fischer M, Orth M. Short-latency sensory afferent inhibition: conditioning stimulus intensity, recording site, and effects of 1 Hz repetitive TMS. Brain Stimul. 2011;4:202–209. doi:10.1016/j.brs.2010.10.005
23. Di Lazzaro V, Oliviero A, Pilato F, et al. Motor cortex hyperexcitability to transcranial magnetic stimulation in Alzheimer’s disease. J Neurol Neurosurg Psychiatry. 2004;75:555–559. doi:10.1136/jnnp.2003.018127
24. Di Lazzaro V, Pilato F, Dileone M, et al. Segregating two inhibitory circuits in human motor cortex at the level of GABAA receptor subtypes: a TMS study. Clin Neurophysiol. 2007;118:2207–22014. doi:10.1016/j.clinph.2007.07.005
25. Turco CV, El-Sayes J, Savoie MJ, Fassett HJ, Locke MB, Nelson AJ. Short- and long- latency afferent inhibition; uses, mechanisms and influencing factor. Brain Stimul. 2018;11:59–74. doi:10.1016/j.brs.2017.09.009
26. Ferreri F, Ponzo D, Hukkanen T, et al. Human brain cortical correlates of short-latency afferent inhibition: a combined EEG-TMS study. J Neurophysiol. 2012;108:314–323. doi:10.1152/jn.00796.2011
27. Epstein LJ, Kristo D, Strollo PJ
28. Kushida CA, Nichols DA, Holmes TH, et al. Effects of continuous positive airway pressure on neurocognitive function in obstructive sleep apnea patients: the Apnea Positive pressure Long-term Efficacy Study (APPLES). Sleep. 2012;35:1593–1602. doi:10.5665/sleep.2226
29. Kylstra WA, Aaronson JA, Hofman WF, Schmand BA. Neuropsychological functioning after CPAP treatment in obstructive sleep apnea: a meta-analysis. Sleep Med Rev. 2013;17:241–347. doi:10.1016/j.smrv.2012.09.002
30. Pecotic R, Dodig IP, Valic M, Ivkovic N, Dogas Z. The evaluation of the Croatian version of the Epworth sleepiness scale and STOP questionnaire as screening tools for obstructive sleep apnea syndrome. Sleep Breath. 2012;16:793–802. doi:10.1007/s11325-011-0578-x
31. Oldfield RC. The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia. 1971;9:97–113. doi:10.1016/0028-3932(71)90067-4
32. Chung F, Abdullah HR, Liao P. STOP-bang questionnaire: a practical approach to screen for obstructive sleep Apnea. Chest. 2016;149:631–638. doi:10.1378/chest.15-0903
33. Kushida CA, Littner MR, Morgenthaler T, Alessi CA, Bailey D, Coleman J
34. Rossini PM, Burke D, Chen R, et al. Non-invasive electrical and magnetic stimulation of the brain, spinal cord, roots and peripheral nerves: basic principles and procedures for routine clinical and research application. Clin Neurophysiol. 2015;126:1071–1107. doi:10.1016/j.clinph.2015.02.001
35. Zomboni J. How to Calculate Percentage Deviation; 2018. Available from: https://sciencing.com.
36. Bishara AJ, Hittner JB. Testing the significance of a correlation with nonnormal data: comparison of Pearson, Spearman, transformation, and resampling approaches. Psychol Methods. 2012;17(3):399–417. doi:10.1037/a0028087
37. Di Lazzaro V, Oliviero A, Profice P, et al. Muscarinic receptor blockade has differential effects on the excitability of intracortical circuits in the human motor cortex. Exp Brain Res. 2000;135:455–461. doi:10.1007/s002210000543
38. Di Lazzaro V, Oliviero A, Pilato F, et al. Neurophysiological predictors of long term response to AChE inhibitors in AD patients. J Neurol Neurosurg Psychiatry. 2005;76:1064–1069. doi:10.1136/jnnp.2004.051334
39. Di Lazzaro V, Oliviero A, Saturno E, et al. Effects of lorazepam on short latency afferent inhibition and short latency intracortical inhibition in humans. J Physiol. 2005;564:661–668. doi:10.1113/jphysiol.2004.061747
40. Aronoff R, Matyas F, Mateo C, Ciron C, Schneider B, Petersen CC. Long-range connectivity of mouse primary somatosensory barrel cortex. Eur J Neurosci. 2010;31:2221–2233. doi:10.1111/j.1460-9568.2010.07264.x
41. Ferezou I, Haiss F, Gentet LJ, Aronoff R, Weber B, Petersen CC. Spatiotemporal dynamics of cortical sensorimotor integration in behaving mice. Neuron. 2007;56:907–923. doi:10.1016/j.neuron.2007.10.007
42. Ballinger EC, Ananth M, Talmage DA, Role LW. Basal Forebrain Cholinergic Circuits and Signaling in Cognition and Cognitive Decline. Neuron. 2016;91:1199–1218. doi:10.1016/j.neuron.2016.09.006
43. Bellingham C, Ireland MF. Contribution of cholinergic systems to state-dependent modulation of respiratory control. Respir Physiol Neurobiol. 2002;131:135–144. doi:10.1016/S1569-9048(02)00043-5
44. Kubin L, Fenik V. Pontine cholinergic mechanisms and their impact on respiratory regulation. Respir Physiol Neurobiol. 2004;143:235–249. doi:10.1016/j.resp.2004.04.017
45. Webster HH, Jones BE. Neurotoxic lesions of the dorsolateral pontomesencephalic tegmentum cholinergic cell area in the cat. II. Effects upon sleepwaking states. Brain Res. 1988;458:285–302. doi:10.1016/0006-8993(88)90471-4
46. Woolf NJ. Cholinergic systems in mammalian brain and spinal cord. Prog Neurobiol. 1991;37:475–524. doi:10.1016/0301-0082(91)90006-M
47. Hedner J, Kraiczi H, Peker Y, Murphy P. Reduction of sleep disordered breathing after physostigmine. Am J Respir Crit Care Med. 2003;168:124–151. doi:10.1164/rccm.200211-1344OC
48. Sukys-Claudino L, Moraes W, Guilleminault C, Tufik S, Poyares D. Beneficial effect of donepezil on obstructive sleep apnea: a double-blind, placebo-controlled clinical trial. Sleep Med. 2012;13:290–296. doi:10.1016/j.sleep.2011.09.014
49. Ferini-Strambi L, Lombardi GE, Marelli S, Galbiati A. Neurological deficits in obstructive sleep Apnea. Curr Treat Options Neurol. 2017;19:16. doi:10.1007/s11940-017-0451-8
50. Polsek D, Gildeh N, Cash D, et al. Obstructive sleep apnoea and Alzheimer’s disease: in search of shared pathomechanisms. Neurosci Biobehav Rev. 2018;86:142–149. doi:10.1016/j.neubiorev.2017.12.004
51. Petersen NT, Pyndt HS, Nielsen JB. Investigating human motor control by transcranial magnetic stimulation. Exp Brain Res. 2003;152:1–16. doi:10.1007/s00221-003-1537-y
52. Ciavarella D, Tepedino M, Chimenti C, et al. Correlation between body mass index and obstructive sleep apnea severity indexes - a retrospective study. Am J Otolaryngol. 2018;39(4):388–391. doi:10.1016/j.amjoto.2018.03.026
53. Pecotic R, Dodig IP, Valic M, et al. Effects of CPAP therapy on cognitive and psychomotor performances in patients with severe obstructive sleep apnea: a prospective 1-year study. Sleep Breath. 2019;23(1):41–48. doi:10.1007/s11325-018-1642-6
54. Turner K, Zambrelli E, Lavolpe S, et al. Obstructive Sleep Apnea: neurocognitive and behavioral functions before and after treatment. Funct Neurol. 2019;34(2):71–78.
55. Silva GE, Vana KD, Goodwin JL, Sherrill DL, Quan SF. Identification of patients with sleep disordered breathing: comparing the four-variable screening tool, STOP, STOP-Bang, and Epworth Sleepiness Scales. J Clin Sleep Med. 2011;7:467–472. doi:10.5664/JCSM.1308
56. Amra B, Rahmati B, Soltaninejad F, Feizi A. Screening questionnaires for obstructive sleep apnea: an updated systematic review. Oman Med J. 2018;33:184–192. doi:10.5001/omj.2018.36
57. Chiu HY, Chen PY, Chuang LP, et al. Diagnostic accuracy of the Berlin questionnaire, STOP-BANG, STOP, and Epworth sleepiness scale in detecting obstructive sleep apnea: a bivariate meta-analysis. Sleep Med Rev. 2017;36:57–70. doi:10.1016/j.smrv.2016.10.004
58. Nishibayashi M, Miyamoto M, Miyamoto T, Suzuki K, Hirata K. Correlation between severity of obstructive sleep apnea and prevalence of silent cerebrovascular lesion. J Clin Sleep Med. 2008;4(3):242–247. doi:10.5664/jcsm.27187
59. Nardone R, Bergmann J, Kronbichler M, et al. Abnormal short latency afferent inhibition in early Alzheimer’s disease: a transcranial magnetic demonstration. J Neural Transm (Vienna). 2008;115(11):1557–1562. doi:10.1007/s00702-008-0129-1
60. Di Lazzaro V, Pilato F, Dileone M, et al. In vivo functional evaluation of central cholinergic circuits in vascular dementia. Clin Neurophysiol. 2008;119(11):2494–2500. doi:10.1016/j.clinph.2008.08.010
61. Bella R, Cantone M, Lanza G, et al. Cholinergic circuitry functioning in patients with vascular cognitive impairment – no dementia. Brain Stimul. 2016;2:225–233. doi:10.1016/j.brs.2015.09.013
62. Tremblay S, Rogasch NC, Premoli I, et al. Clinical utility and prospective of TMS-EEG. Clin Neurophysiol. 2019;130:802–844. doi:10.1016/j.clinph.2019.01.001
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.