Back to Journals » International Journal of Chronic Obstructive Pulmonary Disease » Volume 14
Novel Respiratory Impedance-Based Phenotypes Reflect Different Pathophysiologies in Chronic Obstructive Pulmonary Disease Patients
Authors Matsuo Y , Ogawa E, Seto-Yukimura R , Ryujin Y, Kinose D, Yamaguchi M, Osawa M, Nagao T, Kurosawa H, Nakano Y
Received 31 July 2019
Accepted for publication 11 November 2019
Published 20 December 2019 Volume 2019:14 Pages 2971—2977
DOI https://doi.org/10.2147/COPD.S224902
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Richard Russell
Yumiko Matsuo,1 Emiko Ogawa,1,2 Ruriko Seto-Yukimura,1 Yasushi Ryujin,1,3 Daisuke Kinose,1 Masafumi Yamaguchi,1 Makoto Osawa,1 Taishi Nagao,1 Hajime Kurosawa,4 Yasutaka Nakano1
1Division of Respiratory Medicine, Department of Internal Medicine, Shiga University of Medical Science, Shiga, Japan; 2Health Administration Center, Shiga University of Medical Science, Shiga, Japan; 3Ryujin Medical Clinic, Osaka, Japan; 4Center for Environmental Conservation and Research Safety, Department of Occupation Health, Tohoku University, Miyagi, Japan
Correspondence: Emiko Ogawa
Health Administration Center, Shiga University of Medical Science, Seta Tsukinowa-Cho, Otsu, Shiga 520-2192, Japan
Tel/Fax +81-77-548-2212
Email [email protected]
Purpose: The forced oscillation technique (FOT) is a non-invasive method to measure respiratory impedance, the respiratory resistance (Rrs) and reactance (Xrs). The disease probability measure (DPM) is a useful computed tomography (CT) imaging variable for the assessment of gas trapping and emphysema in patients with chronic obstructive pulmonary disease (COPD) using pairs of inspiratory and expiratory CT images. We aimed to develop FOT-based phenotypes and determine whether the phenotypes and their imaging characteristics could facilitate the understanding of COPD pathophysiology.
Patients and methods: FOT and spirometry were examined in 164 COPD patients and 22 non-COPD smokers. COPD patients were divided into four FOT-based phenotypes (NL, normal group; RD, resistance-dominant group; XD, reactance-dominant group; and MIX, mixed group) based on the 3rd quartile values of R5 (Rrs at 5Hz) and X5 (Xrs at 5Hz) in the non-COPD group. The emphysematous lesions and the airway lesions were quantitatively assessed in CT images by low attenuation volume and the square root of the wall area of a hypothetical airway with an internal perimeter of 10 mm (√Aaw at Pi10), respectively. DPM imaging analysis was also performed in 131 COPD patients. We investigated the differences in COPD parameters between the FOT-based phenotypes.
Results: √Aaw at Pi10 were significantly higher in the RD, XD, and MIX groups than in the NL group. The XD group showed lower pulmonary function and higher dyspnea scores than the RD group. No significant changes in DPM values were observed between the RD and the NL groups. The gas-trapping area was significantly higher in the XD group than in the NL group. The MIX group showed the highest dyspnea score, most emphysematous lesions, and the lowest forced expiratory volume in 1 s % predicted value.
Conclusion: The FOT-based phenotyping may be useful to assess pathophysiological changes of COPD with CT assessments.
Keywords: oscillometry, emphysema, exhalation, quantitative evaluation, COPD
Introduction
Chronic obstructive pulmonary disease (COPD) is a chronic progressive disease with heterogeneous pathophysiology including small airways disease and parenchymal destruction.1,2 Computed tomography (CT) is useful for assessing structural abnormalities of airways and lung parenchyma depending on COPD severity.1–3
Recently, Galbán et al4 established a new voxel-wise image analysis method called parametric response mapping (PRM) to quantitatively distinguish functional small airway disease with air-trapping from emphysema using digitally co-registered inspiration and expiration CT scans. PRM is reported as a useful imaging biomarker for monitoring COPD progression and differentiating COPD phenotypes.5–7 Additionally, the disease probability measure (DPM) is a registered voxel classification approach using inspiratory and expiratory CT scans to quantify gas trapping in functional small airways disease.8 DPM as well as PRM exhibit associations with pulmonary function measurements and scores in a COPD dyspnea scale.8 Although imaging assessment is useful, image analysis takes time and effort.
The forced oscillation technique (FOT), which is used in COPD, is a noninvasive and simple functional assessment method of the airways that measures the respiratory system impedance, consisting of the respiratory system resistance (Rrs) and reactance (Xrs).9,10 Rrs is a measure of the airway caliber, whereas Xrs reflects the abnormality of parenchyma and airways.9 Rrs and Xrs have been independently investigated to ascertain their relationship to other parameters of pulmonary function test and CT scans.9,11 However, the relationship between FOT indices and CT imaging parameters remains controversial.11,12 Clearly, Rrs and Xrs were not just substitutes for existing COPD parameters.
We hypothesized that the combined assessment of Rrs and Xrs may help elucidate COPD pathophysiology from a new viewpoint, different from spirometry. In this study, we aimed to develop FOT phenotyping based on R5 (Rrs at 5 Hz) and X5 (Xrs at 5 Hz) for COPD patients and determine whether the phenotypes and their imaging characteristics could facilitate the understanding of COPD pathophysiology, as FOT-based phenotyping may emphasize individually different pathophysiological heterogeneity.
Materials and Methods
Subjects and Study Design
A total of 164 COPD patients and 22 non-COPD smokers who visited the respiratory medicine outpatient clinic of the Shiga University of Medical Science (SUMS) Hospital between August 2013 and November 2016 were enrolled in the SUMS COPD cohort. The non-COPD smokers in this study visited our respiratory medicine outpatient clinic with symptoms such as cough or were referred from general practitioners in order to rule out the presence of COPD due to their history of smoking. The specialists diagnosed the non-COPD smokers using a post-bronchodilator ratio of forced expiratory volume in 1 s (FEV1)/forced vital capacity (FVC) ≥ 0.70 according to the GOLD recommendations.13 COPD was diagnosed using a post-bronchodilator ratio of FEV1/FVC < 0.70 according to the GOLD recommendations.13,14 The eligibility criteria were 1) smoking history of more than 10 pack-years or long-term biomass exposure, 2) no COPD exacerbation in the previous 4 weeks, and 3) apart from asthma no uncontrolled comorbidities, such as severe heart failure, malignant diseases, or other chronic lung diseases. Treatment difference among the study population was based on clinical judgment.
The scores in the COPD assessment test (CAT)15 and the modified Medical Research Council (mMRC) dyspnea scale16 were recorded for all subjects. Pulmonary function tests, FOT, and paired inspiratory and expiratory chest CT scans were performed in each participant on the same day. This study was approved by the Ethics Committee of SUMS (registration number: 27-11) and conformed to the tenets of the Declaration of Helsinki, and all patients provided written informed consent prior to their participation.
Pulmonary Function Tests
Spirometry was performed after inhalation of 20 µg of procaterol using a FUDAC77® spirometer (Fukuda Denshi, Tokyo, Japan) according to the American Thoracic Society/European Respiratory Society guidelines.17 Carbon monoxide diffusing capacity was measured by the single-breath washout technique. The predicted values for the spirometry were calculated in accordance with the guidelines of the Japanese Respiratory Society.18
Forced Oscillation Technique
FOT indices were measured after inhalation of 20 µg of procaterol using a MostGraph-01® (Chest M.I., Inc., Tokyo, Japan) at baseline and at follow-up after 2 years.19 Briefly, impulse wave signals, which contain multi-frequency spectra (4 Hz to 36 Hz) generated by loudspeakers at intervals of 0.25 seconds, were applied to the subject’s respiratory system via a mouthpiece. The mouth pressure and flow signals were recorded during the patient’s tidal breathing at rest. Patients’ cheeks were supported during measurement of respiratory impedance. The FOT indices at each oscillatory frequency (4–35 Hz), such as Rrs and Xrs at 5 or 20 Hz (R5, R20, and X5), the resonant frequency (Fres), and the low-frequency reactance (ALX), were calculated automatically using fast Fourier transformation methods. Each oscillatory index was expressed as the mean value of five entire respiratory cycles.
Novel FOT-Based Phenotypes
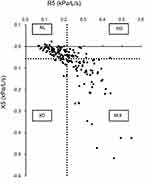
The 164 COPD patients were divided into four phenotypes (NL, normal group; RD, resistance-dominant group; XD, reactance-dominant group; and MIX, mixed group) based on the 3rd quartile values of R5 and X5 in the non-COPD group. With an R5 and X5 cut-off value of 0.214 and −0.057 kPa/L/s, respectively, the numbers of patients in the NL, RD, XD, and MIX groups were 69, 22, 12, and 61, respectively (Figure 1).
Inspiratory and Expiratory Chest CT
Volumetric non-contrast chest CT was performed in the supine position using a 320-detector row CT scanner, the Aquilion ONE® (Canon Medical Systems Corporation, Tochigi, Japan), with either full inspiration or full expiration after inhalation of 20 µg of procaterol. CT images were reconstructed with 1.0-mm slice thickness and 0.5-mm intervals using the FC03 algorithm.
The percentage of low attenuation volume (LAV%), one of the characteristic parameters of emphysematous lesions, were defined as voxels with CT attenuation less than −950 Hounsfield units. LAV% and the square root of the wall area of a hypothetical airway with an internal perimeter of 10 mm (√Aaw at Pi10),3 an index reflecting airway lesions, were quantitatively measured using the Apollo® software (VIDA, Coralville, IA, USA) as previously described.2,20
DPM
DPM is a quantitative voxel analysis based on the registration of paired inspiration and expiration CT scans as previously reported by Kirby et al.8 Briefly, DPM classifies each voxel as normal (DPMNormal), emphysematous (DPMEmph), or gas trapping, referred to as functional small airways disease (DPMGasTrap) by a combination of the probability of gas trapping and the probability of emphysema calculated by inspiratory-to-expiratory intensity differences. DPM analyses were performed by VIDA as previously described.8 Representative colored DPM map images of a non-COPD smoker and a COPD patient are shown in Supplementary Figure 1.
Statistical Analysis
Statistical analyses were performed using the JMP software ver.11 (SAS Institute, Cary, NC, USA). The differences between the COPD and non-COPD groups and comparisons between FOT-based phenotypes were evaluated using the Wilcoxon rank-sum test. A value of p < 0.05 was considered statistically significant.
Results
Characteristics of COPD Patients and Non-COPD Smokers
Table 1 shows the baseline characteristics of the study population. Overall, most participants were men, and most COPD patients had mild or moderate airflow limitation. The FOT indices are presented in Table 2. R5 and R20 were significantly higher in COPD patients than in non-COPD smokers. Although no significant differences in Xrs indices were detected between subjects with and without COPD, X5 tended to be lower while Fres and ALX tended to be higher in COPD patients.
 |
Table 1 Characteristics of the Study Subjects |
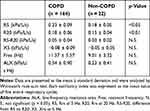 |
Table 2 Comparison of FOT Indices Between COPD Patients and Non-COPD Smokers |
Differences Among FOT-Based Phenotypes
Table 3 shows the comparisons among these FOT-based phenotypes. The XD and MIX groups were older and showed significantly higher scores in the mMRC dyspnea scale than the NL and RD groups. The FEV1% predicted values were significantly lower in the XD and MIX than in the NL and RD groups. The minimum of the LAV% values was determined in the RD group and was significantly different from that in the MIX group, which presented the highest value of all groups (p < 0.05; Table 3). Furthermore, √Aaw at Pi10 was significantly higher in the RD, XD, and MIX groups than in the NL group.
 |
Table 3 Characteristics, Pulmonary Functions, and CT Imaging Biomarkers Among FOT-Based Phenotypes |
DPM
The distribution of DPM parameters in the categories based on the defined FOT phenotypes is presented in Figure 2. No significant differences in DPM parameters were noted between the RD and NL groups. In the XD group, the percentage of DPMEmph did not significantly differ from that in the NL group, but the DPMGasTrap value was significantly increased. The values for DPMNormal and DPMGasTrap differed significantly between the MIX and the NL groups, and DPMEmph was significantly higher than that in the RD group.
Comparison of FOT-Based Phenotypes with GOLD-Based Severity of Airflow Limitation
Figure 3 shows the distribution of FOT-based phenotypes in relation to the severity of airflow limitation according to the GOLD classification. Although subjects in the RD, XD, and MIX groups were present in the GOLD 1 category, it was mostly composed of NL subjects. The percentages of study participants from the XD and MIX groups increased with increased severity of airflow limitation, and the GOLD 4 category consisted only of subjects from the MIX group. The RD group was mainly present in categories GOLD 1 and 2.
Discussion
We proposed a novel FOT-based phenotyping for combined assessment of Rrs and Xrs. The FOT-based phenotyping may emphasize individually on the different pathophysiological backgrounds with the same level of pulmonary function severity. The FOT-based phenotyping may be useful to assess pathophysiological changes of airway lesions and emphysema comprehensively.
In COPD, Rrs tends to increase, while Xrs tends to decrease with an increased degree of airflow obstruction.19 Di Mango et al reported that airway obstruction in the initial phase of COPD can be represented mainly by the resistive parameters, while the reactive parameters gain importance in more advanced stages.21 However, in this study, we revealed a different phenotype which X5 was predominantly decreased. The XD group shows lower pulmonary function and higher dyspnea scores than the RD group. In addition, the increase in the gas-trapping area concomitant with the decrease in normal area was observed in the XD group by imaging analysis. The XD group may be the previous stage of CT-based emphysema type.22–24 On the contrary, the RD group corresponds to CT-based airway dominant type by considering the values of √Aaw at Pi10 and LAV%. The features of the MIX group are in line with our previous observation that a combined airway and parenchymal phenotype in the assessment of CT images is associated with more severe airflow limitation and dyspnea.20 The FOT-based phenotyping is correlated with the assessments of CT parameters. Thus, the pathophysiological heterogeneity of COPD could be reflected to different FOT-based phenotypes.
Recently, Karayama et al reported that emphysematous lesions in chest CT do not associate with Rrs indices but are significantly correlated with some Xrs indices.12 Conversely, Wada et al demonstrated a lack of significant correlation of both Rrs and Xrs with emphysematous lesions, but both were correlated with √Aaw at Pi10 as an indicator of airway wall thickness in COPD.11 Both opinions do not contradict our FOT-based phenotyping. R5 may purely reflect airway lesions and X5 may reflect the both gas-trapping area and airway lesions, both of which eventually shift to emphysema. Destruction of the lung parenchyma leads to the loss of bronchiolar-alveolae attachments. As a result, gas-trapping, emphysematous changes and airway lesions are occurred in COPD patients. FOT-based phenotyping could both assess Rrs and Xrs and reflect pathophysiological heterogeneity.
Spirometry is also a useful functional assessment to reveal airway obstruction such as FEV1/FVC and FEV1%predicted. However, it is difficult to conclusively identify the cause of obstructive functional changes. As shown in Figure 3, severe and very severe GOLD categories comprise mainly the MIX group. However, all FOT-based phenotypes are presented in mild and moderate GOLD categories. With combined assessment of Rrs and Xrs, the FOT-based phenotyping could represent the pathophysiological heterogeneity in COPD from a new perspective.
Notably, the FOT-based phenotyping is a simple and useful assessment which could reflect the pathophysiology of COPD caused by both emphysematous lesions and airway lesions.
This study has several limitations. First, this was a single-center and retrospective study. Second, the non-COPD smokers in this study were ruled out COPD by definitions but they visited our outpatient clinic because they presented symptoms such as cough. For the FOT-based phenotyping, this may have caused stricter cut-off values in our population of non-COPD smokers compared to a healthy control population. Hence, we used the 3rd quartile instead of the mean +2 SD of the R5 and X5 values. Further, the number of the non-COPD patients was relatively small. Finally, two different FOT devices, namely, MasterScreen-IOS® (CareFusion Germany 234 GmbH, Germany) and the MostGraph® (Chest M.I., Inc., Tokyo, Japan), are widely used, and they do not always generate identical respiratory impedance values.25 Further studies are needed to elucidate the influence of functional changes on quantitative imaging parameters.
Conclusion
The FOT-based phenotyping may be safe and useful to assess pathophysiological changes of COPD with CT assessments.
Ethics Approval and Informed Consent
This study was approved by the Ethics Committee of SUMS (registration number: 27-11) and conformed to the tenets of the Declaration of Helsinki, and all patients provided written informed consent prior to their participation.
Acknowledgments
The authors would like to thank the secretary, Ms. Yoko Naito, and the technicians, Ms. Mayuko Ono and Mr. Hayato Okumura, for their continued help throughout the study. This paper was presented at the American Thoracic Society InternationalConference 2019 as a conference talk with interim findings. The abstract was published in “Conference Abstracts” in American Journal of Respiratory and Critical Care Medicine 2019, Volume 199.
Author Contributions
Yumiko Matsuo contributed to the analysis and the interpretation of data and wrote the initial draft of the manuscript. Emiko Ogawa designed the study, contributed to the interpretation of data, and assisted in the preparation of the manuscript. All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; gave final approval of the version to be published; and agree to be accountable for all aspects of the work.
Disclosure
HK reports personal fees from Chest M.I. Inc., during the conduct of the study. In addition, HK has a patent for a 3D graphic technique in forced oscillation technique apparatus of MostGraph and a patent for a noise reduction technique in forced oscillation technique apparatus of MostGraph. The authors report no other conflicts of interest in this work.
References
1. Nakano Y, Muro S, Sakai H, et al. Computed tomographic measurements of airway dimensions and emphysema in smokers. Correlation with lung function. Am J Respir Crit Care Med. 2000;162(3 Pt 1):1102–1108. doi:10.1164/ajrccm.162.3.9907120
2. Tho NV, Ryujin Y, Ogawa E, et al. Relative contributions of emphysema and airway remodelling to airflow limitation in COPD: consistent results from two cohorts. Respirology. 2015;20(4):594–601. doi:10.1111/resp.12505
3. Nakano Y, Wong JC, de Jong PA, et al. The prediction of small airway dimensions using computed tomography. Am J Respir Crit Care Med. 2005;171:142–146. doi:10.1164/rccm.200407-874OC
4. Galban CJ, Han MK, Boes JL, et al. Computed tomography-based biomarker provides unique signature for diagnosis of COPD phenotypes and disease progression. Nat Med. 2012;18(11):1711–1715. doi:10.1038/nm.2971
5. Bhatt SP, Soler X, Wang X, et al. Association between functional small airway disease and FEV1 decline in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2016;194(2):178–184. doi:10.1164/rccm.201511-2219OC
6. Boes JL, Hoff BA, Bule M, et al. Parametric response mapping monitors temporal changes on lung CT scans in the subpopulations and intermediate outcome measures in COPD Study (SPIROMICS). Acad Radiol. 2015;22(2):186–194. doi:10.1016/j.acra.2014.08.015
7. Pompe E, Galban CJ, Ross BD, et al. Parametric response mapping on chest computed tomography associates with clinical and functional parameters in chronic obstructive pulmonary disease. Respir Med. 2017;123:48–55. doi:10.1016/j.rmed.2016.11.021
8. Kirby M, Yin Y, Tschirren J, et al. A novel method of estimating small airway disease using inspiratory-to-expiratory computed tomography. Respiration. 2017;94(4):336–345. doi:10.1159/000478865
9. Shirai T, Kurosawa H. Clinical application of the forced oscillation technique. Intern Med. 2016;55:559–566. doi:10.2169/internalmedicine.55.5876
10. Oostveen E, MacLeod D, Lorino H, et al. The forced oscillation technique in clinical practice: methodology, recommendations and future developments. Eur Respir J. 2003;22(6):1026–1041. doi:10.1183/09031936.03.00089403
11. Wada Y, Kitaguchi Y, Yasuo M, et al. Diversity of respiratory impedance based on quantitative computed tomography in patients with COPD. Int J Chron Obstruct Pulmon Dis. 2018;13:1841–1849. doi:10.2147/COPD
12. Karayama M, Inui N, Mori K, et al. Respiratory impedance is correlated with morphological changes in the lungs on three-dimensional CT in patients with COPD. Sci Rep. 2017;7:41709.
13. Global strategy for the diagnosis, management and prevention of Chronic Obstructive Lung Disease 2019 report. [homepage on the Internet]. Global Initiative for Chronic Obstructive Lung Disease. 2019. Available from: https://goldcopd.org/wp-content/uploads/2018/11/GOLD-2019-v1.7-FINAL-14Nov2018-WMS.pdf.
14. Mirza S, Clay RD, Koslow MA, Scanlon PD. COPD guidelines: a review of the 2018 GOLD report. Mayo Clin Proc. 2018;93(10):1488–1502. doi:10.1016/j.mayocp.2018.05.026
15. Jones PW, Harding G, Berry P, Wiklund I, Chen WH, Kline Leidy N. Development and first validation of the COPD Assessment Test. Eur Respir J. 2009;34(3):648–654. doi:10.1183/09031936.00102509
16. Fletcher CM. Standardised questionnaire on respiratory symptoms: a statement prepared and approved by the MRC Committee on the Aetiology of Chronic Bronchitis (MRC breathlessness score). BMJ Open. 1960;2:1665.
17. Miller MR, Hankinson J, Brusasco V, et al. Standardisation of spirometry. Eur Respir J. 2005;26(2):319–338. doi:10.1183/09031936.05.00034805
18. Sasaki H, Nakamura M, Kida K, et al. Reference values for spirogram and blood gas analysis in Japanese adults. J Japan Respir Soc. 2001;39:S1–S17.
19. Ohishi J, Kurosawa H, Ogawa H, Irokawa T, Hida W, Kohzuki M. Application of impulse oscillometry for within-breath analysis in patients with chronic obstructive pulmonary disease: pilot study. BMJ Open. 2011;1(2):e000184. doi:10.1136/bmjopen-2011-000184
20. Van Tho N, Ogawa E, Trang Le TH, et al. A mixed phenotype of airway wall thickening and emphysema is associated with dyspnea and hospitalization for chronic obstructive pulmonary disease. Ann Am Thorac Soc. 2015;12(7):988–996. doi:10.1513/AnnalsATS.201411-501OC
21. Di Mango AM, Lopes AJ, Jansen JM, Melo PL. Changes in respiratory mechanics with increasing degrees of airway obstruction in COPD: detection by forced oscillation technique. Respir Med. 2006;100(3):399–410. doi:10.1016/j.rmed.2005.07.005
22. Ogawa E, Nakano Y, Ohara T, et al. Body mass index in male patients with COPD: correlation with low attenuation areas on CT. Thorax. 2009;64:20–25. doi:10.1136/thx.2008.097543
23. Higami Y, Ogawa E, Ryujin Y, et al. Increased epicardial adipose tissue is associated with the airway dominant phenotype of chronic obstructive pulmonary disease. PLoS One. 2016;11(2):e0148794. doi:10.1371/journal.pone.0148794
24. Van Tho N, Wada H, Ogawa E, Nakano Y. Recent findings in chronic obstructive pulmonary disease by using quantitative computed tomography. Respir Investig. 2012;50(3):78–87. doi:10.1016/j.resinv.2012.08.002
25. Tanimura K, Hirai T, Sato S, et al. Comparison of two devices for respiratory impedance measurement using a forced oscillation technique: basic study using phantom models. J Physiol Sci. 2014;64(5):377–382. doi:10.1007/s12576-014-0329-4
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.