Back to Journals » Infection and Drug Resistance » Volume 15
NWMN2330 May Be Associated with the Virulence of Staphylococcus aureus by Increasing the Expression of hla and saeRS
Authors Liu L, Wang B, Yu J, Guo Y, Yu F
Received 7 March 2022
Accepted for publication 26 May 2022
Published 2 June 2022 Volume 2022:15 Pages 2853—2864
DOI https://doi.org/10.2147/IDR.S365314
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Prof. Dr. Héctor Mora-Montes
Li Liu,1 Bingjie Wang,2,3 Jingyi Yu,2,3 Yinjuan Guo,2,3 Fangyou Yu2,3
1Department of Transfusion Medicine, Nanchong Central Hospital, The Second Clinical Medical College of North Sichuan Medical College, Nanchong, People’s Republic of China; 2Department of Clinical Laboratory Medicine, Shanghai Pulmonary Hospital, Tongji University School of Medicine, Shanghai, People’s Republic of China; 3Shanghai Key Laboratory of Tuberculosis, Shanghai Pulmonary Hospital, Tongji University School of Medicine, Shanghai, People’s Republic of China
Correspondence: Fangyou Yu, Email [email protected]
Introduction: Staphylococcus aureus is an opportunistic pathogen that can cause life-threatening bloodstream infections such as sepsis and endocarditis. In recent years, the emergence and increase of methicillin-resistant and multidrug-resistant S. aureus has posed a great challenge to the antibiotic treatment of infectious diseases. Anti-virulence strategies targeting virulence factors are an effective new therapy for the treatment of S. aureus infections.
Results: In this study, we constructed a NWMN2330 deletion mutant (Newman-ΔNWMN2330) and a complement (Newman-ΔNWMN2330-C) of S. aureus Newman to study the role of NWMN2330 in the virulence of S. aureus. Through transcriptome sequencing, it was found that the expression of 224 genes in Newman-ΔNWMN2330 was significantly different (> 2-fold) compared with S. aureus Newman, and these differentially expressed genes were related to multiple functions of S. aureus. And we found that NWMN2330 could positively regulate the expression of S. aureus hla gene. Therefore, the deletion mutant Newman-ΔNWMN2330 exhibited lower hemolytic activity and lower α-toxin production than Newman. Newman-ΔNWMN2330 also exhibited lower lethality and pathogenicity in worm survival experiments and nude mouse skin abscess model. RT-qPCR results showed that compared with the wild-type strain, the expression of saeRS and hla in Newman-ΔNWMN2330 strain was significantly reduced at the mRNA level, which preliminarily indicated that NWMN2330 promoted the expression of hla by up-regulating saeRS.
Discussion: In general, our results indicated that NWMN2330 may be associated with the virulence of Staphylococcus aureus by increasing the expression of hla and saeRS.
Keywords: virulence, NWMN2330, Staphylococcus aureus, hla, saeRS
Introduction
Staphylococcus aureus is an asymptomatic human nostril colonizer, permanently colonizing approximately 30% of individuals.1 And it is one of the most notorious and widespread bacterial pathogens, causing incalculable numbers of uncomplicated skin infections and possibly hundreds of thousands to millions of more serious invasive infections worldwide each year.2,3 S. aureus infections are particularly problematic due to the frequent emergence of antibiotic resistance among S. aureus isolates, of which methicillin-resistant S. aureus (MRSA) is the most clinically important.4 Resistance of S. aureus to other antibiotics is also common. For example, resistance to β-lactamase-sensitive traditional β-lactam antibiotics (penicillin and derivatives) is almost ubiquitous in S. aureus.5 Furthermore, S. aureus typically exhibits resistance to nearly all available antibiotics in combination.6 Vancomycin remains the antibiotic of last resort for MRSA infections, and highly vancomycin-resistant strains (VRSA) have emerged but have not spread, probably due to the greatly increased fitness costs imposed by vancomycin resistance genes.7 However, some strains have acquired moderate resistance to vancomycin (VISA).8 Available antibiotics are not sufficiently effective against multidrug-resistant S. aureus strains, and although several S. aureus vaccines have been tested in clinical trials, all vaccine candidates have so far failed clinical trials.9,10 Overreliance on animal models and an incomplete understanding of S. aureus pathogenesis during human infection may explain this failure.11 Based on the failure of all vaccines, people are forced to seek new treatments for S. aureus infections.
Since S. aureus produces a variety of virulence factors that play important roles in infection, including hemolysin, enterotoxins, plasma coagulase, and leukocidal toxins,12,13 anti-virulence strategies for the treatment of S. aureus infections have received increasing attention. The anti-bacterial virulence strategy is to achieve the purpose of anti-infection by reducing the virulence of pathogens. It does not directly kill the bacteria, and it is not easy to cause drug resistance and destroy the beneficial microbiota. Current studies have found that α-hemolysin, SrtA and golden yellow pigment are good virulence targets.14–18 In our previous study, our group found that after sub-inhibitory concentrations of resveratrol reduced the virulence of S. aureus, the expression of the NWMN2330 gene was down-regulated in its transcriptome.19 Therefore, we speculate that this gene may be closely related to the virulence of S. aureus. See Supplementary Material for NWMN2330 gene sequence.
Our study mainly focuses on the role of NWMN2330 in the virulence of S. aureus, which provides a theoretical basis for further understanding the role of NWMN2330 in S. aureus, and also provides a new target for the treatment of S. aureus infection.
Materials and Methods
Bacterial Strains, Plasmids, and Culture Conditions
The bacterial strains and plasmids used in this study were listed in Table 1. According to the manufacturer’s instructions (bioMérieux, Marcy l’Etoile, France), the VITEK-2 microbial analyzer was used to identify the isolates. S. aureus Newman and S. aureus Newman NWMN2330 mutants were grown in tryptic soy broth (TSB)(BD) medium, supplemented in tryptic soy broth (TSB)(BD, NJ, United States) containing 10 mg/l chloramphenicol Grow in a medium, shake 220 rpm at 37°C. Escherichia coli was cultured in Luria broth (LB,)(Oxoid) medium containing appropriate antibiotics (ampicillin 100 mg/L and anhydrotetracycline 50 mg/L).
 |
Table 1 Bacterial Strains and Plasmids Used in This Study |
Construction of S. aureus NWMN2330 Mutant Strain (Newman-ΔNWMN2330) and Its Complemented Strain (Newman-ΔNWMN2330-C)
The accession number of the Newman2330 gene in the NCBI GenBank is BAF68602.1.
The specific function in S. aureus Newman is unclear. We blasted other S. aureus with this gene. In common S. aureus, such as USA300, the gene expresses hypothetical protein, and in some other S. aureus such as 16,405, the gene expresses GtrA family protein. The temperature-sensitive plasmid pKOR1 was used to construct the NWMN2330 deletion mutant of S. aureus Newman through allelic replacement. Use primer sets NWMN2330-UP-F/NWMN2330-UP-R and NWMN2330-DOWN-F/NWMN2330-DOWN-R to amplify the upstream and downstream fragments of NWMN2330 from the genomic DNA of S. aureus Newman (Table 2). The resulting amplicon was digested with XhoI and then ligated with T4 DNA ligase to generate a homology arm fragment with NWMN2330 gene deletion, which was then cloned into pKOR1. The recombinant plasmid pKOR1-ΔNWMN2330 was sequentially transferred to E. coli DH5α and DC10B competent cells, and finally electroporated into S. aureus Newman competent cells. The allelic replacement mutants were selected as described previously.20 Briefly, we amplified the NWMN2330 fragment using Newman genomic DNA as a template. The plasmid pLI50 and the gene fragment were digested with EcoRI and KpnI restriction enzymes, and the plasmid and fragment were ligated with T4 ligase. The ligated products were sequentially thermally transformed into E. coli DH5α and DC10B for amplification and modification. Finally, it was electroporated into Newman-ΔNWMN2330, and each step is verified by PCR and first-generation Sanger DNA sequencing.
 |
Table 2 Primers Used in This Study |
Growth Assay
The strain Newman, the deletion mutant Newman-ΔNWMN2330, and the complementary strain Newman-ΔNWMN2330-C were inoculated on blood agar plates and incubated overnight at 37 ° C incubator. Pick a single clone and inoculate in 5mL of tryptic soy broth (TSB) (BD, NJ, United States) for shaking culture, and then inoculate in 50mL TSB for shaking culture at a ratio of 1: 200. All cultures were induced at 37 °C with shaking at 220 r.p.m. and the OD562 values were measured every hour for a total of 24 h. The assay was performed in triplicate.
RNA-seq and Identification of Differentially Expressed Genes
The wild strains of S. aureus Newman and Newman-ΔNWMN2330 were cultured in TSB for 9h, and then the bacteria were collected by centrifugation at 12,000 g for 1 minute at 4 ° C. The method recommended by the manufacturer (QIAGEN, Berlin, Germany) for RNA extraction. Illumina HiSeq X platform and pe150 (150 bp double-stranded assay) strategy were used to analyze the tested RNA, and DEGseq software was used to analyze differentially expressed genes. log2 (fold-change) |> 1, and p <0.005 represents the difference in gene expression between samples.
Quantitative Enzyme-Linked Immunosorbent Assay for α-Toxin
The bacteria were cultured in TSB with shaking for 24 hours, after which the supernatant was collected by centrifugation at 6000 g for 2 minutes and filtered with a 0.22 μm filter. We used the staphylococcus alpha-toxin enzyme-linked immunoassay kit (Sigma-Aldrich, St. Louis, Missouri, USA) to detect alpha-toxin. The above-extracted supernatant was added to the detection plate, and then horseradish peroxidase (HRP) label was added to the plate, and the above mixture became an antibody-antigenase labeled antibody complex. After washing, TMB (3,30,5,50-tetramethylbenzidine) substrate solution was added, and finally sulfuric acid solution was added to stop the reaction, and the color change was measured by spectrophotometry at 450 nm wavelength. Use the standard curve y = ax + b to calculate the concentration of alpha toxin in each sample and then multiply it by the corresponding dilution factor. Each test was repeated three times.
Determination of Hemolytic Ring
Newman and Newman-ΔNWMN2330 were inoculated on Columbia blood agar medium, Newman-ΔNWMN2330-C was inoculated on the plate containing chloramphenicol, and placed in a 37°C incubator for overnight culture. Bacteria were shaken overnight at 37°C in TSB liquid medium at 220 rpm. Centrifuge at 4000g at room temperature for 5 minutes, discard the supernatant, resuspend the bacterial solution in PBS, and centrifuge again at 4000g at room temperature for 5 minutes. After that, 10 μL of bacteria was dropped on Columbia blood agar medium, placed in a 37°C incubator for 24 hours, and the size of hemolytic ring was observed.
α-Toxin Activity Determination
The experimental method is slightly modified on the basis of the previous literature.15 Briefly, S. aureus strains were cultured in TSB at 37 ° C, and the supernatant was extracted according to the above experimental method. Then add 100 µL supernatant to 875 µL hemolysis buffer, incubate with 25 µL defibrillated rabbit blood at 37 ° C for 1 h, and centrifuge at 6000 g for 2 minutes. Take the supernatant and measure the OD value of the supernatant at 450 nm. Triton X-100 was used as a positive control and 0.9% NaCl was used as a negative control. Each test was performed independently in triplicate.
Real-Time Quantitative Polymerase Chain Reaction (RT-qPCR)
After shaking culture of S. aureus for 9h, RNA was extracted according to the above method. The primer pairs used in real-time RT-PCR are listed in Table 1. The total RNA was reversed to cDNA using Takara RNA PCR kit (Takara, Tokyo, Japan). PCR was performed in 20 µL reaction mixture using Luna Universal qPCR Master Mix (NEB, MA, United States). Each test was performed in triplicate.
Determination of Silkworms Survival Rate
This experiment mainly refers to previous studies with minor modifications.21 Newman and Newman-ΔNWMN2330 were inoculated on Columbia blood agar medium, Newman-ΔNWMN2330-C was inoculated on the plate containing chloramphenicol, and placed in a 37°C incubator for overnight culture. Pick a single clone and add it to 5mL TSB liquid medium, shake it at 220rpm, 37°C, and grow it in the late logarithmic growth period, about 8 hours. The bacteria were centrifuged at 5000 g for 5 min at room temperature. After discarding the supernatant, they were resuspended in PBS, centrifuged again and repeated three times. Adjust the bacterial solution to a consistent OD value, probably at OD562=1.5. The experiment was divided into 4 groups, namely wild bacteria group (Newman), deletion mutant group (Newman-ΔNWMN2330), complementary strain group (Newman-ΔNWMN2330-C), and blank control group, which was injected with PBS. The experimental sample for each group is 12. Between the second and third gastropods of the silkworms, 8×108 CFU/100 μL of bacteria were injected into the body with an insulin needle, and PBS was used as a blank control. Placed in a 37°C incubator, record the survival time of the wax moth, and draw it with drawing software.
Mouse Skin Abscess Model
Animal research was approved by the Institutional Animal Care and Use Committee.
All animal assays were carried out in accordance with The Regulation on the Management of Laboratory Animals for the welfare of the laboratory animals, and the protocol approved by the Ethics Committee of Shanghai pulmonary hospital, Tongji University School of medicine. In the study, 4 to 6 week old male BALB/c nude mice were used, divided into three groups of four. For the specific method, refer to the previously published article.22 BALB/c mice were completely anesthetized with 1% (mass/volume) pentobarbital sodium (50 mg/kg body weight). Then, 100 μL of 5×108 CFU bacteria were inoculated subcutaneously on the back of the mice, and the mice injected with PBS were used as blank controls. The area evaluated by the maximum length and width of the skin damaged part is measured every day. Use the formula A = L × W to calculate the damage area, where L is the length and W is the width.
Statistical Analysis
Experimental data were analyzed using GraphPad Prism 6 software (version 6.00, La Jolla, CA, United States). A p-value less than 0.05 was considered to be statistically significant.
Result
NWMN2330 Did Not Affect the Growth of S. aureus
In order to test whether NWMN2330 had an effect on the growth of S. aureus Newman, we measured the growth curves of S. aureus Newman, Newman-ΔNWMN2330 and Newman-ΔNWMN2330-C. The results showed that the growth of the three strains was similar, with no statistical difference (Figure 1), indicating NWMN2330 had no effect on the growth of S. aureus Newman.
NWMN2330 Participates in the Regulation of Multiple Genes Related to Virulence
Transcriptome data showed that Newman-ΔNWMN2330 has a total of 224 gene expression changes (according to |log2(Fold Change)| >1 and q value<0.05 criteria) relative to Newman, of which 91 genes are up-regulated and 133 genes are down-regulated (Figure 2). After bioinformatics analysis, it was found that these genes are involved in multiple metabolic pathways, including S. aureus infection, cell killing, carotenoid biosynthesis, two-component regulatory system, complement binding, and amino acid anabolism (Figure 3 and Table 3).
 |
Table 3 Newman-ΔNWMN2330 vs Newman Genes with Significantly Changed Expression |
NWMN2330 Inhibits the Expression of Virulence Gene hla
Transcriptome data showed that the expression of hla in Newman-ΔNWMN2330 was significantly down-regulated by 2^10.008-fold compared to Newman. We used RT-qPCR to verify the transcriptome data, the results showed (Figure 4) that hla expression in Newman-ΔNWMN2330 was significantly down-regulated, indicating that NWMN2330 may be linked with hla regulation.
NWMN2330 Enhances the Hemolytic Ability of S. aureus
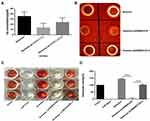
The production of hemolysin is an important part of the virulence of S. aureus, and alpha toxin is the most important toxin in hemolysin. We tested the hemolytic ability of S. aureus Newman, Newman-ΔNWMN2330 and Newman-ΔNWMN2330-C. The amount of α-toxin was quantitatively detected by ELISA, and it was found that the amount of Newman-ΔNWMN2330 produced was significantly less than that of Newman (Figure 5A). And the hemolytic ring produced by Newman was significantly larger than that of Newman-ΔNWMN2330, and the complementary strain reached the level of Newman (Figure 5B). Because alpha toxin is a punch protein, it has the most obvious hemolytic effect on rabbit red blood cells. We used defibrillated rabbit blood to detect hemolytic activity, and used Triton as a positive control and normal saline as a negative control. It was found that the hemolytic activity of Newman-ΔNWMN2330 was significantly lower than that of wild strain Newman (Figure 5C and D). The above results indicate that NWMN2330 can positively regulate the expression of S. aureus hemolysin.
NWMN2330 Enhances the Lethality of S. aureus to Silkworms
Because the production of toxins is closely related to the pathogenicity of S. aureus, after observing the effect of NWMN2330 on hemolysin, we speculate that NWMN2330 may be related to the virulence and pathogenicity of S. aureus. So we measured the survival rate of the silkworms after infection with the three kinds bacteria. The results showed that the survival time of silkworms infected with Newman was significantly shorter than the time of injection of Newman-ΔNWMN2330, which initially indicated that NWMN2330 could enhance the virulence of S. aureus (Figure 6).
Effect on Infection of Subcutaneous Abscess in Mice
S. aureus infection often causes skin abscesses.23,24 We used skin abscess model in nude mice to investigate whether NWMN2330 had an effect on the ability of S. aureus to cause skin abscesses. The results showed that the abscess area of mice infected with the mutant strain (4±0.76) was significantly reduced compared to the wild strain (130±16.16) (Figure 7). The above results indicate that NWMN2330 is associated with the virulence and pathogenicity of S. aureus and can enhance the pathogenicity of S. aureus.
NWMN2330 May Promote hla Expression by Up-Regulating saeRS
The saeRS two-component regulatory system is a regulator that controls the expression of multiple virulences.25–28 According to the transcriptome data, it is speculated that saeRS is also involved in the regulation of hla expression by NWMN2330. RT-PCR results showed that when NWMN2330 was knocked out, the expression of saeRS was significantly down-regulated, and there was no downward trend in other related regulators sarA (Figure 8). The previous speculations have been further confirmed, indicating that NWMN2330 may promote hla expression by up-regulating saeRS.
Discussion
Due to the increasing number of multidrug-resistant S. aureus in recent years, there is an urgent need for new antibacterial agents that can overcome these more robust S. aureus strains. The discovery of new antibiotics has slowed in recent years, and strategies to reduce the virulence of S. aureus to reduce its lethality have attracted great interest.29 In recent years, there have been many studies on the use of anti-virulence strategies for the treatment of S. aureus infections,30–32 but not enough for the rapidly evolving multidrug-resistant bacteria. Our previous study found that NWMN2330 in S. aureus may be related to virulence,19 but the specific role was not clear. The gene annotation of NWMN2330 is “similar to functionally unknown protein”, and its specific function is worth exploring.
In the present study, we mainly focused on the virulence control of S. aureus by NWMN2330. We successfully constructed the S. aureus Newman deletion mutant Newman-ΔNWMN2330 and the complemented strain Newman-ΔNWMN2330-C. In recent years, transcriptome sequencing analysis has been frequently used to study the pathogenic and drug resistance mechanisms of S. aureus.33,34 The results of transcriptome sequencing showed that compared with Newman, the expression of hla in the mutant strain was down-regulated very significantly. We also confirmed by real-time quantitative PCR that the expression of hla at the mRNA level was indeed significantly reduced in the mutant strain. We speculate that NWMN2330 may regulate S. aureus virulence by enhancing hla expression.
Alpha-toxin (encoded by hla) is the most studied pore-forming toxin and plays an important role in S. aureus skin and soft tissue infections.35–37 Alpha-toxin has an obvious hemolytic effect on red blood cells of various mammals, especially rabbit red blood cells.38 Using the above characteristics of α-toxin, we detected the hemolytic activity, hemolytic ring and α-toxin production of Newman, Newman-ΔNWMN2330 and Newman-ΔNWMN2330-C strains, respectively. The deletion mutant Newman-ΔNWMN2330 showed lower hemolytic activity, lower α-toxin production and smaller hemolytic ring than the wild strain in the above three experiments. We know that NWMN2330 also enhance the virulence and pathogenicity of S. aureus from survival experiments in silkworms and skin abscess model in nude mice. The production of S. aureus α-toxin is regulated by multiple regulatory systems, of which saeRS is considered to be the most important one because the sae locus is important for hla transcription.39 The S. aureus sar locus also has alpha and beta hemolysins, and SarA directly increases α-toxin production.40 We found by real-time quantitative PCR that the expression of saeRS decreased significantly in the deletion mutant, but the change of sarA was not significant. We speculate that NWMN2330 may up-regulate the expression of hla by up-regulating the expression of saeRS, thereby enhancing the virulence of S. aureus.
Our research has many limitations. First, it is only tentatively speculated that NWMN2330 up-regulates the expression of hla through saeRS to enhance the virulence of S. aureus, and has not been studied more precisely. Secondly, we only studied the regulatory effect of NWMN2330 on the α-toxin of S. aureus, and there are many other functions that are not covered in this paper. We can see in the transcriptome sequencing data that NWMN2330 also affects the expression of many other important virulence genes. Also, it has not been studied whether the gene has the same effect in other S. aureus strains.
In summary, our study shows that the gene NWMN2330 can up-regulate the expression of hla and enhance the pathogenicity in S. aureus Newman strain. The gene NWMN2330 may be an important target for limiting S. aureus infection in people colonised by this potential pathogen.
Ethics Statement
Animal research was approved by the Institutional Animal Care and Use Committee.
All animal assays were carried out in accordance with The Regulation on the Management of Laboratory Animals for the welfare of the laboratory animals, and the protocol approved by the Ethics Committee of Shanghai pulmonary hospital, Tongji University School of medicine. The approved ethics review number is K20-151Y.
Funding
This work was supported by grants from the Natural Science Fund of China (82072343).
Disclosure
No potential conflict of interest was reported by the authors.
References
1. Wertheim HF, Melles DC, Vos MC, et al. The role of nasal carriage in Staphylococcus aureus infections. Lancet Infect Dis. 2005;5(12):751–762. doi:10.1016/S1473-3099(05)70295-4
2. Rasigade JP, Dumitrescu O, Lina G. New epidemiology of Staphylococcus aureus infections. Clin Microbiol Infect. 2014;20(7):587–588. doi:10.1111/1469-0691.12718
3. Klevens RM, Morrison MA, Nadle J, et al. Active bacterial core surveillance MI. Invasive methicillin-resistant Staphylococcus aureus infections in the United States. JAMA. 2007;298(15):1763–1771. doi:10.1001/jama.298.15.1763
4. Turner NA, Sharma-Kuinkel BK, Maskarinec SA, et al. Methicillin-resistant Staphylococcus aureus: an overview of basic and clinical research. Nat Rev Microbiol. 2019;17(4):203–218. doi:10.1038/s41579-018-0147-4
5. Lowy FD. Antimicrobial resistance: the example of Staphylococcus aureus. J Clin Invest. 2003;111(9):1265–1273. doi:10.1172/JCI18535
6. Cheung GYC, Bae JS, Otto M. Pathogenicity and virulence of Staphylococcus aureus. Virulence. 2021;12(1):547–569. doi:10.1080/21505594.2021.1878688
7. McGuinness WA, Malachowa N, DeLeo FR. Vancomycin resistance in Staphylococcus aureus. Yale J Biol Med. 2017;90(2):269–281.
8. Hiramatsu K, Kayayama Y, Matsuo M, et al. Vancomycin-intermediate resistance in Staphylococcus aureus. J Glob Antimicrob Resist. 2014;2(4):213–224. doi:10.1016/j.jgar.2014.04.006
9. Miller LS, Fowler VG, Shukla SK, Rose WE, Proctor RA. Development of a vaccine against Staphylococcus aureus invasive infections: evidence based on human immunity, genetics and bacterial evasion mechanisms. FEMS Microbiol Rev. 2020;44(1):123–153. doi:10.1093/femsre/fuz030
10. Bagnoli F, Bertholet S, Grandi G. Inferring reasons for the failure of Staphylococcus aureus vaccines in clinical trials. Front Cell Infect Microbiol. 2012;2:16. doi:10.3389/fcimb.2012.00016
11. Cruz AR, van Strijp JAG, Bagnoli F, Manetti AGO. Virulence gene expression of Staphylococcus aureus in human skin. Front Microbiol. 2021;12:692023. doi:10.3389/fmicb.2021.692023
12. Hodille E, Rose W, Diep BA, Goutelle S, Lina G, Dumitrescu O. The role of antibiotics in modulating virulence in Staphylococcus aureus. Clin Microbiol Rev. 2017;30(4):887–917. doi:10.1128/CMR.00120-16
13. Dinges MM, Orwin PM, Schlievert PM. Exotoxins of Staphylococcus aureus. Clin Microbiol Rev. 2000;13(1):16–34, table of contents. doi:10.1128/CMR.13.1.16
14. Zhang B, Teng Z, Li X, et al. Chalcone attenuates Staphylococcus aureus virulence by targeting Sortase A and alpha-hemolysin. Front Microbiol. 2017;8:1715. doi:10.3389/fmicb.2017.01715
15. Teng Z, Shi D, Liu H, et al. Lysionotin attenuates Staphylococcus aureus pathogenicity by inhibiting alpha-toxin expression. Appl Microbiol Biotechnol. 2017;101(17):6697–6703. doi:10.1007/s00253-017-8417-z
16. Chen F, Di H, Wang Y, et al. Small-molecule targeting of a diapophytoene desaturase inhibits S. aureus virulence. Nat Chem Biol. 2016;12(3):174–179. doi:10.1038/nchembio.2003
17. Mu D, Xiang H, Dong H, Wang D, Wang T. Isovitexin, a potential candidate inhibitor of Sortase A of Staphylococcus aureus USA300. J Microbiol Biotechnol. 2018;28(9):1426–1432. doi:10.4014/jmb.1802.02014
18. Nitulescu G, Nicorescu IM, Olaru OT, et al. Molecular docking and screening studies of new natural Sortase A inhibitors. Int J Mol Sci. 2017;18(10):2217. doi:10.3390/ijms18102217
19. Duan J, Li M, Hao Z, et al. Subinhibitory concentrations of resveratrol reduce alpha-hemolysin production in Staphylococcus aureus isolates by downregulating saeRS. Emerg Microbes Infect. 2018;7(1):136. doi:10.1038/s41426-018-0142-x
20. Zhu Q, Wen W, Wang W, Sun B. Transcriptional regulation of virulence factors Spa and ClfB by the SpoVG-Rot cascade in Staphylococcus aureus. Int J Med Microbiol. 2019;309(1):39–53. doi:10.1016/j.ijmm.2018.10.006
21. Kaito C, Kurokawa K, Matsumoto Y, et al. Silkworm pathogenic bacteria infection model for identification of novel virulence genes. Mol Microbiol. 2005;56(4):934–944. doi:10.1111/j.1365-2958.2005.04596.x
22. Shang W, Rao Y, Zheng Y, et al. Beta-Lactam antibiotics enhance the pathogenicity of methicillin-resistant Staphylococcus aureus via SarA-controlled lipoprotein-like cluster expression. mBio. 2019;10(3). doi:10.1128/mBio.00880-19
23. McCaig LF, McDonald LC, Mandal S, Jernigan DB. Staphylococcus aureus-associated skin and soft tissue infections in ambulatory care. Emerg Infect Dis. 2006;12(11):1715–1723. doi:10.3201/eid1211.060190
24. Moran GJ, Krishnadasan A, Gorwitz RJ, et al; Group EMINS. Methicillin-resistant S. aureus infections among patients in the emergency department. N Engl J Med. 2006;355(7):666–674. doi:10.1056/NEJMoa055356
25. Nygaard TK, Pallister KB, Ruzevich P, Griffith S, Vuong C, Voyich JM. SaeR binds a consensus sequence within virulence gene promoters to advance USA300 pathogenesis. J Infect Dis. 2010;201(2):241–254. doi:10.1086/649570
26. Voyich JM, Vuong C, DeWald M, et al. The SaeR/S gene regulatory system is essential for innate immune evasion by Staphylococcus aureus. J Infect Dis. 2009;199(11):1698–1706. doi:10.1086/598967
27. Watkins RL, Pallister KB, Voyich JM, Diep B. The SaeR/S gene regulatory system induces a pro-inflammatory cytokine response during Staphylococcus aureus infection. PLoS One. 2011;6(5):e19939. doi:10.1371/journal.pone.0019939
28. Montgomery CP, Daum RS. Transcription of inflammatory genes in the lung after infection with community-associated methicillin-resistant Staphylococcus aureus: a role for panton-valentine leukocidin? Infect Immun. 2009;77(5):2159–2167. doi:10.1128/IAI.00021-09
29. Kong C, Neoh HM, Nathan S. Targeting Staphylococcus aureus toxins: a potential form of anti-virulence therapy. Toxins. 2016;8(3):72. doi:10.3390/toxins8030072
30. Sharifi A, Mohammadzadeh A, Salehi TZ, Mahmoodi P, Nourian A. Cuminum cyminum L. Essential Oil: a promising antibacterial and antivirulence agent against multidrug-resistant Staphylococcus aureus. Front Microbiol. 2021;12:667833. doi:10.3389/fmicb.2021.667833
31. Slavetinsky CJ, Hauser JN, Gekeler C, et al. Sensitizing Staphylococcus aureus to antibacterial agents by decoding and blocking the lipid flippase MprF. Elife. 2022;11. doi:10.7554/eLife.66376
32. Zheng J, Shang Y, Wu Y, et al. Loratadine inhibits Staphylococcus aureus virulence and biofilm formation. iScience. 2022;25(2):103731. doi:10.1016/j.isci.2022.103731
33. Mi S, Tang Y, Dari G, et al. Transcriptome sequencing analysis for the identification of stable lncRNAs associated with bovine Staphylococcus aureus mastitis. J Anim Sci Biotechnol. 2021;12(1):120. doi:10.1186/s40104-021-00639-2
34. Slany M, Oppelt J, Cincarova L, Schaffner DW. Formation of Staphylococcus aureus Biofilm in the presence of sublethal concentrations of disinfectants studied via a transcriptomic analysis using transcriptome sequencing (RNA-seq). Appl Environ Microbiol. 2017;83(24):24. doi:10.1128/AEM.01643-17
35. Nygaard TK, Pallister KB, DuMont AL, et al. Alpha-toxin induces programmed cell death of human T cells, B cells, and monocytes during USA300 infection. PLoS One. 2012;7(5):e36532. doi:10.1371/journal.pone.0036532
36. Shallcross LJ, Williams K, Hopkins S, Aldridge RW, Johnson AM, Hayward AC. Panton-Valentine leukocidin associated staphylococcal disease: a cross-sectional study at a London hospital, England. Clin Microbiol Infect. 2010;16(11):1644–1648. doi:10.1111/j.1469-0691.2010.03153.x
37. Seilie ES, Bubeck Wardenburg J. Staphylococcus aureus pore-forming toxins: the interface of pathogen and host complexity. Semin Cell Dev Biol. 2017;72:101–116. doi:10.1016/j.semcdb.2017.04.003
38. Bhakdi S, Tranum-Jensen J. Alpha-toxin of Staphylococcus aureus. Microbiol Rev. 1991;55(4):733–751. doi:10.1128/mr.55.4.733-751.1991
39. Liu Q, Yeo WS, Bae T. The SaeRS two-component system of Staphylococcus aureus. Genes. 2016;7:10. doi:10.3390/genes7100081
40. Cheung AL, Ying P. Regulation of alpha- and beta-hemolysins by the sar locus of Staphylococcus aureus. J Bacteriol. 1994;176(3):580–585. doi:10.1128/jb.176.3.580-585.1994
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.