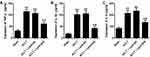Back to Journals » Journal of Inflammation Research » Volume 14
Nrf2 Regulates CHI3L1 to Suppress Inflammation and Improve Post-Traumatic Osteoarthritis
Authors Song Y, Hao D, Jiang H, Huang M, Du Q, Lin Y, Liu F, Chen B
Received 12 March 2021
Accepted for publication 18 August 2021
Published 24 August 2021 Volume 2021:14 Pages 4079—4088
DOI https://doi.org/10.2147/JIR.S310831
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 4
Editor who approved publication: Professor Ning Quan
Yang Song,1,2 Dake Hao,3 Huan Jiang,4 Mingguang Huang,2 Qingjun Du,2 Yi Lin,2 Fei Liu,1 Bin Chen1
1Division of Orthopaedics and Traumatology, Department of Orthopaedics, Nanfang Hospital, Southern Medical University, Guangzhou, 510515, People’s Republic of China; 2Division of Traumatology and Joint, Department of Orthopaedics, Shunde Hospital, Southern Medical University, Foshan, 528308, People’s Republic of China; 3Department of Surgery, School of Medicine, University of California Davis, Sacramento, CA, 95817, USA; 4Department of Anesthesiology, Shunde Hospital, Southern Medical University, Foshan, 528308, People’s Republic of China
Correspondence: Bin Chen
Division of Orthopaedics and Traumatology, Department of Orthopaedics, Nanfang Hospital, Southern Medical University, Guangzhou, 510515, People’s Republic of China
Email [email protected]
Introduction: Post-traumatic osteoarthritis (PTOA) is an inflammatory condition that occurs following mechanical joint trauma and that results in joint degeneration. This study sought to evaluate the regulatory function of nuclear factor erythroid 2-related factor 2 (Nrf2) in a murine model of anterior cruciate ligament transection (ACLT)-induced PTOA and in an in vitro model of synoviocyte inflammation induced by LPS treatment with the goal of exploring the role of chitinase 3-like-1 (CHI3L1) in this pathogenic context.
Methods: PTOA model mice were intra-articularly injected with Nrf2 overexpression lentiviral vector, and safranin O-fast green staining as well as the Osteoarthritis Research Society International (OARSI) Scoring System were used to evaluate the severity of cartilage damage. Protein expression in the synovial tissue was evaluated by Western blotting, immunohistochemical staining, and ELISA. Additionally, murine synoviocytes were infected with Nrf2 overexpression lentivirus and stimulated with LPS. The levels of inflammatory cytokines were detected by ELISA. ROS levels were measured using dihydroethidium (DHE) dye.
Results: We determined that the overexpression of Nrf2 was sufficient to reduce cartilage degradation in the context of PTOA in vivo, and we observed a significant decrease in the expression of matrix metalloproteinase 13 (MMP13) in the articular cartilage of samples from mice overexpressing Nrf2 relative to control mice. Synovial CHI3L1 expression and serum TNF-α, IL-1β, and IL-6 levels were reduced in animals overexpressing this transcription factor relative to PTOA model controls. Consistent with these findings, murine synoviocytes treated with LPS exhibited dose-dependent increases in ROS, TNF-α, IL-1β, IL-6, Nrf2, and CHI3L1 levels, whereas Nrf2 overexpression was sufficient to suppress these increases.
Conclusion: Our data indicated that Nrf2 negatively regulates CHI3L1, suggesting that this signaling axis may regulate PTOA progression and may thus be a viable therapeutic target in individuals affected by this condition.
Keywords: post-traumatic osteoarthritis, synovium, Nrf2, CHI3L1, oxidative stress, inflammation
Introduction
Post-traumatic osteoarthritis (PTOA) is a condition that arises as a consequence of trauma, resulting in pathological changes including articular cartilage ossification and degenerative chondrogenic hyperplasia, resulting in joint pain and dysfunction.1 While PTOA can affect individuals of any age, it is most common in young adults following traumatic injury or in the context of unbalanced or excessive load-bearing.2 As the ability of the articular cartilage to undergo self-renewal is very limited, treatment options for PTOA remain unsatisfactory.3
When reactive oxygen species (ROS) are produced at a rate faster than the rate at which the articular cartilage is able to clear them, this tissue is affected by oxidative stress. Oxidative stress levels are high in the cartilage in the context of PTOA, resulting in chronic inflammation,4 which can, in turn, drive synovial inflammation, intraarticular lesion formation, chondrocyte anabolic processed, and cartilage degeneration.5 Nuclear factor E2-related factor 2 (Nrf2) is a transcription factor that serves as a primary regulator of cellular antioxidant responses. Under normal conditions in the absence of ongoing oxidative stress, Nrf2 is sequestered in the cytoplasm in an inactive state by Kelch-like ECH-associated protein 1 (Keap1). When oxidative stress occurs, however, Nrf2 dissociates from Keap1, becomes activated, and translocates to the nucleus wherein it is able to regulate antioxidant-related gene expression to protect cells against this deleterious form of stress.6 Osteoarthritis patients exhibit high levels of Nrf2 expression in the synovial tissues and cartilage,7,8 and the Nrf2/HO-1 signaling pathway has been shown to protect chondrocytes against hydrogen peroxide-induced oxidative damage and consequent apoptotic cell death.9 ROS generation and inflammation are closely linked processes that regulate one another, and which represent promising targets for PTOA treatment.10 Yan et al11 found that Nrf2 activation was sufficient to alleviate osteoarthritis via the inhibition of inflammasome activation.
Chitinase 3-like 1 (CHI3L1, also known as YKL-40) is a proinflammatory glycoprotein biomarker that has been linked to tissue remodeling and bone resorption.12 CHI3L1 is secreted by macrophages, synoviocytes, and chondrocytes,13–16 and can be detected at increased levels in the synovial fluid and serum of patients with inflammatory conditions, in addition to correlating with the extent of joint degradation in rheumatoid arthritis (RA) patients.17 Inhibiting CHI3L1 has previously been shown to suppress inflammation and cortical bone degeneration in a murine osteomyelitis model system,18 while Kim et al19 similarly determined that knocking down this glycoprotein protected against hyperoxia-induced apoptotic death in human airway epithelial cells, whereas CHI3L1 overexpression increased the susceptibility of these cells to such apoptosis. As such, these prior data highlight CHI3L1 as an important regulator of inflammatory and oxidative stress responses. Its association with Nrf2, however, has yet to be defined.
Herein, we explored the relationship between Nrf2 and CHI3L1 by comparing the expression of these genes in synovial samples from PTOA model mice. We then explored the mechanistic basis for this relationship by conducting Nrf2 knockdown experiments both in vitro and in vivo.
Materials and Methods
Animals
C57BL/6 male mice (8-weeks-old) from Jiesijie Experimental Animal Co., Ltd (Shanghai, China) were housed in a climate-controlled facility (23±1°C; 50‑60% relative humidity; 12 h light/dark cycles). The Ethics Committee of Shunde Hospital approved these studies. This animal experiments was performed according to the guideline of Animal Care and Use Committee of Shunde Hospital and following internationally approved laboratory animal care principles published by the National Institute of Health.
Nrf2 Overexpression
The Nrf2 coding sequence (GI: 927028865) was synthesized, and both control and Nrf2 overexpression lentiviral vectors were prepared by Hanbio Biotechnology Co., LTD (Shanghai, China) at 3×108 transducing units (TU)/mL.
PTOA Model Animal Treatment
Mice were randomized into the following four groups (n=5/group): a sham group, an ACLT group, an ACLT+Lenti-NC group, and an ACLT+Lenti-Nrf2 group. All ACLT model animals were anesthetized with 3% sodium pentobarbital (i.p.; Sigma‑Aldrich, MO, USA). ACLT of the right knee was then conducted to cause joint instability, thereby inducing PTOA. In sham control mice, a skin and capsule incision was instead conducted. Animals in the lentiviral treatment groups were intra-articularly injected with 10 μL of the appropriate negative control (Lenti-NC) or Nrf2 overexpression (Lenti-Nrf2) vectors on days 0, 15, 30, and 45 after ACTL surgery, as reported previously.20 At eight weeks post-surgery, all mice were euthanized, and knee joints were collected for histological assessment.
Histochemical and Immunohistochemical Staining
Samples of paraffin-embedded tissue were cut to yield 4-µm thick sagittal sections which were then subjected to Hematoxylin and Eosin (HE), Masson’s trichrome and Safranin O-Fast Green staining. Immunohistochemical staining for matrix metalloproteinase (MMP)-13 and alpha-smooth muscle actin (α-SMA) were conducted by deparaffinizing tissue sections, rehydrating them with an ethanol gradient, and conducting antigen retrieval by processing samples for 30 min with citrate buffer. Samples were then washed with PBS, treated with 3% H2O2 to quench endogenous peroxidase activity, blocked for 1 h in 10% sheep serum at 37°C, and treated overnight with anti-MMP13 (1/500, Abcam, USA) or anti-α-SMA (1/200, Abcam) at 4°C. Sections were then probed with an appropriate HRP-linked secondary antibody (Abcam) for 45 min, washed with PBS, stained with the DAB substrate solution, and counterstained for 30 s with hematoxylin. A light microscope (Olympus Optical, Tokyo, Japan) was then used to image sections, which were evaluated using ImageJ. Two investigators blinded to experimental conditions calculated the percentage of staining positive area in six randomly selected fields of view for each articular cartilage section at a magnification of ×400.
Mouse OARSI Scoring
In order to quantify the degree of cartilage degeneration, mouse cartilage degeneration was graded for Safranin O/Fast Green-stained knee sections using the Osteoarthritis Research Society International (OARSI) scoring system. A subjective score of 0–6 was applied as previously described.21 Grading was performed by three blinded observers. The three grades for each section were then averaged, and the data for mice in each group were collated. Higher scores were indicative of more serious cartilage damage.
Immunofluorescent Staining
Tissue sections were blocked for 1 h using 5% goat serum at room temperature, and were then incubated overnight with anti-Nrf2 (1/500, Abcam) or anti-CHI3L1 (1/50, Santa Cruz, USA). Sections were then washed thrice with PBS and stained for 1 h with secondary goat anti-mouse AF488 (1/200, Invitrogen, USA) and rhodamine-conjugated goat anti-rabbit IgG (1/100, Abcam). Nuclei were then stained with DAPI, and cells were imaged via laser-scanning confocal microscopy (Olympus, Tokyo, Japan).
Synovial Fibroblast Culture
Minced synovial tissue from C57BL/6 mice was used to prepare primary murine synovial fibroblasts as in prior reports.22 Briefly, these issues were digested for 3 h in serum-free DMEM containing 1 mg/mL of collagenase (Roche, Meylan, France) at 37°C. Cells were then washed twice with PBS prior to being cultured in DMEM containing 10% fetal calf serum (Life Technologies), 50 mg/mL L-glutamine, and penicillin/streptomycin in a humidified 5% CO2 incubator. Synoviocytes used in the present study were obtained between passages 3 and 9, were positive for Vimentin immunofluorescent staining (anti-vimentin, 1:100, Abcam), and exhibited typical synovial fibroblast morphology. Lentiviral transduction was conducted by combining these cells (1×105) with Lenti-Nrf2 or Lenti-NC at a multiplicity of infection (MOI) of 100 for 48 h in DMEM. Changes in Nrf2 protein expression were then evaluated via Western blotting.
Intracellular ROS Assessment
Murine synovial fibroblasts were treated for 24 h with a range of LPS concentrations, after which the dihydroethidium (DHE, Santa Cruz) dye was used to evaluate ROS production. Briefly, cells grown on glass coverslips were incubated in HBSS (Gibco) containing CaCl2, MgCl2, and DHE (10 μmol/L) for 30 min at 37 °C in the dark. Flow cytometry was then used to evaluate ROS production based on median fluorescence intensity values for 10,000 cells.
ELISAs
On day 7 post-surgery, samples of blood were obtained from experimental animals. Blood was spun for 15 min at 3000 rpm at 4°C to yield serum. Levels of TNF-α (#MTA00B), IL-1β (#MLB00C), and IL-6 (#M6000B) in serum and in samples of supernatants from cultured synovial fibroblasts were measured with commercial ELISA kits (R&D Systems, MN, USA) based on provided directions. The absorbance of each standard and sample was measured at 450 nm. A standard concentration gradient was used as a standard curve.
Western Blotting
RIPA buffer supplemented with protease inhibitor cocktail (Pierce, IL, USA) was used to lyse cells, after which nuclear and cytoplasmic proteins were isolated with a commercially available nuclear and cytoplasmic extraction kit (Thermo Fisher Inc., IL, USA) based on provided instructions. A BCA assay (Beyotime Biotechnology, China) was then used to measure protein levels, and equal amounts of protein (40μg) per sample were separated via 10% SDS-PAGE and transferred onto PVDF membranes (Millipore, Germany) that were then blocked using 5% non-fat milk in TBST for 1 h. Next, blots were probed overnight with anti-Nrf2 (1:1000, Abcam), anti-CHI3L1 (1:200, Santa Cruz), anti-LaminB (1:1000, Sigma), anti-α-tubulin (1:1000, Santa Cruz), or anti-GAPDH (1:1000, Santa Cruz) at 4°C. Blots were then washed using TBST and probed with an appropriate secondary antibody for 2 h at room temperature, after with the ECL Plus reagent (Amersham Biosciences, Little Chalfont, UK) and autoradiographic film were used to detect protein bands. A gel image analysis system (BioRad, CA, USA) was used for densitometric analyses of developed blots.
Statistical Analysis
Data are means ± SD, and were compared through Student’s t-tests or one-way ANOVAs with Tukey’s post hoc test, as appropriate. P < 0.05 was the significance threshold for this study.
Results
Overexpressing Nrf2 Prevents Articular Cartilage Degeneration in a Murine Model of PTOA
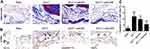
Western blotting was performed to determine the expression of Nrf2 in knee joints of mice. Compared with that in the sham group, the expression of Nrf2 in ACLT group was increased. Notably, the expression of Nrf2 in the Nrf2 overexpression (ACLT+Lenti-Nrf2) group was increased significantly compared with that in the ACLT group (Figure 1A). Safranin O/fast green staining was used to evaluate articular cartilage structures in our experimental mice. In sham control animals, the articular cartilage was intact with a smooth surface and uniform organization (Figure 1B). In contrast, animals in the ACLT and ACLT+Lenti-NC groups exhibited a reduction in safranin O staining consistent with a loss of articular cartilage. However, ACLT+Lenti-Nrf2 treatment significantly decreased the severity of articular cartilage degeneration in these PTOA model animals. The ACLT+Lenti-Nrf2 group also exhibited significantly lower cartilage OARSI grade scores relative to the ACLT and ACLT+Lenti-NC groups (Figure 1C). We then conducted immunohistochemical staining for MMP13 in synovial tissue samples from these mice (Figure 1D), revealing significant increases in MMP13-positive cartilage area in ACLT and ACLT+Lenti-NC group animals relative to sham controls, whereas this area was significantly decreased in animals in the ACLT+Lenti-Nrf2 group relative to those in the ACLT model group (Figure 1E).
Overexpressing Nrf2 Prevents Synovial Fibrosis and Inflammation in PTOA Model Mice
Masson’s trichrome staining was next conducted to assess synovial fibrosis in these mice at 8 weeks post-surgery. This analysis revealed that Nrf2 overexpression markedly suppressed ACLT-induced increases in the fibrotic area (Figure 2A). Immunohistochemical staining also confirmed that the expression of the fibrosis marker α-SMA was significantly increased in the ACLT group in comparison with the Sham group, while overexpression of Nrf2 in the ACLT+Lenti-Nrf2 group appeared to reduce their expression against the ACLT group (Figure 2B and C). HE staining displayed pathological changes among groups, and immunofluorescent staining of synovial tissues revealed that ACLT surgery induced Nrf2 translocation to the nucleus, and this localization was increased in mice injected with Lenti-Nrf2. We also observed significantly higher CHI3L1 levels in PTOA model mice, and found that this protein colocalized with Nrf2 in synovial tissue samples (Figure 3). We additionally collected serum samples from experimental mice on day 7 post-surgery and measured TNF-α, IL-1β, and IL-6 levels therein. This analysis revealed that ACLT surgery increased the circulating levels of all three of these cytokines, whereas Lenti-Nrf2 administration markedly suppressed their expression relative to ACLT model control mice (Figure 4A–C), suggesting that Nrf2 can inhibit inflammatory mediator expression.
LPS Treatment of Murine Synoviocytes Induces Inflammation and ROS Production
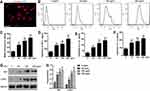
Figure 5A displays the immunofluorescence-based identification of synoviocytes. We treated murine synoviocytes with LPS (0, 50, 100, or 200 ng/mL) for 24 h. DHE staining of these cells then revealed that ROS levels were significantly increased in a dose-dependent fashion upon LPS treatment (Figure 5B and C), as were TNF-α, IL-1β, and IL-6 levels (Figure 5D–F). We then evaluated Nrf2 and CHI3L1 protein levels in these synoviocytes via Western blotting, revealing both to be upregulated by LPS in a dose-dependent fashion (Figure 5G and H). In particular, extremely significant increases in all the above indicators were observed in cells treated with LPS concentrations ≥100 ng/mL, as compared to cells not stimulated with LPS. As such, 100 ng/mL of LPS was selected as the concentration used in subsequent experiments.
Overexpressing Nrf2 Suppresses LPS-Induced Synoviocyte CHI3L1 Protein Expression, Inflammation, and ROS Production
Lastly, we explored the functional role of Nrf2 in murine synoviocytes by transducing these cells for 48 h with Lenti-NC or Lenti-Nrf2 and then treating them for an additional 24 h with LPS (100 ng/mL). Western blotting revealed that nuclear Nrf2 levels were significantly higher in Nrf2 overexpressing cells relative to those transduced with Lenti-NC, whereas CHI3L1 levels were significantly reduced upon Lenti-Nrf2 infection (Figure 6A and B). Following LPS treatment, ROS levels in these cells were assessed via DHE staining (Figure 6C), revealing significant reductions in ROS levels in cells overexpressing Nrf2 (Figure 6D). We additionally evaluated inflammatory cytokine levels in supernatants collected from these cells following LPS treatment, revealing that TNF-α (Figure 6E), IL-1β (Figure 6F), and IL-6 (Figure 6G) levels were all significantly decreased by Nrf2 overexpression compared to Lenti-NC transduction.
Discussion
PTOA is a form of osteoarthritis (OA), a chronic disease that can cause synovial joint pain and even disability in severe cases.23 The knee is the most common site of traumatic synovitis in the body.24 Herein, we utilized a murine model of PTOA via conducting ACLT of the right knee joint. At 8 weeks post-ACLT, articular cartilage degeneration was clearly evident in the operated knee of these mice. MMP13 is a major driver of cartilage degradation,25 and we found that the ACLT procedure was associated with a significant increase in MMP13 expression in the articular cartilage of these mice relative to sham-operated controls. We also found that serum TNF-α, IL-1β, and IL-6 levels were higher in ACLT model mice. Together these data are consistent with clinical signs of osteoarthritis such as synovitis and cartilage degeneration.1,2,23
The synovium plays a key role in maintaining joint homeostasis, lubricating the joint and facilitating the flow of synovial fluid therein.26 Herein, we observed clear signs of synovial fibrosis in ACLT model mice, consistent with the impairment of synovial function. Coleman et al27 determined that intra-articular N-acetylcysteine (NAC) injection can effectively prevent PTOA via suppressing oxidative stress in model experimental systems. As a key regulator of oxidative stress responses, Nrf2 is primarily localized to the cytoplasm under normal physiological conditions, but rapidly localizes to the nucleus in response to oxidative stress whereupon it can promote the upregulation of antioxidant stress response protein.6,9 Su et al28 showed that the activation of p62/Nrf2 signaling was sufficient to inhibit ROS production, thus alleviating bone erosion in rheumatoid arthritis. Moreover, Lal et al29 confirmed that Nrf2/HO-1 signaling pathway activation improved arthritis in rats. Our data indicated that Nrf2 activation occurs in the synovial tissue of ACLT model mice, and this activity was enhanced by Nrf2 overexpression. In addition, Nrf2 overexpression inhibited fibrosis, cartilage degeneration, and systemic inflammation in these ACLT model mice, indicating that activation of Nrf2 is beneficial to the development of PTAO. In our study, CHI3L1 expression was also increased in the synovium of mice following ACLT, whereas Nrf2 overexpression suppressed this CHI3L1 upregulation. CHI3L1 has been shown to be a proinflammatory biomarker that is reflective of the extent of synovial inflammation and cartilage degradation.17,30 These data indicate that Nrf2 can downregulate CHI3L1 and thereby suppress inflammation in the context of PTOA.
ROS have been identified as key regulators of inflammation, controlling oxidative stress and antioxidant enzyme activity and thereby promoting an increase in inflammatory activity.31 We found that ROS and inflammatory cytokine levels were increased in a dose-dependent manner in murine synoviocytes treated with LPS, and we found Nrf2 and CHI3L1 expression to be similarly increased. We then treated Nrf2-overexpressing synovial fibroblasts with LPS in order to explore the association between CHI3L1 and Nrf2 in this pathogenic context. We found that CHI3L1 expression was significantly reduced in cells overexpressing this transcription factor relative to cells transduced with a control lentivirus. ROS can trigger Nrf2 activation, which can suppress oxidative stress and thereby inhibit inflammation.32 Our data indicate that Nrf2 overexpression may suppress CHI3L1 expression and ROS production, thereby inhibiting inflammatory responses.
Conclusion
Together, our data indicate that Nrf2 and CHI3L1 are expressed at high levels in the murine PTOA model system. Importantly, we determined that Nrf2 overexpression downregulates CHI3L1 expression and suppresses inflammation in vivo and in vitro. Nrf2 may therefore be a viable target for therapeutic efforts aimed at treating or preventing PTOA through mechanisms linked to the regulation of CHI3L1. However, one noteworthy limitation of this study is that the mechanisms and pathways whereby Nrf2 affects the cartilage and synoviocytes were not analyzed, and future research will be required to validate and expand upon the findings of this study in order to fully clarify the mechanisms and pathways underlying this Nrf2-mediated activity and the relationship between Nrf2 and CHI3L1 in the synovium and articular cartilage in the context of PTOA.
Funding
The authors received no funding for this work.
Disclosure
The authors declare no conflicts of interest.
References
1. Whittaker JL, Roos EM. Infographic. Risk profile for sport-related post-traumatic knee osteoarthritis. Br J Sports Med. 2020;54(6):362–363.
2. Chery DR, Han B, Li Q, et al. Early changes in cartilage pericellular matrix micromechanobiology portend the onset of post-traumatic osteoarthritis. Acta biomaterialia. 2020;111:267–278. doi:10.1016/j.actbio.2020.05.005
3. Dai C, Kuang Z. An invited commentary on: lingering risk: a meta-analysis of outcomes following primary total knee arthroplasty for patients with post-traumatic arthritis. Int j Surgery. 2020;78:103. doi:10.1016/j.ijsu.2020.04.036
4. Liang R, Zhao J, Li B, et al. Implantable and degradable antioxidant poly(epsilon-caprolactone)-lignin nanofiber membrane for effective osteoarthritis treatment. Biomaterials. 2020;230:119601. doi:10.1016/j.biomaterials.2019.119601
5. Leimer EM, Tanenbaum LM, Nettles DL, et al. Amino Acid profile of synovial fluid following intra-articular ankle fracture. Foot Ankle Int. 2018;39(10):1169–1177. doi:10.1177/1071100718786163
6. Dodson M, de la Vega MR, Cholanians AB, Schmidlin CJ, Chapman E, Zhang DD. Modulating NRF2 in Disease: timing Is Everything. Annu Rev Pharmacol Toxicol. 2019;59:555–575. doi:10.1146/annurev-pharmtox-010818-021856
7. Khan NM, Ahmad I, Haqqi TM. Nrf2/ARE pathway attenuates oxidative and apoptotic response in human osteoarthritis chondrocytes by activating ERK1/2/ELK1-P70S6K-P90RSK signaling axis. Free Radic Biol Med. 2018;116:159–171. doi:10.1016/j.freeradbiomed.2018.01.013
8. Chen Z, Zhong H, Wei J, et al. Inhibition of Nrf2/HO-1 signaling leads to increased activation of the NLRP3 inflammasome in osteoarthritis. Arthritis Res Ther. 2019;21(1):300. doi:10.1186/s13075-019-2085-6
9. Kim EN, Lee HS, Jeong GS. Cudratricusxanthone O Inhibits H2O2-Induced Cell Damage by Activating Nrf2/HO-1 Pathway in Human Chondrocytes. Antioxidants. 2020;9(9):788. doi:10.3390/antiox9090788
10. Ansari MY, Ahmad N, Haqqi TM. Oxidative stress and inflammation in osteoarthritis pathogenesis: role of polyphenols. Biomed Pharmacother. 2020;129:110452. doi:10.1016/j.biopha.2020.110452
11. Yan Z, Qi W, Zhan J, et al. Activating Nrf2 signalling alleviates osteoarthritis development by inhibiting inflammasome activation. J Cell Mol Med. 2020;24(22):13046–13057. doi:10.1111/jcmm.15905
12. Steinke J, Samietz S, Friedrich N, et al. Associations of plasma YKL-40 concentrations with heel ultrasound parameters and bone turnover markers in the general adult population. Bone. 2020;141:115675. doi:10.1016/j.bone.2020.115675
13. Kim EG, Kim MN, Hong JY, et al. Chitinase 3-Like 1 Contributes to Food Allergy via M2 Macrophage Polarization. Allergy Asthma Immunol Res. 2020;12(6):1012–1028. doi:10.4168/aair.2020.12.6.1012
14. Holst CB, Christensen IJ, Skjoth-Rasmussen J, Hamerlik P, Poulsen HS, Johansen JS. Systemic Immune Modulation in Gliomas: prognostic Value of Plasma IL-6, YKL-40, and Genetic Variation in YKL-40. Front Oncol. 2020;10:478. doi:10.3389/fonc.2020.00478
15. Wang R, Xu C, Zhong H, et al. Inflammatory-sensitive CHI3L1 protects nucleus pulposus via AKT3 signaling during intervertebral disc degeneration. FASEB J. 2020;34(3):3554–3569. doi:10.1096/fj.201902096R
16. Kazakova MH, Batalov AZ, Mateva NG, Kolarov ZG, Sarafian VS. YKL-40 and cytokines - A new diagnostic constellation in rheumatoid arthritis? Folia Med (Plovdiv). 2017;59(1):37–42. doi:10.1515/folmed-2017-0013
17. Karalilova R, Kazakova M, Batalov A, Sarafian V. Correlation between protein YKL-40 and ultrasonographic findings in active knee osteoarthritis. Med Ultrason. 2018;1(1):57–63. doi:10.11152/mu-1247
18. Chen X, Jiao J, He X, et al. CHI3L1 regulation of inflammation and the effects on osteogenesis in a Staphylococcus aureus-induced murine model of osteomyelitis. FEBS J. 2017;284(11):1738–1747. doi:10.1111/febs.14082
19. Kim MN, Lee KE, Hong JY, et al. Involvement of the MAPK and PI3K pathways in chitinase 3-like 1-regulated hyperoxia-induced airway epithelial cell death. Biochem Biophys Res Commun. 2012;421(4):790–796. doi:10.1016/j.bbrc.2012.04.085
20. Zheng G, Zhan Y, Li X, et al. TFEB, a potential therapeutic target for osteoarthritis via autophagy regulation. Cell Death Dis. 2018;9(9):858. doi:10.1038/s41419-018-0909-y
21. Schott EM, Farnsworth CW, Grier A, et al. Targeting the gut microbiome to treat the osteoarthritis of obesity. JCI Insight. 2018;3(8). doi:10.1172/jci.insight.95997.
22. Gao B, Calhoun K, Fang D. The proinflammatory cytokines IL-1beta and TNF-alpha induce the expression of Synoviolin, an E3 ubiquitin ligase, in mouse synovial fibroblasts via the Erk1/2-ETS1 pathway. Arthritis Res Ther. 2006;8(6):R172. doi:10.1186/ar2081
23. Lai-Zhao Y, Pitchers KK, Appleton CT. Transient anabolic effects of synovium in early post-traumatic osteoarthritis: a novel ex vivo joint tissue co-culture system for investigating synovium-chondrocyte interactions. Osteoarthritis Cartilage OARS. 2021;29(7):1060–1070. doi:10.1016/j.joca.2021.03.010
24. Bailey KN, Furman BD, Zeitlin J, et al. Intra-articular depletion of macrophages increases acute synovitis and alters macrophage polarity in the injured mouse knee. Osteoarthritis Cartilage OARS. 2020;28(5):626–638. doi:10.1016/j.joca.2020.01.015
25. Takahashi A, de Andres MC, Hashimoto K, et al. DNA methylation of the RUNX2 P1 promoter mediates MMP13 transcription in chondrocytes. Sci Rep. 2017;7(1):7771. doi:10.1038/s41598-017-08418-8
26. Bao J, Yan W, Xu K, et al. Oleanolic Acid Decreases IL-1beta-Induced Activation of Fibroblast-Like Synoviocytes via the SIRT3-NF-kappaB Axis in Osteoarthritis. Oxid Med Cell Longev. 2020;2020:7517219. doi:10.1155/2020/7517219
27. Coleman MC, Goetz JE, Brouillette MJ, et al. Targeting mitochondrial responses to intra-articular fracture to prevent posttraumatic osteoarthritis. Sci Transl Med. 2018;10(427):eaan5372. doi:10.1126/scitranslmed.aan5372
28. Su X, Guo W, Yuan B, et al. Artesunate attenuates bone erosion in rheumatoid arthritis by suppressing reactive oxygen species via activating p62/Nrf2 signaling. Biomed Pharmacother. 2021;137:111382. doi:10.1016/j.biopha.2021.111382
29. Lal R, Dhaliwal J, Dhaliwal N, Dharavath RN, Chopra K. Activation of the Nrf2/HO-1 signaling pathway by dimethyl fumarate ameliorates complete Freund’s adjuvant-induced arthritis in rats. Eur J Pharmacol. 2021;899:174044. doi:10.1016/j.ejphar.2021.174044
30. Dundar U, Asik G, Ulasli AM, et al. Assessment of pulsed electromagnetic field therapy with Serum YKL-40 and ultrasonography in patients with knee osteoarthritis. Int J Rheum Dis. 2016;19(3):287–293. doi:10.1111/1756-185X.12565
31. Kim GY, Jeong H, Yoon HY, et al. Anti-inflammatory mechanisms of suppressors of cytokine signaling target ROS via NRF-2/thioredoxin induction and inflammasome activation in macrophages. Bmb Rep. 2020;53(12):640–645. doi:10.5483/BMBRep.2020.53.12.161
32. Kahroba H, Ramezani B, Maadi H, Sadeghi MR, Jaberie H, Ramezani F. The role of Nrf2 in Neural stem/Progenitors cells: from maintaining stemness and self-renewal to promoting differentiation capability and facilitating therapeutic application in neurodegenerative disease. Ageing Res Rev. 2020;2:101211.
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.