Back to Journals » Journal of Pain Research » Volume 14
Objective Real-World Outcomes of Patients Suffering from Painful Neurogenic Claudication Treated with the mild® Procedure: Interim 6-Month Report of a Randomized Controlled Trial
Authors Deer T , Kim C , Wahezi SE, Qu H, Sayed D
Received 24 March 2021
Accepted for publication 26 May 2021
Published 10 June 2021 Volume 2021:14 Pages 1687—1697
DOI https://doi.org/10.2147/JPR.S312573
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 5
Editor who approved publication: Dr Krishnan Chakravarthy
Timothy Deer,1 Christopher Kim,1 Sayed Emal Wahezi,2 Huaguang Qu,3 Dawood Sayed4 On Behalf of The MOTION Study Investigators
1The Spine & Nerve Centers of the Virginias, Charleston, WV, USA; 2Montefiore Health System, Bronx, NY, USA; 3Pennsylvania Pain & Spine Institute, Chalfont, PA, USA; 4The University of Kansas Medical Center, Kansas City, KS, USA
Correspondence: Timothy Deer
The Spine & Nerve Centers of the Virginias, 400 Court Street, Suite 100, Charleston, WV, 25301, USA
Tel +1 304 347 6120
Fax +1 304 347 6126
Email [email protected]
Background: Lumbar spinal stenosis (LSS) is a serious degenerative condition of the spine that can cause significant functional disability. Therapies for these patients generally begin with conservative management, since more invasive interventions such as open surgery and spinal implants are associated with higher complication rates. Early in the treatment algorithm for LSS patients, multiple conventional medical management (CMM) therapies are often combined as an initial low-risk treatment strategy. This composite first-line treatment plan may include conservative care together with early interventional treatment options such as epidural steroid injections, radiofrequency ablation and the mild® Procedure.
Methods: This prospective randomized controlled trial evaluates patients aged 50 to 80 years treated with mild plus CMM, compared to those treated with CMM alone, as the active control. Walking tolerance test outcomes and incidence of subsequent disallowed procedures provided objective real-world outcome data. The incidence of device or procedure-related adverse events was analyzed. Follow-up includes 6-month, 1-year and 2-year assessments, with 1-year being primary. Patients in the mild+CMM group are followed at 3, 4, and 5 years. This is a report of interim 6-month outcomes.
Results: Of 155 patients enrolled at 19 US interventional pain management centers, 78 were allocated to CMM-Alone, and 77 to mild+CMM. At 6-months, the validated walking tolerance test demonstrated statistical superiority of mild+CMM versus CMM-Alone (p< 0.001). The incidence of patients receiving a subsequent disallowed procedure, and thereby considered treatment failures in their study group, was statistically significantly higher in CMM-Alone versus mild+CMM (p< 0.001). There were no device or procedure-related adverse events in either group.
Conclusion: At 6-months, the mild Procedure combined with CMM provided statistically superior objective real-world outcomes versus CMM-Alone. There were no device or procedure-related adverse events reported in either study group. With its excellent safety profile and superior efficacy, mild is uniquely positioned as early first-line therapy.
Keywords: mild®, minimally invasive lumbar decompression, lumbar spinal stenosis, neurogenic claudication, ligamentum flavum
Introduction
Lumbar spinal stenosis (LSS), a serious degenerative condition of the spine, is associated with significant functional disability and chronic pain. LSS is caused by structural narrowing of the spinal canal and resulting ischemic compression of neural elements leading to symptomatic neurogenic claudication. Intervertebral disc degeneration, hypertrophic ligamentum flavum (HLF), and bony pathology are consistently present in patients with neurogenic claudication.1,2 This range of pathologies can lead to central canal stenosis as well as foraminal and lateral recess stenosis. HLF is a major cause of neurogenic claudication for LSS patients, and contributes up to 85% of spinal canal narrowing.3 LSS patients suffering from neurogenic claudication commonly present with multiple types of stenosis and numerous spinal comorbidities.4 Therapies for these patients generally begin with conservative management, since more invasive interventions such as interspinous spacers, surgical decompression and spinal fusion are associated with higher complication rates.4–7 Early in the treatment algorithm for LSS patients, multiple conventional medical management (CMM) therapies are often combined as an initial low-risk treatment strategy. This composite first-line treatment plan may include physical therapy, home exercise programs, and pain medication, together with early interventional procedures such as epidural steroid injections, radiofrequency ablation and the mild® Procedure (Vertos Medical, Aliso Viejo, CA, USA).
The MOTION study is a prospective, multicenter, randomized controlled trial (RCT) evaluating functional improvement in LSS patients aged 50–80 years with neurogenic claudication who are treated with the mild Procedure plus CMM (mild+CMM), compared to those treated with CMM alone (CMM-Alone), as the active control. Patients in the control group are able to crossover and receive mild after completion of 1-year follow-up and prior to 2-years. The goal of the MOTION study is to provide Level 1 evidence of the safety and effectiveness of mild+CMM versus CMM-Alone in managing neurogenic claudication symptoms in patients with verified HLF as a contributing factor. Validated functional improvement measures provide objective outcome data for patients treated with the mild Procedure as first-line therapy in a real-world setting that does not restrict the use of adjunctive conventional medical management measures in either study group. The MOTION study is registered with the US Clinical Trial Registry (NCT03610737). Interim 6-month objective real-world outcomes for patients enrolled in this study are reported.
Patients and Methods
Nineteen interventional pain management centers in the United States are participating in the MOTION study, providing real-world data from academic medical centers, community hospitals and ambulatory surgical centers (ASCs) (See Appendix). All investigators have undergone training on the mild Procedure and are experienced users of mild instrumentation. Institutional Review Board approval was obtained for all sites and Consolidated Standards of Reporting Trials (CONSORT) guidelines were followed.8 All patients provided written informed consent to participate in this study, and the study is being conducted in accordance with the Declaration of Helsinki.
Primary inclusion and exclusion criteria, as well as study symptomatic diagnosis screening requirements to confirm symptoms of neurogenic claudication,9 are presented in Table 1. Patients with comorbid conditions commonly associated with spinal stenosis, such as osteophytes, facet hypertrophy, minor spondylolisthesis (Grade I without instability), foraminal stenosis, and/or disc protrusion were included unless the condition was deemed to be too advanced. The study Medical Monitor reviewed the medical records of all potential patients to confirm eligibility and approve for enrollment. Patients were 50 to 80 years of age, with evidence of ligamentum flavum ≥2.5mm10 confirmed by preoperative imaging.
 |
Table 1 Selection Criteria and Neurogenic Claudication Symptomatic Diagnosis |
Patients were randomly assigned in equal number (1:1) to the mild+CMM treatment arm or CMM-Alone as the active control. Patient allocation to study arm was accomplished by the site using an interactive electronic data capture (EDC) system. Prior to randomization, each patient’s inclusion/exclusion criteria data points were entered into the EDC system. Once the patient was determined to be eligible for the study based on the selection criteria responses provided, the EDC system automatically generated the assigned treatment group.
Patients in both study groups are required to complete 6-month, 1-year and 2-year follow-up assessments, with 1-year being the primary endpoint of the study. Further, patients in the mild+CMM group, together with those in the CMM-Alone group that crossover to receive mild prior to 2-year follow-up, will be followed at 3, 4, and 5 years.
Interventions
The mild Procedure
The mild Procedure is used to treat LSS patients suffering from neurogenic claudication by selectively removing small portions of lamina and HLF, and thereby relieving pressure on nerve roots. mild devices are designed to percutaneously access the interlaminar space through a 5.1mm port positioned in the patient’s posterior lumbar spine. Generally, only moderate sedation and local anesthetic are required, and the small access port minimizes trauma to tissue and bony structures of the spine. mild treatment can be performed unilaterally or bilaterally, and at multiple levels. mild leaves no implants behind, and patients typically resume normal activity within 24 hours with no restrictions.4,7,11 The mild Procedure has been previously described.7 All study mild Procedures were conducted in accordance with the product labeling and indications for use.
Conventional Medical Management
Conventional Medical Management involves treatment with one or more conservative or interventional therapies that are commonly used for early treatment of LSS with neurogenic claudication. The study investigators at each site determine what CMM is optimal for each patient. CMM therapies may include physical therapy, home exercise programs, pain medication, epidural steroid injections (ESIs), nerve blocks, facet joint injections, medial branch injections, trigger point injections, sacroiliac joint injections, radiofrequency ablation and other conservative therapies such as back brace, walking aid, and chiropractic care. All CMM therapies performed are documented and reported throughout the course of the study.
Outcome Measures
Clinical outcome measures for this interim report focus on objective measurements of function and pain. These objective outcome measures included the validated walking tolerance test described by Deen et al,12 which is designed to define baseline functional status and response to treatment at follow-up. During this test, patients walk upright on a flat surface for up to 15 minutes at their preferred speed. The examination is stopped after 15 minutes or at onset of severe symptoms. In this study, the walking tolerance test is administered to all patients at baseline and at all follow-ups.
Another important outcome measure of the MOTION study protocol is the rate of subsequent disallowed procedures which is used as a proxy for study treatment failure. Patients receiving a disallowed procedure to treat continuing symptoms of neurogenic claudication are deemed to be non-responders in their treatment group and are considered study failures. This objective outcome measure is reported and compared as an incidence rate of patients receiving subsequent disallowed procedures in each study group. Lumbar therapies that are disallowed in this study include adhesiolysis, kyphoplasty, neurostimulator, interspinous spacers, and spine surgery. mild Procedures are disallowed for patients in the CMM-Alone study arm.
For all outcome measures, statistical superiority is tested for mild+CMM versus CMM-Alone, as the active control. Safety is reported as the incidence of device/procedure-related adverse events, as adjudicated by an independent clinical event adjudicator. All serious adverse events regardless of relationship are also reported. All subjective clinical outcomes, including the composite primary endpoint, will be reported at 1-year to minimize bias.
Sample Size and Power
Sample size was calculated to obtain at least 90% power for testing the primary superiority hypothesis. The total sample size of 155 is sufficient to meet this objective under the assumption of a 2-sided hypothesis, type 1 error of 0.05, power (1-β) at least 90%, randomization ratio of 1:1, and accounting for dropouts.
Statistical Methods
Descriptive summaries are presented by randomized group for all baseline and outcome measures. Continuous data is summarized using means and standard deviations, while categorical variables are summarized using frequency counts and percentages. For events that can occur more than once in a single subject (eg, adverse events), the percentage is based on subjects experiencing the event, and both patient and event counts are reported. All p-values presented are two-sided using the Fisher Exact Probability Test, with values less than 0.05 considered statistically significant.
Results
Patients
A total of 181 patients signed study informed consent for study evaluation, and of those, 26 were excluded because they did not meet the study selection criteria. The remaining 155 patients were enrolled in the MOTION study from September 2018 through December 2019. Randomization placed 78 patients in the CMM-Alone group and 77 in mild+CMM. The number of patients in each study group was balanced within investigational sites. Following randomization, two CMM-Alone and five mild+CMM patients were not treated, and four CMM-Alone patients voluntarily withdrew for unrelated reasons. Of the remaining patients, two CMM-Alone and one mild+CMM missed their 6-month follow-up visit, resulting in 70 CMM-Alone and 71 mild+CMM patients. Figure 1 presents the participant flow through 6 months.
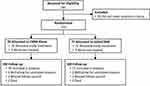 |
Figure 1 Study patient flow through 6-month follow-up. |
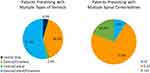
Gender distribution was similar between groups, and while the difference did not reach statistical significance, patients in the CMM-Alone group were on average 2 years older than patients in the mild+CMM group (CMM-Alone: 66.8 years, mild+CMM: 64.7 years, p=0.077). Overall, 91% of the patients presented with five or more spinal comorbidities. The most common lumbar spine comorbidities in addition to HLF were foraminal narrowing, bulging disc, facet arthropathy and facet hypertrophy. All presenting spinal comorbidities occurred at a similar rate between groups. There were no significant baseline differences between the groups. (See Table 2.) It is important to note that MOTION patients also presented with multiple types of stenosis. While 100% of the patients had central stenosis, 93% and 55% also suffered from foraminal stenosis and lateral stenosis, respectively. Only 5% of the patients had central stenosis alone. Figure 2 presents the categorical frequencies of multiple types of stenosis and spinal comorbidities for MOTION patients at baseline presentation.
 |
Table 2 Patient Demographics and Presenting Spinal Comorbidities |
Procedures
mild Procedure data for the mild+CMM group is provided in Table 3. Approximately two-thirds of mild cases were conducted in the hospital outpatient setting, with one-third performed in an ASC. The most common lumbar level treated with mild was L4-L5 (76.4%), followed by L3-L4 (52.8%), L2-L3 (13.9%), and L5-S1 (5.6%). Over 90% of the patients were treated bilaterally, and 59.7% were treated at one level, 31.9% at two levels, and the remaining 8.3% at three levels. The majority of mild patients (93%) underwent monitored anesthesia care (MAC) sedation, moderate sedation, and/or local anesthesia, with the balance receiving a combination of general/local anesthesia. All mild patients were discharged within 24 hours of the procedure.
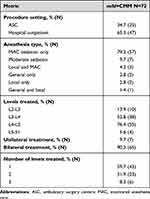 |
Table 3 mild Procedure Information |
Per the study protocol, patients in both treatment arms were able to receive conventional medical management therapies. These CMM therapies included conservative treatments such as physical therapy, home exercise programs, and walking aids, and specified interventional treatments which included ESIs, nerve blocks, other steroid injections, and radiofrequency ablation. Overall, the only difference between the study groups in the incidence of CMM treatments received during 6-month follow-up is facet injections which were significantly higher in the CMM-Alone group (8/70 in CMM-Alone versus 1/71 in mild+CMM, p=0.017). The number of patients receiving CMM conservative and interventional therapies is presented in Table 4.
 |
Table 4 CMM Therapy Received by Patients in Both Study Groups Through 6-Month Follow-Up |
Functional Efficacy Outcomes
With a specific focus on objective measurement of function, the walking tolerance test revealed a marked difference between the two study groups. The number of mild+CMM patients able to walk the full 15 minutes increased more than three-fold from baseline to 6 months, while the number of CMM-Alone patients decreased by one patient over the same period. This difference at 6 months demonstrated statistical superiority of the mild+CMM group versus CMM-Alone (p<0.001). (See Figure 3.) During 6-month follow-up, 17 patients (24.3%) in the CMM-Alone group and two patients (2.8%) in the mild+CMM group received a subsequent disallowed procedure (Figure 4). The incidence of patients receiving a subsequent disallowed procedure, and thereby considered treatment failures in their study group, was statistically significantly higher in the CMM-Alone group versus the mild+CMM group (p<0.001). Table 5 presents the specific disallowed therapies received by patients in both study groups.
 |
Table 5 Disallowed Therapies Received by Each Study Group Through 6-Month Follow-Up |
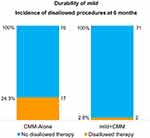 |
Figure 4 Patients receiving disallowed therapy during 6-month follow-up. In this study, disallowed procedures are used as a proxy for study group treatment failure. |
Safety
There were no device or procedure-related adverse events in either group, and the incidence of all serious, non-related adverse events was not statistically significantly different between groups (5.1% and 3.9% for CMM-Alone and mild+CMM, respectively) (p=1.000).
Discussion
The MOTION study was designed to provide objective functional improvement data for patients treated with the mild Procedure as first-line therapy in a real-world setting. In this study, centers were chosen to reflect real-world practice, and CMM is allowed in both study arms at the full discretion of the investigators, which is standard of care. Therefore, study patients are treated exactly as they would be outside of the study. The only difference between study groups is that the mild Procedure is disallowed in the CMM-Alone arm and it is allowed in the mild+CMM arm. Results from this RCT provide Level 1 evidence of the difference in patient outcomes for patients able to receive mild, versus those who are not able to receive mild, in a setting where all other CMM treatment options are allowed.
The MOTION study relies on multiple validated outcome measures, with a focus on analyzing and reporting objective outcome data. The strength of objective outcomes is that they are independent of judgement and are therefore less susceptible to bias.12–16 At 6-month follow-up, two objective outcome measures demonstrated statistically significant superiority of mild+CMM versus CMM-Alone. First, the walking tolerance test resulting in an over three-fold increase in mild+CMM patients able to walk for the full 15 minutes of the test, versus a decrease for CMM-Alone patients. By the 6-month follow-up time point, half of the mild+CMM patients were able to walk the full 15 minutes versus only 13% of the patients in the CMM-Alone arm. The ability to walk further without severe symptoms provides a valuable quality of life improvement for these patients. Second, the use of subsequent disallowed procedures as a proxy for study treatment failure provides a second objective outcome measure that is widely accepted and often used in the real-world analysis of Medicare Claims Data.6 The incidence rate of patients receiving subsequent disallowed procedures is over eight times higher in the CMM-Alone group (24.3%) compared to mild+CMM (2.8%). This pronounced difference is remarkable and demonstrates a distinctly different treatment path between the two study groups.
Patients in this study presented with multiple comorbidities and multiple types of stenosis. Nearly all patients (91%) suffered from five or more spinal comorbidities, and over 50% presented with all three types of stenosis—central, lateral and foraminal (Figure 2). In addition to ligamentum flavum thickening, other spinal comorbidities may contribute to spinal narrowing including intervertebral disc degeneration and bone overgrowth. While the spinal degenerative cascade is complex and introduces numerous potential spinal comorbidities and areas of stenosis, debulking the ligamentum flavum with mild has successfully treated LSS narrowing due to multiple etiologies in the clinical setting. Similar to the MOTION study, the large majority of patients in the ENCORE RCT presented with foraminal stenosis, bulging disc or facet hypertrophy, and these comorbid conditions were determined to be positive predictors of success with mild.4,11
Mekhail et al studied functional improvements in Standing Time and Walking Distance following treatment with the mild Procedure. At 1-year follow-up, 40 patients in this study experienced a mean seven-fold increase in Standing Time and 16-fold increase in Walking Distance. These objective outcome measures directly evaluated patient quality of recovery and provided a real-world report of statistically significant functional improvement for these patients.17
There were no device or procedure-related adverse events reported for either arm in the MOTION study. This level of safety for the mild Procedure is supported by 13 clinical studies, including 2 Level 1 RCTs, that have demonstrated statistically significant efficacy improvement, superiority of mild versus ESIs, and low complication rates.4,7,18–20 In fact, the safety of mild has been shown to be similar to ESIs at a 1.3% rate of device or procedure-related complications for both study groups in the ENCORE RCT.19 It is important to note that all conservative and interventional therapies included in CMM in the MOTION study are positioned early in the treatment algorithm due to their low risk (Table 4). With its excellent safety profile and superior efficacy, mild is uniquely positioned as early first-line therapy.
In the event these first-line CMM therapies do not provide adequate relief for symptoms of neurogenic claudication, LSS patients may choose to undergo more invasive treatments such as interspinous spacers, laminectomy, and spinal fusion that are associated with higher complication rates. These refractory patients represent a significantly different patient profile than those early in the continuum of care.4,7 While the clinical superiority of mild to ESIs/CMM has been established by this and two other RCTs,19,20 a suggested comparison of mild to open surgery presents several methodological challenges. First, these patient populations are at completely different phases on the treatment algorithm. Early patients are seeking to delay or avoid surgery altogether.21 Further, the considerable difference in invasiveness would lead to difficulty with enrollment and post randomization withdrawals due to patients opting out to receive their desired therapy. Due to these fundamental barriers, a valid RCT of this comparison is not feasible. Almost one-third of the US population is covered for reimbursement of the mild Procedure. This includes all Medicare carriers, the Veterans Administration, and multiple commercial plans. It is also covered by Walmart’s Centers of Excellence program for the treatment of LSS.
Limitations of this study include the lack of blinding which was not possible due to the use of an active comparator that allows for a broad range of both conservative and interventional treatments in both study groups. It is anticipated that patients in both arms may continue to receive CMM therapies throughout the study period. The real-world nature of this study, which allows the use of CMM in both study arms at the full discretion of the investigator, is also a limitation due to use of various standard of care treatment algorithms. All CMM treatments as well as subsequent disallowed procedures are recorded. Patients who receive a disallowed procedure remain in the study and are assessed at all follow-ups.
Conclusion
Six-month results of this RCT demonstrate that the mild Procedure combined with CMM provides statistically superior objective real-world outcomes versus CMM-Alone for LSS patients with neurogenic claudication and verified ligamentum flavum hypertrophy. There were no device or procedure-related adverse events reported in either MOTION study group. This strong overall level of safety supports the positioning of these conservative and interventional therapies early in the treatment algorithm due to their established low risk. With its excellent safety profile and superior efficacy, mild is uniquely positioned as early first-line therapy.
Data Sharing Statement
The datasets related to the current study are available from the corresponding author.
Ethics Approval and Informed Consent
Institutional Review Board approval was obtained for all sites. All patients provided written informed consent to participate in this study, and the study is being conducted in accordance with the Declaration of Helsinki.
Acknowledgment
The authors wish to thank Dennis Gilman, PhD, of ClinDM for statistical analysis.
Author Contributions
All authors made significant contributions to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation of data, or in all of these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
This study is sponsored by Vertos Medical. The sponsorship includes study-related supplies and expenses, as well as funding for statistical analysis services by an independent provider.
Disclosure
Timothy Deer is the research principal investigator on this study and is a consultant for Abbott, Axonics, Ethos, Flowonix, Mainstay, SpineThera, SPR Therapeutic, SI Bone, Nevro, Saluda, CornerLoc, PainTEQ, Nalu, Boston Scientific (Vertiflex), Vertos, and Medtronic (Stimgenics). He has received research funding from Abbott, Boston Scientific, Vertos, and Avanos. Christopher Kim is a consultant for Boston Scientific (Vertiflex), Vertos, PainTEQ, and Abbott. Sayed Emal Wahezi is a consultant for Boston Scientific and a research investigator for Abbott. Huaguang Qu is a consultant for Boston Scientific, Vertos, and Flowonix. Dawood Sayed is a consultant for Abbott, Medtronic, Nevro, SPR, Vertos, and PainTEQ, and has received research funding and holds minority equity in Vertos. The authors report no other conflicts of interest in this work.
References
1. Katz JN, Harris MB. Lumbarspinal stenosis. N Engl J Med. 2008;358(8):818–825. doi:10.1056/NEJMcp0708097
2. Porter RW. Spinal stenosis and neurogenic claudication. Spine. 1996;21(17):2046–2052. doi:10.1097/00007632-199609010-00024
3. Hansson T, Suzuki N, Hebelka H, Gaulitz A. The narrowing of the lumbar spinal canal during loaded MRI: the effects of the disc and ligamentum flavum. Eur Spine J. 2009;18:679–686. doi:10.1007/s00586-009-0919-7
4. Staats PS, Chafin TB, Golovac S, et al. Long-term safety and efficacy of minimally invasive lumbar decompression procedure for the treatment of lumbar spinal stenosis with neurogenic claudication: 2-year results of MiDAS ENCORE. Reg Anesth Pain Med. 2018;43:789–794. doi:10.1097/AAP.0000000000000868
5. Weinstein JN, Tosteson RD, Lurie JD, et al. Surgical versus nonsurgical therapy for lumbar spinal stenosis. N Engl J Med. 2008;358:794–810. doi:10.1056/NEJMoa0707136
6. Deyo RA, Martin BI, Ching A, et al. Interspinous spacers compared with decompression or fusion for lumbar stenosis: complications and repeat operations in the Medicare population. Spine. 2013;38(10):865–872. doi:10.1097/BRS.0b013e31828631b8
7. Jain S, Deer TR, Sayed D, et al. Minimally invasive lumbar decompression: a review of indications, techniques, efficacy and safety. Pain Manag. 2020;10:331–348. doi:10.2217/pmt-2020-0037
8. Moher D, Hopewell S, Schulz KF, et al. CONSORT 2010 explanation and elaboration: updated guidelines for reporting parallel group randomized trials. BMJ. 2010;340:c869. doi:10.1136/bmj.c869
9. Sandella DE, Haig AJ, Tomkins-Lane C, Yamakawa K. Defining the clinical syndrome of lumbar spinal stenosis: a recursive specialist survey process. PMR. 2013;5(6):491–495. doi:10.1016/j.pmrj.2012.10.001
10. Park JB, Chang H, Lee JK. Quantitative analysis of transforming growth factor-beta 1 in ligamentum flavum of lumbar spinal stenosis and disc herniation. Spine. 2001;26:E492–E495. doi:10.1097/00007632-200111010-00007
11. Deer TR, Grider JS, Pope JE, et al. The MIST guidelines: the Lumbar Spinal Stenosis Consensus Group guidelines for minimally invasive spine treatment. Pain Pract. 2019;19(3):250–274. doi:10.1111/papr.12744
12. Deen HG, Zimmerman RS, Lyons MK, McPhee MC, Verheijde JL, Lemens SM. Measurement of exercise tolerance on the treadmill in patients with symptomatic lumbar spinal stenosis: a useful indicator of functional status and surgical outcome. J Neurosurg. 1995;83(1):27–30. doi:10.3171/jns.1995.83.1.0027
13. Bowyer A, Royse C. A matter of perspective – objective versus subjective outcomes in the assessment of quality of recovery. Best Pract Res Clin Anaesthesiol. 2018;32(3–4):287–294. doi:10.1016/j.bpa.2018.02.003
14. Page MJ, Higgins JPT, Clayton G, Sterne JAC, Hróbjartsson A, Savović J. Empirical evidence of study design biases in randomized trials: systematic review of meta-epidemiological studies. PLoS One. 2016;11:e0159267. doi:10.1371/journal.pone.0159267
15. Moustgaard H, Bello S, Miller FG, Hróbjartsson A. Subjective and objective outcomes in randomized clinical trials: definitions differed in methods publications and were often absent from trial reports. J Clin Epidemiol. 2014;67(12):1327–1334. doi:10.1016/j.jclinepi.2014.06.020
16. Savovic J, Jones HE, Altman DG, et al. Influence of reported study design characteristics on intervention effect estimates from randomized, control trials. Ann Intern Med. 2012;156:429–438. doi:10.7326/0003-4819-157-6-201209180-00537
17. Mekhail N, Costandi S, Abraham B, Samuel SW. Functional and patient-reported outcomes in symptomatic lumbar spinal stenosis following percutaneous decompression. Pain Pract. 2012;12(6):417–425. doi:10.1111/j.1533-2500.2012.00565.x
18. Staats PS, Benyamin RM. MiDAS ENCORE: randomized controlled clinical trial report of 6-month results. Pain Physician. 2016;19:25–37. doi:10.36076/ppj/2016.19.25
19. Benyamin RM, Staats PS. mild® is an effective treatment for lumbar spinal stenosis with neurogenic claudication: miDAS ENCORE Randomized Controlled Trial. Pain Physician. 2016;19:229–242. doi:10.36076/ppj/2019.19.229
20. Brown L. A double-blind, randomized, prospective study of epidural steroid injection vs. the mild® procedure in patients with symptomatic lumbar spinal stenosis. Pain Pract. 2012;12(5):333–341. doi:10.1111/j.1533-2500.2011.00518.x
21. Mekhail NA, Costandi SJ, Armanyous S, et al. The impact of age on the outcomes of minimally invasive lumbar decompression for lumbar spinal stenosis. Med Devices. 2020;13:151–161. doi:10.2147/MDER.S251556
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.