Back to Journals » Drug Design, Development and Therapy » Volume 16
Novel Liposomal Rolipram Formulation for Clinical Application to Reduce Emesis
Authors Gobejishvili L, Rodriguez WE, Bauer P, Wang Y, Soni C, Lydic T, Barve S, McClain C, Maldonado C
Received 13 January 2022
Accepted for publication 20 April 2022
Published 3 May 2022 Volume 2022:16 Pages 1301—1309
DOI https://doi.org/10.2147/DDDT.S355796
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Anastasios Lymperopoulos
Leila Gobejishvili,1 Walter E Rodriguez,1 Philip Bauer,2 Yali Wang,1 Chirag Soni,2 Todd Lydic,3 Shirish Barve,1 Craig McClain,1 Claudio Maldonado4
1Department of Medicine, School of Medicine, University of Louisville, Louisville, KY, USA; 2Endoprotech, Inc., Louisville, KY, USA; 3Lipidomics Center, Michigan State University, East Lansing, MI, USA; 4Department of Physiology, School of Medicine, University of Louisville, Louisville, KY, USA
Correspondence: Claudio Maldonado, Department of Physiology, School of Medicine, University of Louisville, 500 S. Preston Street, HSC A-1115, Louisville, KY, 40292, USA, Tel +1 (502) 852-1078, Email [email protected] Leila Gobejishvili, Department of Medicine, School of Medicine, University of Louisville, 505 S. Hancock Street, CTR 516, Louisville, KY, 40202, USA, Tel +1 (502) 852-0361, Fax +1 (502) 852-8927, Email [email protected]
Introduction: The phosphodiesterase 4 (PDE4) inhibitor, rolipram, has beneficial effects on tissue inflammation, injury and fibrosis, including in the liver. Since rolipram elicits significant CNS side-effects in humans (ie, nausea and emesis), our group developed a fusogenic lipid vesicle (FLV) drug delivery system that targets the liver to avoid adverse events. We evaluated whether this novel liposomal rolipram formulation reduces emesis.
Methods: C57Bl/6J male mice were used to compare the effect of three doses of free and FLV-delivered (FLVs-Rol) rolipram in a behavioral correlate model of rolipram-induced emesis. Tissue rolipram and rolipram metabolite levels were measured using LC-MS/MS. The effect of FLVs-Rol on brain and liver PDE4 activities was evaluated.
Results: Low and moderate doses of free rolipram significantly reduced anesthesia duration, while the same doses of FLVs-Rol had no effect. However, the onset and duration of adverse effects (shortening of anesthesia period) elicited by a high dose of rolipram was not ameliorated by FLVs-Rol. Post-mortem analysis of brain and liver tissues demonstrated that FLVs affected the rate of rolipram uptake by liver and brain. Lastly, administration of a moderate dose of FLVs-Rol attenuated endotoxin induced PDE4 activity in the liver with negligible effect on the brain.
Discussion: The findings that the low and moderate doses of FLVs-Rol did not shorten the anesthesia duration time suggest that FLV delivery prevented critical levels of drug from crossing the blood-brain barrier (BBB) to elicit CNS side-effects. However, the inability of high dose FLVs-Rol to prevent CNS side-effects indicates that there was sufficient unencapsulated rolipram to cross the BBB and shorten anesthesia duration. Notably, a moderate dose of FLVs-Rol was able to decrease PDE4 activity in the liver without affecting the brain. Taken together, FLVs-Rol has a strong potential for clinical application for the treatment of liver disease without side effects.
Keywords: liposomes, rolipram, side-effects, PDE4, liver
Introduction
Chronic liver disease is a significant health problem worldwide and has a high mortality. Alcohol associated liver disease (ALD) and non-alcoholic fatty liver disease (NAFLD) are among the major causes of chronic liver disease.1–3 Experimental studies have provided a rationale for targeting phosphodiesterase 4 (PDE4) as a therapeutic strategy for liver disease, including for ALD and NAFLD.4–6 PDE4 inhibitors have been shown to be effective in reducing inflammation, injury and fibrosis in the liver in animal studies.7–12 However, the clinical application of PDE4 inhibitors, such as rolipram, is hampered by dose-associated side-effects, including severe nausea and emesis caused by increased cyclic adenosine monophosphate (cAMP) levels in the central nervous system (CNS).13,14 Rolipram, which is lipophilic, easily crosses the blood-brain barrier (BBB)15 and is able to inhibit PDE4 in the emetic centers of the brainstem,16 causing nausea and emesis. To ameliorate the nausea and emetic response accompanied by systemic rolipram administration, our group developed a nanoliposome-based fusogenic lipid vesicle (FLV) system for rolipram delivery (FLVs-Rol). The FLV carrier system was designed to target rolipram to hepatic tissue, and at the same time, to restrict levels of free drug from circulating in the blood and, thus, impede its crossing of the BBB. Bio-distribution studies of FLVs without drug documented the major uptake of FLVs by the liver in mice.10 Importantly, previous work demonstrated that FLV delivery of rolipram was very effective in attenuating hepatic injury in a mouse model of ALD.10
Examining rolipram-induced side effects in rodents is difficult because they are a non-vomiting species.17 The mechanism of inducing the emetic reflex associated with PDE4 inhibitors is thought to be analogous to the pharmacological response of presynaptic α2A-adrenoceptor inhibition, which leads to elevated intracellular levels of cAMP in noradrenergic neurons.18 Consequently, PDE4 inhibitors remove an inhibitory mechanism that modulates the release of mediators (5-hydroxytryptamine (5-HT), substance-P, and noradrenaline) involved in the onset of the emetic reflex.19 Robichaud et al developed a behavioral correlate of the emesis model in mice.20 In this model, rolipram was used to reduce the duration of α2A-adrenoceptor-mediated xylazine/ketamine-induced anesthesia, which is quantified by restoration of the righting reflex indicated by the time it takes the animal to turn spontaneously to the prone position. We hypothesized that the encapsulation of rolipram in FLVs would reduce the ability of the drug to cross the BBB, and thereby largely prevent its inhibitory effect on xylazine/ketamine anesthesia. Using the model developed by Robichaud et al, the present study compared the effect of rolipram vs FLVs-Rol in blocking the effects of xylazine/ketamine anesthesia. Specifically, the aims of the present study were to examine: 1) the effect of different FLVs-Rol doses on the duration of xylazine/ketamine anesthesia; 2) the time course of a high dose of FLVs-Rol on the onset and duration response of potential CNS adverse effects; and 3) whether FLVs-Rol has an inhibitory effect on PDE4 activity in the liver without affecting the brain.
Materials and Methods
Animals
Twelve-week-old male C57BL/6 mice (25–30 grams body weight) were obtained from the Jackson Laboratory (Bar Harbor, ME). Mice were housed in a temperature- and humidity-controlled environment, in groups of five, with chow and water available ad libitum. A period of 7 days of acclimatization was allowed prior to experimentation. All animals were cared for in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals (Department of Health and Human Services, Publication No. [NIH] 86–23), and with approval from the University of Louisville Institutional Animal Care and Use Committee (IACUC). Animals were euthanized by anesthetizing to a deep plane of anesthesia followed by exsanguination via the inferior vena cava. Livers and brains were harvested, and flash frozen in liquid nitrogen for future analysis. Carcasses were safely disposed of by placing in a labeled plastic bag, which was placed in a designated freezer in the vivarium until incineration according to IACUC guidelines.
Preparation of FLVs and FLVs-Rolipram
Preparation of FLVs was performed as previously described by Goga et al with a few minor modifications.21 In brief, FLVs were formulated with 1,2-dioleoyl-sn-glycerol-3-phosphocholine (DOPC) and 1-palmitoyl-2-oleol-sn-glycerol-3-phosphate (POPA) (Cat. #s 850375C and 840875C, respectively, Avanti Lipids International, Alabaster, AL) mixed in chloroform at a mole ratio of 3:2. The chloroform was removed using a vacuum pump overnight and by blowing nitrogen gas. The lipid material was hydrated in PBS to a concentration of 12.5 mg/mL of lipid. The buffer and lipid mixture were vortexed for a period of 1 min to create multilamellar vesicles, placed in a 37°C bath for 5 min, and repeated 3 times. The resulting solution was sonicated with a Branson Sonifier 450A (Branson Ultrasonics Corp, Danbury, CT) at 50% duty cycle for 1 min/mL to form smaller unilamellar FVLs. The solution was repeatedly extruded through membranes with progressively smaller pore sizes (400 nm, 200 nm and 100 nm) using a Thermobarrel Extruder (Northern Lipids, Burnaby, BC, Canada). To prepare FLVs-Rol, two concentrations of rolipram were dissolved in phosphate buffered saline (PBS) solution. To deliver 1.6 mg/kg of rolipram, the PBS hydrating solution contained 0.375 mg/mL of rolipram, and to deliver 3.3 and 6.6 mg/kg of rolipram, the rolipram concentration in PBS solution for both was 0.75 mg/mL. The FLV lipid concentration in PBS for all doses was 12.5 mg/mL. Solutions were prepared freshly for each experiment, and FLV characteristics including particle size, concentration of particles, and zeta potential were quantified using a ZetaView Multiple Parameter Particle Tracking Analyzer (Particle Metrix Inc, Mebane, NC). Encapsulation efficiency percent (EE%) of rolipram in FLVs was quantified as follows. A stock solution of 0.75 mg/mL Rolipram in DMSO was prepared. Dilutions of this stock solution (5x dilution of stock, followed by 2x dilutions) in 1:9 water/DMSO were measured for absorbance at 280 nm. A standard curve was created by plotting the absorbance of a 2-fold dilution series of standard rolipram solution vs the concentration in μg/mL. To determine EE%, 100 μL of each sample was filtered using a centrifugal filtration unit (CFU, 30 minutes at 2000 x g). 10 μL of the filtrate was diluted in 90 μL DMSO, in triplicate, and absorbance at 280 nm was determined. Values were compared to the calibration curve to determine the concentration of rolipram in the CFU filtrate. The following equation was used to determine encapsulation efficiency: EE% = (Ct – Cf)/Ct x 100, where Ct is the total rolipram in the mixture, and Cf is the rolipram in the mixture that is not associated with FLVs.
Dose Response Studies
A total of 36 mice were assigned to six groups (n=6 per group) receiving either rolipram or FLVs-Rol at the following Intravenous (i.v.) doses: 1.6, 3.3 or 6.6 mg/kg of body weight. A paired study design was used, each mouse served as its own control. A baseline anesthesia duration time was first quantified. Mice were then anesthetized using a mixture of xylazine (10 mg/kg, Bayer Health Care, Animal Health Division, Shawnee Mission, KS) and ketamine (80 mg/kg, Mylan Institutional LLC, Rockford, IL), prepared using the following volumes: xylazine 1.5 mL; ketamine 4.8 mL, and saline 13.7 mL (total volume: 20 mL). After 15 min of receiving the anesthetic, the depth of anesthesia was evaluated by the pedal reflex, and mice were placed on a controlled heating pad in the dorsal recumbent position. The anesthesia duration time was determined by the return of the righting reflex (when the mouse turned itself spontaneously to a prone position). Four days after baseline measurements, mice were re-anesthetized, and at 15 min of anesthesia injection, either rolipram or FLVs-Rol was administered via the tail vein (volume ranged from 150 to 300 μL), and anesthesia duration time was quantified.
Safety Pharmacology Studies
To assess whether FLVs protect against the adverse CNS effects induced by a high dose of rolipram safety pharmacology studies were performed. A total of 60 mice were assigned to 10 groups (n=6 per group) receiving either rolipram or FLVs-Rol at a high i.v. dose of 6.6 mg/kg. A paired study design was used, each mouse served as its own control. A baseline xylazine/ketamine anesthesia duration time was performed first, and four days later, rolipram or FLVs-Rol was administered via the tail vein and was followed by anesthesia induction at 35, 60-, 80-, 100-, and 120-min post-dosing. Anesthesia duration time was determined by the return of the righting reflex.
Rolipram and Rolipram Metabolites Tissue Levels
At the end of dose response experiments (~100 min post-dosing), livers and brains from the treatment groups receiving 3.3 mg/kg of rolipram were harvested, sectioned, flash frozen, and stored at −80°C. Extraction of rolipram and the metabolites hydroxy-pyrrolidinone rolipram (HPR), decyclopentylated rolipram (DCR) and hydroxy-cyclopentyl rolipram (HCR) were performed according to Cai and Li.22 Briefly, frozen tissues on dry ice were placed into 400 μL of ice-cold HPLC-grade water and spiked with 40 μL/200 mg tissue of internal standard (10 ng/μL Salbutamol). Tissues were homogenized using zirconium oxide beads in an air-cooled Bullet Blender homogenizer. 400 μL of methanol and 400 μL of acetone were added to the homogenates and vortexed for 30 min. Homogenates were precipitated for 2 h at −80°C and centrifuged for 30 min at ~14,000 rpm. The supernatant was collected and transferred to a 1.5 mL plastic test tube. The solvent was extracted via evaporation using a speedvac (Savant SC210A, ThermoFisher Scientific, Waltham, MA). The drug extracts were resuspended using 1 μL of HPLC-grade methanol per mg of tissue extracted. Tubes containing extracts were filled with nitrogen and stored at −80°C until ready for analysis. Rolipram, HPR, DCR, and HCR were analyzed by multiple reaction monitoring using precursor-product ion pairs as performed by Thevis et al23 using an HILIC-Triple Quadrupole LC-MS/MS (TSQ Vantage, ThermoFisher Scientific, Waltham, MA). Extracts were centrifuged at high speed and the supernatant was diluted 1:1 with HPLC-grade methanol in small vial glass insert placed inside an LC-MS vial. The LC (Michrom Paradigm MS4, Michrom Bioresources, Auburn, CA) was set up to inject 5 μL of each extract. The HILIC column (Phenomenex HILIC, 2.0 mm x 100 mm, 3 μ particles, Torrence, CA) equipped with a matching guard cartridge was used for compound separation using a linear gradient of (A) water + 50mM ammonium formate) and (B) acetonitrile, from 95% to 50% B over 13 min. The autosampler temperature was set at 10°C. Column flow during the first 1.5 min was diverted to waste. Gradient was set as follows: time 0 to 3 min=95% B, 3–23 min it was ramped to 50% B, 23.1 min it was returned to 95% B, and it was held at 95% B to re-equilibrate column until 32 min.
Lipopolysaccharide (LPS) Treatment
24 hours after administering FLVs-Rol, wild type C57BL/6J male mice were intraperitoneally (i.p.) injected with a single dose of sterile PBS or 5 mg/kg LPS (Escherichia coli 0111: B4, Difco, Detroit, MI) for 3 hours. Before use, LPS was dissolved in sterile, pyrogen-free water, sonicated, and diluted with sterile PBS. Sterile PBS was administered to 6 mice, which served as vehicle controls, 9 mice were used in LPS treatment group, and 9 mice were used in FLVs-Rol followed by LPS treatment group.
PDE4 Enzymatic Activity Assay
PDE4 activity of brain and liver tissues was analyzed as described by us previously.5,24 Briefly, 50 mg of tissues were lysed in 1 mL of lysis buffer containing 20 mM HEPES, 150 mM NaCl, 2 mM EDTA, 5% Glycerol and 1% NP-40 supplemented with protease and phosphatase inhibitors. PDE4 specific enzymatic activity was determined using PDE4 assay kit (FabGennix Inc. International, Frisco, TX).
Data Analysis and Statistics
Data are presented as the mean ± standard deviation (SD). Differences between means of treated and paired controls were compared using paired Student’s t-tests or Mann–Whitney Rank Sum tests. Multiple group analyses were preformed using One Way ANOVA followed by Tukey's post-hoc multiple comparison test. A P-value ≤ 0.05 was considered significant.
Results
Preparation of FLVs and FLVs-Rolipram
FLV nano-tracking analysis demonstrated that at a concentration of 12.5 mg/mL of lipid the particle mean size was 107±44.8 nm, the concentration of particles was 2.3×1013 per mL, and the zeta potential at 25°C was −67.47 mV. The EE%, or the amount of rolipram that was encapsulated by FLVs, was 76.02±2.54%.
Dose Response Studies
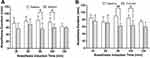
To determine whether the encapsulation of rolipram in FLVs would reduce the drug’s CNS mediated side-effects, experiments in a surrogate mouse model of emesis that quantifies the inhibitory effect of rolipram on shortening xylazine/ketamine anesthesia duration time were performed. The effect of three i.v. doses of encapsulated and non-encapsulated rolipram (1.6, 3.3 and 6.6 mg/kg) were compared. Within 15 min of the administration of anesthesia mice experience a loss of the righting reflex. One animal in the 1.6 mg/kg group had to be excluded due to technical problems during the tail vein injection. The results showed that free rolipram significantly shortened anesthesia duration for all three doses (Figure 1A). However, when rolipram was encapsulated in FLVs the anesthesia duration time for 1.6 and 3.3 mg/kg doses was not significantly reduced, but it was reduced for the 6.6 mg/kg dose (Figure 1B).
Safety Pharmacology Studies
To determine the onset and duration response of potential CNS adverse effects elicited by rolipram or FLVs-Rol, a time course study was performed using a 6.6 mg/kg i.v. dose followed by anesthesia induction at 35, 60, 80, 100, and 120 min. This high dose was selected because it was near the upper limits of rolipram solubility in water and near the maximum safe volume of PBS that may be injected as a bolus into 25–30 g mice (Organic solvents were not used to dissolve rolipram because they would disrupt FLV integrity). The results demonstrated that the mean baseline anesthesia duration for groups administered a high dose of rolipram was 77.9±4.1 min. One animal in the 100 min FLVs-Rol group had to be excluded due to technical problems during the tail vein injection. The onset of CNS side-effects occurred between 60 and 80 min of anesthesia induction and lasted less than 60 min (Figure 2A). Similarly, the mean baseline anesthesia duration for groups administered a high dose of FLVs-Rol was 83.2±7.7 min and the onset of CNS side effects were similar to those of free rolipram, lasting less than 60 min (Figure 2B). These findings suggest that a high dose of rolipram will elicit side-effects (whether encapsulated or not) starting ~1 h post-dosing and lasting less than an hour.
Tissue Rolipram and Rolipram Metabolite Levels
To gain a better understanding of the effect of delivering rolipram with and without FLVs on the distribution of drug in liver and brain, tissue levels of rolipram and its metabolites (HPR, DCR and HCR) at the time of harvest were quantified. The rolipram and metabolite liver-to-brain ratios were calculated and compared in mice receiving 3.3 mg/kg of rolipram in the dose response studies. In general, the ratios elicited by rolipram administration tended to be larger than those produced by FLVs-Rol, however, only the DCR and HCR metabolites were significantly different between the two modes of rolipram delivery (Figure 3).
Effect of FLVs-Rol on Brain and Liver PDE4 Activity
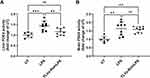
Our group showed that FLV delivery of rolipram was as effective as free rolipram in attenuating liver injury in a mouse model of ALD.10 Notably, a single dose of rolipram prevented alcohol mediated loss of hepatic cAMP levels demonstrating that FLVs-Rol successfully inhibited hepatic PDE4 activity.10 However, whether FLVs-Rol treatment affects PDE4 activity in the brain is unclear. The present observation that FLVs-Rol shortens anesthesia time indicates that FLV delivery of rolipram reduces the drug’s ability to cross the BBB to inhibit PDE4 activity in the brain. To confirm that FLVs-Rol inhibits liver PDE4 activity without affecting the brain, we measured LPS-inducible PDE4 activities in mice with and without FLVs-Rol treatments. We kept the FLVs-Rol dose the same as in our ALD study (3.3 mg/kg body weight). Mice were left untreated or treated with FLVs-Rol at 3.3 mg/kg dose 24 hours before administering LPS at a dose of 5 mg/kg body weight. Based on previous observations this dose of LPS significantly increases PDE4 expression in the brain.25 Indeed, LPS administration resulted in a marked increase in brain and liver PDE4 activities after 3 hours (Figure 4). FLVs-Rol treatment significantly decreased LPS-inducible PDE4 activity in the liver (Figure 4A). At the same time, the effect of FLVs-Rol was not significant in decreasing PDE4 activity in the brain (Figure 4B).
Discussion
The major finding of this study was that low and moderate doses of FLVs-Rol significantly prevented the shortening of anesthesia duration compared to free rolipram, suggesting that the levels of drug that crossed the BBB were not critically high enough to reverse α2A-adrenoceptor-mediated xylazine/ketamine anesthesia. However, safety pharmacology studies using a high dose of FLVs-Rol demonstrated that the onset and duration response for potential CNS adverse effects were not averted by FLV delivery. Lastly, analysis of tissue levels of rolipram and its metabolites demonstrated that administration of FLVs-Rol decreased the liver-to-brain ratios of drug uptake compared to free drug without altering the entry into the CNS at the time of tissue harvest. Importantly, moderate dose of FLVs-Rol was still able to decrease liver PDE4 activity 24 hours after administration without affecting the brain.
In the present study, equivalent i.v. doses of rolipram delivered free or as FLVs-Rol were compared to determine whether the presence of FLVs reduced the entry of rolipram into the CNS. The findings that the low and moderate doses of FLVs-Rol did not shorten the anesthesia duration time demonstrate that FLV delivery of rolipram was able to prevent a critical level of drug from crossing the BBB to mediate CNS side-effects. However, high dose FLVs-Rol had the same effect as free rolipram on anesthesia time, indicating that circulating levels of unencapsulated drug were sufficiently elevated to exceed the critical threshold level of drug in the CNS.
Our group has previously demonstrated that a moderate dose of FLVs-Rol attenuated hepatic injury and steatosis in a mouse model of ALD.10 Specifically, FLVs-Rol prevented the ethanol-mediated decrease in hepatic cAMP indicating that FLV delivery of rolipram elicited the intended therapeutic effect in liver.10 Analysis of liver and brain tissues in the present study demonstrated that a moderate dose of FLVs-Rol produced smaller liver-to-brain ratios of rolipram and its metabolites compared to administration of free rolipram (Figure 3). These findings suggest that FLVs modulated or delayed the rate of rolipram uptake by both tissues, perhaps by prolonging the circulation time of encapsulated drug or by altering the compartmental distribution of drug. A moderate dose of rolipram delivered with FLVs appears to have attenuated the rate of drug uptake by the brain and prevented critical threshold levels of drug from being reached, and thereby the anesthesia duration was not significantly reduced. It is important to note that levels of rolipram and its metabolites used to calculate the liver-to-brain ratios were measured from tissues that were harvested after mice had regained their righting reflex post-dosing. Thus, the critical levels of rolipram that would have affected anesthesia time were no longer present, and only higher levels of what appeared to be non-active rolipram metabolites remained. The safe delivery of rolipram with FLVs to prevent side effects appears to be only limited to the low and moderate doses, since in the pharmacology safety studies, the delivery of a high dose of FLVs-Rol did not prevent the shortening of time compared to administration of free rolipram.
The impetus for the development an FLV carrier system to ameliorate rolipram-induced side-effects was the ability of the drug to be hepatoprotective. This was based on our group’s previous work demonstrating the pathogenic role of PDE4 enzymes in the development of cholestatic liver injury and fibrosis and significant protection by the PDE4 specific inhibitor, rolipram.9 Several other studies have also shown that PDE4 inhibition is beneficial in various liver injury models, where it ameliorates inflammation, fibrogenesis and hepatocyte survival (reviewed in)4,26 Importantly, a significant upregulation of hepatic PDE4 expression in the livers of alcoholic patients was also demonstrated, indicating the clinical relevance of targeting hepatic PDE4.10 Indeed, alcohol induced liver injury was significantly attenuated by FLVs-Rol treatment. Our group also reported that FLVs labeled with near-infrared dye localized in the liver of mice.10 This finding along with the effect of FLVs-Rol on preventing an alcohol induced decrease in cAMP levels demonstrated that FLVs delivery of rolipram was effective in decreasing PDE4 activity in the liver.
Although the present study is based on the behavioral correlate rodent model of emesis developed by Robichaud et al,20 there were differences in anesthesia duration and study methods. The mean baseline xylazine/ketamine anesthesia duration reported by their group was ~22 min shorter than in our study. This discrepancy may be due, in part, to behavioral or phenotypic differences in the mice used in the studies. Although both studies used C57BL/6 wild type mice, it has been reported that inbred mice from different breeding colonies may develop substrains or polymorphisms that contribute to behavioral or phenotypic differences (eg, C57BL/6N vs C57BL/6J).27 In the current study, mice were purchased from Jackson Laboratory and were C57BL/6J. Robichaud’s group purchased their mice from Taconic, which usually are the N strain, C57BL/6N. Additionally, there were substantial differences in study methods regarding the type of compound used, dosing, route of administration, and time point of sample collection. The Robichaud group administered their compound subcutaneously a dose of 1 mg/kg of pure (R)- or (S)-rolipram,20 whereas in the present study, an i.v. dose of 3.3 mg/kg of a 50:50 racemic mixture of rolipram was administered. Their group measured brain and blood rolipram levels at 60 min post-dosing, and in the current work, brain and liver rolipram levels were measured at ~90 min post-dosing. Interestingly, Robichaud’s group reported that the tendency of rolipram to reduce the anesthesia period was correlated with the proportion of compound present in the brain relative to plasma.20 This finding could be explained by the passive diffusion of the drug out of the brain into the blood stream and/or due to metabolism within brain tissue. Additionally, their measurements were performed at ~23 min after the wake-up time (~83 min post drug administration). While, in the present study, brain rolipram levels were measured at ~20 min after the wake-up time (~110 min post drug administration). Results of our study suggest that FLVs attenuated the rate of uptake and the critical threshold levels of rolipram in the brain to interfere with anesthesia duration.
Conclusions
Administration of FLVs-Rol significantly and specifically reduced the duration of anesthesia induced by the xylazine/ketamine combination. FLVs-Rol appeared to reduce the rate of rolipram uptake by liver and brain tissue and prevented critical threshold levels of rolipram from crossing the BBB. FLVs-Rol appears to reduce CNS adverse effects in a behavioral correlate mouse model of rolipram-induced emesis. Importantly, FLVs-Rol had an inhibitory effect on the liver PDE4 activity without significantly affecting the brain. Taken together, our results demonstrate that this novel liposomal rolipram formulation reduces emesis and may be suitable for clinical application to treat liver injury.
Study Limitations
This is a behavioral correlate rodent model of emesis, and the study must be repeated in a large animal with an emetic reflex. Tissue samples represent a single time point post-dosing and were collected at the end of experiments with some variability in the timing. Therefore, depending on the modality of drug delivery, the information that tissue levels of rolipram and its metabolites provide is limited since temporal analysis is not possible. However, drug distribution into tissues was clearly different at the time of euthanasia, and we can only speculate that FLVs delayed the rate of rolipram uptake by brain, and thus prevented a shortening of anesthesia duration.
Acknowledgment
We thank Marion McClain for editing the manuscript. This work was supported by the following grants: R43AA021331, R44AA021331, 1R01AA029798-01.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure
Drs. Maldonado and Bauer declare a conflict of interest in employment and having equity in EndoProtech, Inc. The authors report no other conflicts of interest in this work.
References
1. Singal AK, Mathurin P. Diagnosis and treatment of alcohol-associated liver disease: a review. JAMA. 2021;326:165–176. doi:10.1001/jama.2021.7683
2. Makri E, Goulas A, Polyzos SA. Epidemiology, pathogenesis, diagnosis and emerging treatment of nonalcoholic fatty liver disease. Arch Med Res. 2021;52:25–37. doi:10.1016/j.arcmed.2020.11.010
3. Loomba R, Friedman SL, Shulman GI. Mechanisms and disease consequences of nonalcoholic fatty liver disease. Cell. 2021;184:2537–2564. doi:10.1016/j.cell.2021.04.015
4. Wahlang B, McClain C, Barve S, Gobejishvili L. Role of cAMP and phosphodiesterase signaling in liver health and disease. Cell Signal. 2018;49:105–115. doi:10.1016/j.cellsig.2018.06.005
5. Gobejishvili L, Barve S, Joshi-Barve S, McClain C. Enhanced PDE4B expression augments LPS-inducible TNF expression in ethanol-primed monocytes: relevance to alcoholic liver disease. Am J Physiol Gastrointest Liver Physiol. 2008;295:G718–724. doi:10.1152/ajpgi.90232.2008
6. Avila DV, Barker DF, Zhang J, McClain CJ, Barve S, Gobejishvili L. Dysregulation of hepatic cAMP levels via altered Pde4b expression plays a critical role in alcohol-induced steatosis. J Pathol. 2016;240:96–107. doi:10.1002/path.4760
7. Jin SL, Conti M. Induction of the cyclic nucleotide phosphodiesterase PDE4B is essential for LPS-activated TNF-alpha responses. Proc Natl Acad Sci U S A. 2002;99:7628–7633. doi:10.1073/pnas.122041599
8. Jin SL, Lan L, Zoudilova M, Conti M. Specific role of phosphodiesterase 4B in lipopolysaccharide-induced signaling in mouse macrophages. J Immunol. 2005;175:1523–1531. doi:10.4049/jimmunol.175.3.1523
9. Gobejishvili L, Barve S, Breitkopf-Heinlein K, et al. Rolipram attenuates bile duct ligation-induced liver injury in rats: a potential pathogenic role of PDE4. J Pharmacol Exp Ther. 2013;347:80–90. doi:10.1124/jpet.113.204933
10. Rodriguez WE, Wahlang B, Wang Y, et al. Phosphodiesterase 4 inhibition as a therapeutic target for alcoholic liver disease: from bedside to bench. Hepatology. 2019;70:1958–1971. doi:10.1002/hep.30761
11. Feng H, Chen J, Wang H, et al. Roflumilast reverses polymicrobial sepsis-induced liver damage by inhibiting inflammation in mice. Lab Invest. 2017;97:1008–1019. doi:10.1038/labinvest.2017.59
12. Essam RM, Ahmed LA, Abdelsalam RM, El-Khatib AS. Phosphodiestrase-1 and 4 inhibitors ameliorate liver fibrosis in rats: modulation of cAMP/CREB/TLR4 inflammatory and fibrogenic pathways. Life Sci. 2019;222:245–254. doi:10.1016/j.lfs.2019.03.014
13. Fleischhacker WW, Hinterhuber H, Bauer H, et al. A multicenter double-blind study of three different doses of the new cAMP-phosphodiesterase inhibitor rolipram in patients with major depressive disorder. Neuropsychobiology. 1992;26:59–64. doi:10.1159/000118897
14. Spina D. PDE4 inhibitors: current status. Br J Pharmacol. 2008;155:308–315. doi:10.1038/bjp.2008.307
15. Krause W, Kuhne G. Pharmacokinetics of rolipram in the rhesus and cynomolgus monkeys, the rat and the rabbit. Studies on species differences. Xenobiotica. 1988;18:561–571. doi:10.3109/00498258809041693
16. Nelissen E, van Goethem NP, Bonassoli VT, et al. Validation of the xylazine/ketamine anesthesia test as a predictor of the emetic potential of pharmacological compounds in rats. Neurosci Lett. 2019;699:41–46. doi:10.1016/j.neulet.2019.01.026
17. Robichaud A, Savoie C, Stamatiou PB, et al. Assessing the emetic potential of PDE4 inhibitors in rats. Br J Pharmacol. 2002;135:113–118. doi:10.1038/sj.bjp.0704457
18. Robichaud A, Savoie C, Stamatiou PB, Tattersall FD, Chan CC. PDE4 inhibitors induce emesis in ferrets via a noradrenergic pathway. Neuropharmacology. 2001;40:262–269. doi:10.1016/S0028-3908(00)00142-8
19. Robichaud A, Tattersall FD, Choudhury I, Rodger IW. Emesis induced by inhibitors of type IV cyclic nucleotide phosphodiesterase (PDE IV) in the ferret. Neuropharmacology. 1999;38:289–297. doi:10.1016/s0028-3908(98)00190-7
20. Robichaud A, Stamatiou PB, Jin SL, et al. Deletion of phosphodiesterase 4D in mice shortens alpha(2)-adrenoceptor-mediated anesthesia, a behavioral correlate of emesis. J Clin Invest. 2002;110:1045–1052. doi:10.1172/JCI15506
21. Goga L, Pushpakumar SB, Perez-Abadia G, et al. A novel liposome-based therapy to reduce complement-mediated injury in revascularized tissues. J Surg Res. 2011;165:e51–57. doi:10.1016/j.jss.2010.09.033
22. Cai X, Li R. Concurrent profiling of polar metabolites and lipids in human plasma using HILIC-FTMS. Sci Rep. 2016;6:36490. doi:10.1038/srep36490
23. Thevis M, Krug O, Schanzer W. Monitoring phosphodiesterase-4 inhibitors using liquid chromatography/(tandem) mass spectrometry in sports drug testing. Rapid Commun Mass Spectrom. 2013;27:993–1004. doi:10.1002/rcm.6539
24. Gobejishvili L, Avila DV, Barker DF, et al. S-adenosylmethionine decreases lipopolysaccharide-induced phosphodiesterase 4B2 and attenuates tumor necrosis factor expression via cAMP/protein kinase A pathway. J Pharmacol Exp Ther. 2011;337:433–443. doi:10.1124/jpet.110.174268
25. Avila DV, Myers SA, Zhang J, et al. Phosphodiesterase 4b expression plays a major role in alcohol-induced neuro-inflammation. Neuropharmacology. 2017;125:376–385. doi:10.1016/j.neuropharm.2017.08.011
26. Elnagdy M, Barve S, McClain C, Gobejishvili L. cAMP Signaling in pathobiology of alcohol associated liver disease. Biomolecules. 2020;10:1433. doi:10.3390/biom10101433
27. Bryant CD, Zhang NN, Sokoloff G, et al. Behavioral differences among C57BL/6 substrains: implications for transgenic and knockout studies. J Neurogenet. 2008;22:315–331. doi:10.1080/01677060802357388
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.