Back to Journals » Journal of Hepatocellular Carcinoma » Volume 10
Novel Prognostic Nomogram to Predict Progression-Free Survival of Patients with Hepatocellular Carcinoma After Transarterial Chemoembolization
Authors Xi D, Xu M, Han M, Guan Q, Guo Q, Yan F, Yao J, Ning Q
Received 15 March 2023
Accepted for publication 13 June 2023
Published 16 June 2023 Volume 2023:10 Pages 909—920
DOI https://doi.org/10.2147/JHC.S412643
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Manal Hassan
Dong Xi,1 Mengying Xu,1 Meiwen Han,1 Qianting Guan,1 Qinghao Guo,1 Fangfei Yan,1 Junxia Yao,2 Qin Ning1
1Department and Institute of Infectious Disease, Tongji Hospital, Tongji Medical College and State Key Laboratory for Diagnosis and Treatment of Severe Zoonotic Infectious Disease, Huazhong University of Science and Technology, Wuhan, Hubei, 430030, People’s Republic of China; 2Institute of Hematology, Union Hospital, Tongji Medical College, Huazhong University of Science and Technology, Wuhan, Hubei, 430030, People’s Republic of China
Correspondence: Junxia Yao, Institute of Hematology, Union Hospital, Tongji Medical College, Huazhong University of Science and Technology, Wuhan, People’s Republic of China, Tel +86-18502711578, Email [email protected] Qin Ning, Department and Institute of Infectious Disease, Tongji Hospital, Tongji Medical College and State Key Laboratory for Diagnosis and Treatment of Severe Zoonotic Infectious Disease, Huazhong University of Science and Technology, Wuhan, People’s Republic of China, Tel/Fax +86-27-83665959, Email [email protected]
Purpose: A retrospective analysis of hepatocellular carcinoma (HCC) patients treated with transarterial chemoembolization (TACE) to identify risk factors was conducted, and a novel predictive nomogram model was constructed.
Patients and Methods: A total of 346 HCC patients who underwent TACE as initial treatment were retrospectively included, of which 208 were randomly allocated to the derivation cohort and 138 were allocated to the validation cohort. Progression-free survival (PFS) was used as the follow-up endpoint according to mRECIST. Kaplan‒Meier analysis and the Cox regression model screened out some indicators associated with short-term prognosis, and R language was further used to construct a nomogram model. The nomogram was compared with the classical BCLC staging system.
Results: The independent predictors affecting PFS in HCC patients undergoing TACE included the following: 1. Baseline indicators: age (P=0.013), albumin-bilirubin (ALBI) grade (grade 2 vs grade 1, P=0.029; grade 3 vs grade 1, P< 0.001), and portal vein tumour thrombus (PVTT, P< 0.001); 2. Indicators at the 1-month follow-up: Neutrophil To Lymphocyte Ratio (NLR, P=0.032) and changes in alpha-fetoprotein (AFP, P< 0.05) and des-γ-carboxy prothrombin (DCP, P< 0.001); and 3. Cumulative treatment numbers of TACE in 6 months (P=0.007). In the derivation cohort, the calibration curve of the nomogram showed a high consistency between the predicted and actual PFS probability, and the nomogram outperformed the BCLC staging system (P=0.004). This result was also confirmed in the validation cohort (P=0.012).
Conclusion: The constructed nomogram was suggested to have good predictive efficacy and could be used as a complementary assessment to predict the survival and prognosis of HCC patients treated with TACE.
Keywords: primary liver cancer, transarterial chemoembolization, progression-free survival, modified response evaluation criteria in solid tumours, prognosis, nomogram
Introduction
Primary liver cancer (PLC) ranks sixth in the incidence of common malignant tumours worldwide and is also one of the most common malignant tumours causing death and seriously threatens human life. China has the highest incidence of PLC, and Chinese cases account for approximately 50% of the new cases of PLC and PLC-related mortality in the world.1,2
Due to the insidious onset of PLC and the lack of obvious early symptoms, many patients have already entered the middle or advanced stage when they are diagnosed, thus losing the opportunity for surgery. Transarterial chemoembolization (TACE) has gradually become one of the most common methods for the nonsurgical treatment of liver cancer. Randomized controlled studies have shown that TACE is more beneficial than supportive therapy for patients who are not amenable to surgical resection and that it can improve their quality of life to a large extent.3,4 According to the AASLD5 and EASL6 guidelines, TACE is currently recommended as a first-line treatment for patients with intermediate and advanced hepatocellular carcinoma (HCC) with good liver function. However, individual differences in liver function and tumour characteristics are evident in patients with unresectable HCC, which is the main reason for the large differences in patient prognosis and survival.7
For HCC patients treated with TACE, the correct and accurate assessment of prognosis is the key to guiding subsequent treatment. Since the Barcelona Clinic Liver Cancer (BCLC) staging system was proposed, various new biomarkers and scoring systems have emerged internationally. Unfortunately, in predicting the prognostic survival of patients with unresectable HCC, both the biomarkers and staging systems that have been reported thus far have significant limitations, and most of them are not specific to the specific HCC treatment. Therefore, we need to explore a more practical and reliable prognostic scoring system based on objective data to stage and stratify patients according to their underlying conditions.
Nomograms are excellent prediction tools that have been commonly used in recent years to estimate patient survival and prognosis. A nomogram can improve the accuracy of correlation predictions from the group level to the individual level.8 Currently, nomograms have been used in various tumour-related diseases,9 and some studies have reported their usefulness in the diagnosis and treatment of liver cancer.10,11 These simple tools can be used by clinicians to predict the probability of disease recurrence or the approximate survival time of patients during diagnosis and treatment planning.
Most of the currently reported nomograms for predicting the prognosis of liver cancer include only baseline data of patients,12,13 and less consideration has been given to the dynamic changes in indicators throughout the disease. However, the prognostic survival of HCC patients is influenced by several factors. Therefore, in this study, by analysing some unresectable HCC patients with TACE as the initial treatment, we established a practical, multifactorial, more comprehensive and objective nomogram that includes the dynamic changes in basic liver function, tumour characteristics and important indicators of HCC patients after treatment.
Patients and Methods
Patients
This study retrospectively included 346 patients with primary HCC who underwent TACE as the initial treatment at Tongji Hospital, Huazhong University of Science and Technology between January 2019 and January 2022, and 208 of these patients were used as the derivation cohort to construct the nomogram. The other 138 patients were used as the validation cohort to validate the model. The study was approved by the Ethics Committee of Tongji Hospital. All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation in Tongji Hospital and with the Helsinki Declaration of 1975, as revised in 2008 (5). The identities of patients included in our study were kept anonymous to the researchers by computer-generated ID numbers, and therefore consent from the patients was waived.
Inclusion and Exclusion Criteria
The inclusion criteria were as follows: patients (1) who were aged between 18 and 75 years; (2) had an ECOG performance status score of 0 or 1; (3) had not received any form of antitumour therapy prior to enrolment; (4) had TACE as their initial treatment; and (5) had a clinical or pathological diagnosis consistent with PLC.
The exclusion criteria were as follows: patients (1) with benign or malignant liver lesions that cannot be clearly distinguished or combined with precancerous liver lesions; (2) who had combined primary tumours of other sites in addition to liver tumours; (3) who had metastatic liver cancer; (4) with severe malnutrition or other factors such as the use of warfarin that may affect the level of des- γ-carboxy prothrombin (DCP) or other indicators to be studied; and (5) with incomplete clinical information, poor compliance or those who had not been followed up regularly or treated.
General Clinical Data
Clinical information was collected from the enrolled patients and included general information, past medical history, laboratory test data within 7 days before treatment and 1 month and 12 months after the initial TACE, imaging examinations, and the cumulative number of TACE treatments within the 6 months after the initial treatment. The imaging examinations included liver ultrasound, computed tomography and magnetic resonance imaging.
TACE Method
All HCC patients in this study were treated with TACE in the Department of Radiation Intervention. Digital subtraction angiography (DSA) was used for examination. The right femoral artery was percutaneously punctured, and the catheter and microcatheter were successively placed. The tumour site, size, number and blood supplying artery were clarified according to the DSA images of the hepatic artery, and the superselective cannula was inserted into the tumour supplying artery for perfusion chemotherapy. The chemotherapeutic drugs were mainly anthracyclines and platinum. The appropriate embolic agent was selected according to the specific conditions of the tumour, and superiodinated oil was generally mixed with chemotherapeutic drugs. After embolization, reimaging was needed. The catheter and catheter sheath were removed at the end of the procedure.
Study Endpoints and Follow-Up
Due to the short enrolment of patients, the primary study endpoint was progression-free survival (PFS), defined as the time from the date of the first TACE until the onset of tumour progression, patient death, or the last follow-up visit, whichever came first. The enrolled HCC patients or their families were followed up from admission until January 2022 through outpatient, inpatient, and telephone visits. Tumour response to treatment was determined based on imaging data and according to the mRECIST, which was reviewed and published by two senior physicians in the Department of Radiology and Interventional Medicine.
Statistical Analysis
All continuous variables in the study were converted into dichotomous or ordered categorical variables according to the cut-off values, which were determined with X-tile software when risk factor analysis was performed. The Kaplan‒Meier method was used to draw PFS curves for the univariate analyses, and the log-rank test was performed in parallel. The variables with P≤0.1 were included in the subsequent Cox multivariate regression model analyses, and the forwards LR method was used for variable screening.
Based on the results of the univariate analyses, a nomogram was constructed using the rms data package in R software to visualize the Cox multivariate regression. The study used the bootstrap self-sampling method 1000 times with put-back sampling for internal validation of the nomogram and external validation of the model by a validation cohort. The consistency index (C-index) and the prediction model calibration curve were used to illustrate the accuracy of the model predictions. SPSS 24.0, GraphPad Prism 5 and R software were used for statistical analysis and graphing. The Shapiro‒Wilk test was performed to assess whether the samples conformed to a normal distribution, those variables with a normal distribution were expressed as the mean±standard deviation, and those variables with a skewed distribution were expressed as the median and quadratic spacing. All hypothesis tests were two-sided, and P<0.05 indicated that the differences were statistically significant.
Results
Clinical Characteristics of the Enrolled Patients
The mean age of all 346 patients was 53 years, and the patients were predominantly male, accounting for 87.9%. The mean age was 54 years in the derivation cohort and 53 years in the validation cohort. Of the 208 patients in the derivation cohort, 185 were males, accounting for 88.9%. There were 119 male patients in the validation cohort, accounting for 86.2%. When including all the patients, they had a high chronic hepatitis B virus (HBV) infection rate of 95.4%. The hepatitis C virus (HCV) infection rate was 4% (Table 1).
 |
Table 1 Baseline Characteristics of the Enrolled Patients |
The albumin-bilirubin (ALBI) grade is classified as follows: <-2.6 is grade 1, −2.60 ≤ grade ≤ −1.39 is grade 2, and > −1.39 is grade 3. There were 222 patients who were ALBI grade 1, accounting for 64.2%, 29.4% were grade 2, and the least number of patients, only 6.4%, were grade 3 (Table 1), which suggested that most patients had good liver function and could tolerate invasive operations such as TACE.
From the pretreatment imaging data, 79.8% of the patients had more than a single tumour lesion (Table 1). There were 165 patients with ≥2 multiple tumour lesions in the original dataset, accounting for 79.4%, and only 27 patients had a single tumour lesion in the validation cohort, accounting for 19.6% (Table 2). In addition, when the patients were evaluated for vascular invasion based on imaging data, portal vein tumour thrombus (PVTT) was present in 87 of 346 patients, 50 in the original dataset and 37 in the validation dataset, accounting for 24.0% and 26.8% of each dataset, respectively (Table 2).
 |
Table 2 Characteristics of the Derivation Cohort and Validation Cohort at Baseline |
Based on the BCLC stage, the majority of the patients were BCLC stage B, with 246 patients (71.1%), followed by BCLC stage C patients, which was 87 patients (25.1%), and BCLC stage A patients, which is the group with the lowest number of patients (Table 1).
The laboratory test data showed that the majority of the patients had normal blood counts and good baseline liver function before treatment. The tumour markers directly related to HCC (AFP, DCP) varied greatly among the patients on an individual basis, with values fluctuating between the lowest detected value and the highest upper limit, with a median value of 846.7 ng/mL for AFP and 412.6 ng/mL for DCP (Table 1).
The patients’ follow-up data 1 month after the first TACE treatment were analysed and compared with the baseline data: the Neutrophil To Lymphocyte Ratio (NLR) was significantly higher (P=0.001), which implied that the inflammatory response of the body was enhanced. The total bilirubin (TBIL) levels were not significantly different (P=0.890). However, the patients were evaluated for ALBI grading, and there was a significant increase in ALBI grade 2 versus grade 3 (P<0.001), suggesting that liver reserve function was significantly impaired after TACE. The patients’ serum tumour markers decreased significantly, with the median value of AFP decreasing to 476.3 ng/mL (P<0.001) and that of DCP decreasing to 101.3 ng/mL (P<0.001), suggesting that TACE treatment controlled tumour development in the short term (Table 1).
A total of 1181 TACE procedures were performed in 346 patients within 6 months of the initial treatment. Data from patients at the 12-month follow-up visit were analysed; the NLR levels remained significantly higher than those at baseline (P=0.021). Liver reserve function improved compared to the first month follow-up but remained significantly lower compared to baseline. There was a statistically significant difference in patient ALBI grading, with a significant increase in grades 2 and 3 (P<0.001). The patients had significantly lower AFP (P=0.003) and DCP (P<0.001) (Table 1).
At the endpoint of the observation, 170 of 348 patients had no significant progression of tumour lesions, with a 12-month PFS rate of 48.9%. The 12-month PFS rates were 48.5% and 50.0% in the derivation and validation cohorts, respectively.
The baseline data of the derivation and validation datasets were subjected to statistical and difference analysis. The results showed that no significant differences were observed between the two cohorts, which shows that these cohorts could be used in the follow-up study (Table 2).
Prognostic Factors Associated with PFS After TACE in HCC Patients
These indicators were analysed in the derivation cohort according to the patients’ follow-up results, including the patients’ basic information, laboratory tests, imaging, and the cumulative number of TACE treatments. The univariate analysis revealed that the baseline indicators, including age (P=0.003), aspartate transaminase (AST, P=0.024), ALBI (P=0.011), prothrombin time (P=0.049), number of tumours (P=0.031), and PVTT (P<0.001); the 1-month follow-up indicators, including NLR (P=0.012), AFP change (P<0.001, and DCP change (P<0.001); and the cumulative number of TACE treatments in 6 months (P=0.003) affected progression-free survival (PFS).
As shown in Table 3, the indicators with P≤0.1 were included in the Cox regression multivariate analysis to obtain independent predictors associated with PFS after TACE treatment in the derivation cohort: 1. Baseline indicators: age (HR=2.104, 95% CI=1.327–3.076, P=0.013), ALBI (grade 2 vs grade 1, HR=1.977, 95% CI= 1.215–3.712, P=0.029; grade 3 vs grade 1, HR=2.011, 95% CI=1.664–2.871, P<0.001), PVTT (HR=2.541, 95% CI=1.662–3.491, P<0.001); 2. Indicators at the 1-month follow-up: NLR (HR=1.675, 95% CI=1.475–2.396, P=0.032), change in AFP*(1 vs 0, HR=2.065, 95% CI=1.568–3.715, P=0.015; 2 vs 0, HR=2.224, 95% CI=1.456–3.585, P=0.005), change in DCP*(1 vs 0, HR=3.343, 95% CI=1.79–5.95, P<0.001; 2 vs 0, HR=3.669, 95% CI=2.471–5.877, P<0.001); and 3. cumulative number of TACE treatments in 6 months (HR=0.387, 95% CI=0.134–0.521, P=0.007).
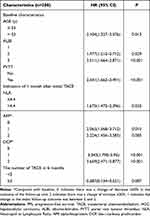 |
Table 3 Prognostic Factors Associated with PFS After TACE in Patients with HCC |
Establishment of a Prognostic Nomogram for PFS in HCC Patients After TACE
The above seven screened independent prognosis-related indicators were integrated, and the data were run through the rms package of R software to generate a nomogram model (Figure 1). We tested the constructed nomogram, and the C-index for predicting the PFS probability in the original dataset was 0.712 (95% CI=0.533–0.931), indicating that the nomogram was used for prediction with high accuracy. Each included index in the nomogram was assigned a certain score, and the scores corresponding to these indices according to the actual situation of each patient were obtained. Finally, these scores were summed to obtain a total score, which corresponded to the PFS probability.
Validation of the PFS Nomogram Model for HCC Patients Treated with TACE
After the internal validation, the C-index of the nomogram developed in this study was 0.712 (95% CI=0.533–0.931), and the standard curve of the derivation cohort of the nomogram showed that the model predicted PFS after treatment well (Figure 2A). The model was then externally validated with a validation cohort, and the C-index of the validation cohort was 0.734 (95% CI=0.636–0.842), with a good fit of the standard curve to the dashed line (Figure 2B). This indicates that the nomogram constructed in this study has good predictive efficacy for the PFS probability after TACE in HCC patients.
Compared with the BCLC Staging System, the Constructed Nomogram Had Better Predictive Efficacy for PFS in HCC Patients
In the derivation cohort, the C-index was 0.712 (95% CI=0.533–0.931) for the nomogram and 0.688 (95% CI=0.542–0.867) for the BCLC staging, which was significantly different (P=0.004). In the validation cohort, the nomogram C-index was 0.734 (95% CI=0.636–0.842), and the BCLC staging C-index was 0.663 (95% CI=0.525–0.791), which was also significantly different (P=0.012). In both cohorts, the nomogram had better predictive efficacy than BCLC staging (Table 4).
 |
Table 4 Comparison of the Concordance Index Between the Constructed Nomogram and BCLC Stage for PFS Prediction in the Derivation and Validation Cohorts |
Figure 3 shows the comparison of the receiver operating characteristic curves (ROCs) of the subjects between the BCLC stage and the constructed nomogram to predict PFS at 12 months in the two cohorts. As in the derivation cohort, the AUC of the nomogram was also greater than that of BCLC stage in the validation cohort. This indicated that the performance of the nomogram for predicting 12-month PFS in TACE-treated HCC patients was better than that of BCLC staging.
Discussion
In this study, we conducted a retrospective analysis of HCC patients treated with TACE to identify relevant risk factors and constructed a predictive nomogram model that better fit the change in patients’ disease to assess the survival prognosis of patients treated with TACE and thus to guide subsequent treatments.
Age is one of the main factors affecting the development and prognosis of HCC. The older patients are, the worse their tolerance to surgical resection, radio frequency ablation, TACE and other treatments, and the worse their tumour response.14,15 Since a single indicator related to liver function is obviously one-sided in the survival prognosis of patients with HCC,16,17 some staging systems including multiple indicators have been established, such as Child-Turcott-Pugh (CTP) grading, the end-stage liver disease (MELD) score, and ALBI grading. Although these systems have good predictive efficacy, they have some obvious shortcomings. The ALBI grading model is an objective method to judge liver damage in patients with liver disease18 and can be used to predict the long-term survival of patients with HCC undergoing surgical treatment.19 For HCC patients who had received TACE treatment, it was reported that the ALBI grading model was better than CTP grading in predicting survival and prognosis.20 PVTT reflects the aggressiveness of the tumour to some extent. In highly selected patients with unresectable liver cancer, patients treated with TACE had better survival benefits than those receiving supportive care,4,21 and subsequent studies reported that patients with PVTT benefited from TACE treatment.22 In our nomogram, the PFS probability was significantly reduced in patients with PVTT. This might be because once PVTT was formed, it was more likely to fall off and enter the blood circulation, resulting in intrahepatic metastasis and distant metastasis, which directly affects the progression of the disease and ultimately affects the prognosis and survival of patients.
The NLR is a good prognostic factor for cancer.23 In this study, the baseline NLR value of most of the HCC patients treated with TACE was low. After receiving TACE, the patients had a relatively strong inflammatory response, and the NLR changed significantly, which is of great significance for the later disease outcome. AFP, as a relatively inexpensive and mature indicator, has always been considered an important indicator of disease progression and survival prognosis after treatment for liver cancer.24 At present, AFP used to predict survival is mostly the baseline value before treatment, but AFP levels are not constant and are affected by many factors. A study showed that serum AFP levels before treatment had no significant effect on the mortality of patients with liver cancer.25 In this study, we selected the degree of change in the AFP levels one month after the initial TACE operation to evaluate the response to treatment. Similar to AFP, DCP can be used as an excellent supplemental marker for AFP in both early diagnosis and prognosis prediction of survival in HCC. In this study, we also included the dynamic changes in DCP in the nomogram model after performing the multivariate COX regression analysis.
The optimal interval for the initial follow-up is recommended to be 1 month clinically. However, due to economic, regional and various problems, it is difficult for patients to return to the doctor regularly. The cumulative number of TACE treatments in 6 months was included in our analysis. Our results showed that it was very important whether the patient’s condition could be evaluated through follow-up and whether necessary repeated TACE treatment could be effectively carried out in time, which could effectively control disease progression to a certain extent.
Although the predictive performance of the nomogram is better than that of BCLC staging, BCLC staging, as a classical liver cancer staging system, has been modified, updated and repeatedly validated over a long period of time, and its status in clinical application is irreplaceable.
The model established in this study has some shortcomings. First, due to the time limitation of the study, the number of enrolled patients was small, and the sample size still needs to be expanded for further analysis and validation of the model. Second, the best follow-up endpoint for the study should be overall survival (OS), but due to the short enrolment time, we chose PFS as a surrogate. Finally, this is a single-centre retrospective study. HCC is a complex disease with very clear heterogeneity in different countries and regions, from epidemiology to tumour development characteristics. The results need to be further validated by more samples and other centre cohorts due to the limitations of sample size and being a single-centre study.
Conclusion
The nomogram constructed in our study not only included pretreatment baseline data but also incorporated some posttreatment follow-up indicators, and our nomogram had significant dynamic advantages compared to previously reported models. It was tested and suggested to have good ability to predict disease progression in the short term, indicating that the model has some clinical significance.
Abbreviations
ALB, albumin; ALBI, albumin-bilirubin; ALP, alkaline phosphatase; ALT, alanine aminotransferase; AFU, alpha-L-fucosidase; AUC, area under curve; BCLC, Barcelona Clinic Liver Cancer; CTP, Child-Turcott-Pugh; CEA, carcinoembryonic antigen; CA199, carbohydrate antigen 199; CI, confidence interval; C-index, consistency index; DSA, Digital subtraction angiography; DCP, des-γ-carboxy prothrombin; γ-GT, γ-glutamyl transpeptidase; HR, hazard ratio; Hb, Hemoglobin; HBV, hepatitis B virus; HCV, hepatitis C virus; HCC, hepatocellular carcinoma; IQR, interquartile range; MELD, end-stage liver disease; mRECIST, modified Response Evaluation Criteria in Solid Tumours; NLR, Neutrophil to Lymphocyte Ratio; OS, overall survival; PLT, Platelet; PFS, progression-free survival; PLC, primary liver cancer; PT, prothrombin time; PTA, prothrombin time activity; PVTT, portal vein tumour thrombus; TACE, transarterial chemoembolization; TBIL, total bilirubin; WBC, white blood cell.
Data Sharing Statement
All data in the study are included in the published article.
Ethics Approval and Informed Consent
The study was approved by the Ethics Committee of Tongji Hospital. All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation in Tongji Hospital and with the Helsinki Declaration of 1975, as revised in 2008 (5). The identities of patients included in our study were kept anonymous to the researchers by computer-generated ID numbers, and therefore consent from the patients was waived.
Acknowledgments
We thank the patients who participated in this study.
Funding
This work was supported by The National Key Research and Development Program of China (2021YFC2600200).
Disclosure
The authors declare that they have no competing interests in this work.
References
1. Sung H, Ferlay J, Siegel RL, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71(3):209–249. doi:10.3322/caac.21660
2. Llovet JM, Kelley RK, Villanueva A, et al. Hepatocellular carcinoma. Nat Rev Dis Primers. 2021;7(1):6. doi:10.1038/s41572-020-00240-3
3. Sieghart W, Hucke F, Peck-Radosavljevic M. Transarterial chemoembolization: modalities, indication, and patient selection. J Hepatol. 2015;62(5):1187–1195. doi:10.1016/j.jhep.2015.02.010
4. Lo CM, Ngan H, Tso WK, et al. Randomized controlled trial of transarterial lipiodol chemoembolization for unresectable hepatocellular carcinoma. Hepatology. 2002;35(5):1164–1171. doi:10.1053/jhep.2002.33156
5. Heimbach JK, Kulik LM, Finn RS, et al. AASLD guidelines for the treatment of hepatocellular carcinoma. Hepatology. 2018;67(1):358–380. doi:10.1002/hep.29086
6. European Association for the Study of the Liver. EASL clinical practice guidelines: management of hepatocellular carcinoma. J Hepatol. 2018;69(1):182–236. doi:10.1016/j.jhep.2018.03.019
7. Bruix J, Llovet JM. Major achievements in hepatocellular carcinoma. Lancet. 2009;373(9664):614–616. doi:10.1016/S0140-6736(09)60381-0
8. Balachandran VP, Gonen M, Smith JJ, et al. Nomograms in oncology: more than meets the eye. Lancet Oncol. 2015;16(4):e173–e180. doi:10.1016/S1470-2045(14)71116-7
9. Cho U, Park HS, Im SY, et al. Prognostic value of systemic inflammatory markers and development of a nomogram in breast cancer. PLoS One. 2018;13(7):e0200936. doi:10.1371/journal.pone.0200936
10. Ni JY, Fang ZT, Sun HL, et al. Nomogram to predict survival of patients with intermediate-stage hepatocellular carcinoma after transarterial chemoembolization combined with microwave ablation. Eur Radiol. 2020;30(4):2377–2390. doi:10.1007/s00330-019-06438-8
11. Hsu CY, Liu PH, Ho SY, et al. Using nomogram of the Barcelona clinic liver cancer system for treatment selection in patients with stage C hepatocellular carcinoma. BMC Cancer. 2018;18(1):289. doi:10.1186/s12885-018-4202-3
12. Shen J, He L, Li C, et al. Prognostic nomograms for patients with resectable hepatocelluar carcinoma incorporating systemic inflammation and tumor characteristics. Oncotarget. 2016;7(49):80783–80793. doi:10.18632/oncotarget.13038
13. Feng LH, Dong H, Lau WY, et al. Novel microvascular invasion-based prognostic nomograms to predict survival outcomes in patients after R0 resection for hepatocellular carcinoma. J Cancer Res Clin Oncol. 2017;143(2):293–303. doi:10.1007/s00432-016-2286-1
14. Takuma Y, Shota I, Miyatake H, et al. Nomograms to predict the disease-free survival and overall survival after radiofrequency ablation for Hepatocellular carcinoma. Intern Med. 2018;57(4):457–468. doi:10.2169/internalmedicine.9064-17
15. Berardi G, Morise Z, Sposito C, et al. Development of a nomogram to predict outcome after liver resection for hepatocellular carcinoma in Child-Pugh B cirrhosis. J Hepatol. 2020;72(1):75–84. doi:10.1016/j.jhep.2019.08.032
16. Antkowiak M, Gabr A, Das A, et al. Prognostic role of albumin, bilirubin, and ALBI Scores: analysis of 1000 patients with hepatocellular carcinoma undergoing radioembolization. Cancers. 2019;11(6):879. doi:10.3390/cancers11060879
17. Zhou L, Wang SB, Chen SG, et al. Prognostic value of ALT, AST, and AAR in Hepatocellular carcinoma with B-type hepatitis-associated cirrhosis after radical hepatectomy. Clin Lab. 2018;64(10):1739–1747. doi:10.7754/Clin.Lab.2018.180532
18. Johnson PJ, Berhane S, Kagebayashi C, et al. Assessment of liver function in patients with hepatocellular carcinoma: a new evidence-based approach-The ALBI grade. J Clin Oncol. 2015;33(6):550–558. doi:10.1200/JCO.2014.57.9151
19. Toyoda H, Lai PB, O’Beirne J, et al. Long-term impact of liver function on curative therapy for hepatocellular carcinoma: application of the ALBI grade. Br J Canc. 2016;114(7):744–750. doi:10.1038/bjc.2016.33
20. Khalid MA, Achakzai IK, Hanif FM, et al. To determine the prognostic value of the albumin-bilirubin grade (ALBI) in patients underwent transarterial chemoembolization for unresectable hepatocellular carcinoma. Gastroenterol Hepatol Bed Bench. 2019;12(2):110–115.
21. Llovet JM, Real MI, Montana X, et al. Arterial embolisation or chemoembolisation versus symptomatic treatment in patients with unresectable hepatocellular carcinoma: a randomised controlled trial. Lancet. 2002;359(9319):1734–1739. doi:10.1016/S0140-6736(02)08649-X
22. Xue TC, Xie XY, Zhang L, et al. Transarterial chemoembolization for hepatocellular carcinoma with portal vein tumor thrombus: a meta-analysis. BMC Gastroenterol. 2013;13:60. doi:10.1186/1471-230X-13-60
23. Stotz M, Gerger A, Eisner F, et al. Increased neutrophil-lymphocyte ratio is a poor prognostic factor in patients with primary operable and inoperable pancreatic cancer. Br J Cancer. 2013;109(2):416–421. doi:10.1038/bjc.2013.332
24. Chang SK, Hlaing WW, Yu RQ, et al. Value of alpha-foetoprotein for screening of recurrence in hepatocellular carcinoma post resection. Singapore Med J. 2012;53(1):32–35.
25. Chen JG, Parkin DM, Chen QG, et al. Screening for liver cancer: results of a randomized controlled trial in Qidong, China. J Med Screen. 2003;10(4):204–209. doi:10.1258/096914103771773320
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.



