Back to Journals » Journal of Pain Research » Volume 17
Novel Implantation Technique for Thoracoabdominal Peripheral Nerve Stimulation via a Transversus Abdominal Plane Approach for Treatment of Chronic Abdominal Pain
Authors Lam CM , Keim SA , Sayed D , Abd-Elsayed A, Gulati A , Schatman ME , Deer T , Latif U
Received 27 November 2023
Accepted for publication 7 March 2024
Published 12 March 2024 Volume 2024:17 Pages 981—987
DOI https://doi.org/10.2147/JPR.S451955
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Professor Krishnan Chakravarthy
Christopher M Lam,1 Sarah A Keim,2 Dawood Sayed,1 Alaa Abd-Elsayed,3 Amitabh Gulati,4 Michael E Schatman,5,6 Timothy Deer,7 Usman Latif1
1Department of Anesthesiology, Pain and Perioperative Medicine, University of Kansas Medical Center, Kansas City, KS, USA; 2Department of Surgery, University of Kansas Medical Center, Kansas City, KS, USA; 3Department of Anesthesiology and Perioperative Medicine, University of Wisconsin, Madison, WI, USA; 4Department of Anesthesiology and Critical Care, Memorial Sloan Kettering Cancer Center, New York, NY, USA; 5Department of Anesthesiology, Perioperative Care, and Pain Medicine, NYU Grossman School of Medicine, New York, NY, USA; 6Department of Population Health – Division of Medical Ethics, NYU Grossman School of Medicine, New York, NY, USA; 7The Spine and Nerve Center of the Virginias, Charleston, WV, USA
Correspondence: Christopher M Lam, University of Kansas Medical Center, 3901 Rainbow Boulevard, Mail Stop 1034, Kansas City, KS, 66160, USA, Tel +1-(704)-488-5665, Fax +1-913-588-3365, Email [email protected]
Background: Chronic abdominal pain (CAP) is a common and challenging to treat condition with a global prevalence of up to 25%. Despite extensive evaluation, approximately 40% of patients with CAP have an unknown diagnosis. Medications may be ineffective, and surgery is rarely indicated. Interventional treatment including sympathetic blocks, sympathetic neurolysis, and transversus abdominal plane (TAP) blocks may be an option, but their efficacy can wane over time. Neuromodulation has emerged as an option for these patients, as there is evidence of success with dorsal column spinal cord and dorsal root ganglion (DRG) stimulation. Peripheral nerve stimulation (PNS) may be an alternative option, particularly in higher risk patients or in patients for whom neuraxial access may be unsafe or too technically challenging. Thoracoabdominal nerve peripheral nerve stimulation via a TAP approach may be more specifically targeted in comparison to dorsal column or DRG stimulation. In this short report, we detail a technique that the authors have successfully used for thoracoabdominal nerve PNS via a TAP approach for management of CAP.
Methods: This article describes a novel medial to lateral ultrasound guided thoracoabdominal nerve PNS via a TAP approach technique for lead placement and implantation.
Results: A medial to lateral ultrasound guided TAP approach as described to successfully implant percutaneous thoracoabdominal nerve PNS leads for management of CAP.
Conclusion: The thoracoabdominal nerve PNS via a TAP approach lead placement technique noted in this report has been used as a means for management of CAP utilizing peripheral neuromodulation. Here, we present a short report detailing a potential technique for PNS utilization for management of CAP. Further studies are needed to validate the safety and efficacy of this therapy modality, although the authors have found it to be a viable management option for patients with medically refractory neuropathic CAP.
Keywords: peripheral nerve stimulator, neuromodulation, neuropathic pain, ultrasound, thoracic nerves
Introduction
Chronic abdominal pain (CAP) is a diverse, pervasive pain condition affecting individuals globally, with an international prevalence estimated between 22% and 25%.1 Various diagnoses and etiologies are associated with CAP, including visceral disease, genetic conditions, spine diseases, abdominal wall conditions, cancer, and vascular disease.1,2 In the US alone, CAP constituted $10.2 billion dollars in healthcare expenditures attributed to diagnostic workups, emergency room visits, hospital admissions, and ambulatory visits.3 A 2018 United States national survey indicated that 34.5% of all responders reported abdominal pain lasting more than 1 day and required urgent medical attention, while 61.5% of all respondents had discussed their abdominal pain with a healthcare professional. Of those seeking health care, 72.4% had undergone testing and/or imaging to evaluate their pain.4 Despite exhaustive work ups, the diagnosis in up to 40% of patients remains unknown.1
Treatment of CAP is determined by the diagnosis and etiology. Disease state management optimization and surgical intervention in certain conditions may improve CAP. Medication management (oral analgesics, muscle relaxants, and antispasmodic medications) often results in inconsistent improvement.2 Interventional options have classically included sympathetic nerve blocks and/or neurolysis, intrathecal pump therapy, dorsal root ganglion (DRG) stimulation, and spinal cord stimulation (SCS).5 The transversus abdominis plane (TAP) block has been popularized for the management of CAP.6–9 Indicated conditions have varied from chronic post-operative abdominal pain, anterior cutaneous entrapment syndrome, and chronic neuropathic abdominal pain. Although effective, there exists a patient population for whom repeated blocks are not desired or the efficacy of TAP blocks is short lived, and a more permanent treatment approach, such as neuromodulation, is desired.
DRG or SCS may not be feasible for some patients due to anatomy or coexisting disease. In such patient populations, peripheral nerve stimulation (PNS) may represent an option. In this report, we detail a novel medial to lateral percutaneous thoracoabdominal nerve PNS via a TAP approach technique for implantation for neuropathic (non-visceral or gynecologic) CAP management, based on our real-world experience, with discussion of relevant anatomic considerations from cadaveric studies.
Methods
This technical guide describes a novel medial to lateral ultrasound guided thoracoabdominal nerve PNS via a TAP approach implantation technique for CAP. The Nalu Medical PNS system (Nalu Medical, Carlsbad, CA) platform was used. Written consent to publish the photographs in this manuscript was provided by the patient. IRB formal review was waived by the University of Kansas Medical Center IRB as this is a technical paper detailing technique and no patients or patient data were involved. Therefore, the study was deemed not to constitute human subject research.
Results
Anatomic Description
The abdominal anterior cutaneous nerves are branches from the seventh to eleventh intercostal, subcostal (twelfth thoracic), and first lumbar nerves.10 The anterior (ventral) rami of the seventh through twelfth intercostal nerves travel in their respective intercostal and subcostal spaces and into the abdominal wall. The first lumbar nerve enters the anterior abdominal wall at the anterior superior iliac spine (ASIS).11 Once in the abdominal wall, the nerves travel in the TAP fascia located between the transversus abdominis and the internal oblique muscles (Figure 1). The fascial layer is not adherent to the internal oblique, and neurovascular structures are located on its deep surface adjacent to the transversus abdominis muscle. This fascia extends medially to the linea semilunaris.11 In the TAP, the thoracoabdominal nerves branch and communicate with at least two other nerves including branches from other T9-L1 segmental nerves.11 The nerves reform and traverse the linea semilunaris to enter the rectus sheath posterior to the rectus abdominus. They form a second plexus before suppling the rectus abdominis and passing through the anterior rectus sheath as the anterior cutaneous nerves, supplying the skin.10
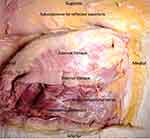 |
Figure 1 Cadaveric dissection revealing the nerves in the TAP. |
Description of Technique
Prior to trial and implant, the patient is monitored in compliance with the American Society of Anesthesiologist sedation and anesthesia guidelines. To facilitate intraoperative testing, monitored anesthesia care is suggested for implantation.
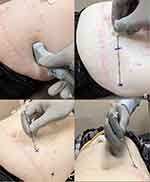
The patient is positioned supine prior to being prepped and draped in usual fashion. Peri-operative antibiotics are administered. Utilizing a sterilely covered ultrasound probe, the underlying abdominal wall structures from the midclavicular to midaxillary region are visualized over the region where the patient reports their pain (Figure 2A). Skin overlying the planned lateral-most aspect for lead placement is marked, and the blunt Tuohy needle is placed flat in a lateral to medial fashion on the skin to determine the planned trajectory. Once positioned, the spot ¼ of the needle length lateral from the medial end of the needle is marked as the starting access point (Figure 2B). The overlying skin and muscle trajectory is infiltrated with local anesthetic, with care not to introduce local anesthetic into the target fascial layer. For implantation, a sub-centimeter incision is made at the starting access point prior to needle placement. The needle is inserted (Figure 2C and D) and advanced medially to laterally under ultrasound guidance until the needle is under the internal oblique and on top of the fascial plane (Figure 3). The needle is flattened out with bevel up and gradually walked or slid under the internal oblique, superficial to the fascia rather than puncturing it as seen in traditional TAP blocks, until the planned lateral-most point is reached. A non-tined PNS lead is advanced under ultrasound guidance, and the needle is withdrawn partially to allow for intra-operative testing. Typical initial programming parameters include 80-Hz frequency, 400 μs pulse width, and 1500–3000 mA amplitude with subsequent customization to meet patient needs. Once paresthesia based dermatomal thoracoabdominal nerve stimulation is confirmed, the entire needle and stylet are removed. If incomplete coverage with single lead placement for unilateral abdominal pain occurs, a second lead can be placed to provide additional coverage. Alternately, a single lead can be inserted somewhat diagonally in order to achieve larger cephalad to caudad coverage with the 8 contacts. For bilateral lead placement, the process is repeated on the opposite side. For the trial, the leads are subsequently secured to the skin prior to application of sterile dressing.
 |
Figure 3 Ultrasound image of needle access of the fascia underlying the internal oblique and the fascial layers of the internal oblique and transverse abdominis muscles. |
For the implant, the lead is secured to the fascia with an anchor and sutures. The leads are subsequently tunneled subcutaneously to the microimplantable pulse generator (IPG) implant site located lateral to the midline of the epigastrium. This area is preferred as it is generally more rigid and less likely to result in flipping or migration of the micro IPG with fluctuations in weight. The leads are then attached to the micro IPG, and impedances checked again before the micro IPG is secured under the skin. If all is satisfactory, the skin is closed in the usual 2-layer fashion prior to application of sterile dressings.
Discussion
SCS and DRG stimulation have had some success in managing CAP, although much of the supportive literature is from case series.5 Factors may preclude patients from these interventions, including prohibitive anatomy for neuraxial access or concomitant use of anticoagulants/antiplatelet medications. PNS may carry a lower risk compared to neuraxial stimulation (SCS or DRG) given the placement of non-neuraxial leads and ultrasound visualization allowing for procedural planning in order to avoid other neurovascular structures. Furthermore, thoracoabdominal nerve PNS via a TAP approach may allow for more specific targeting of the painful area than does neuraxial neuromodulation. Given the success of TAP and other nerve blocks in the management of CAP, thoracoabdominal nerve PNS via a TAP approach may represent an option for long-term management of CAP.
Our approach accounts for the vasculature of the anterior abdominal wall. The superficial inferior epigastric artery (SIEA) and superficial circumflex iliac artery (SCIA) are located both superficial and deep to Scarpa’s fascia.12 The SIEA ascends laterally to the linea semilunaris and 40.4 mm medial to the ASIS, and usually terminates inferior to the umbilicus.12 The SCIA travels toward the ASIS, with median distances of 13.9 mm.12 Thus, use of ultrasound and the location of the needle placement is likely to avoid these structures (Figure 4A and B, Figure 5). This approach was selected to coincide with the commonly utilized approach for TAP peripheral nerve block to allow for ease of visualization and safety while allowing for capture of nerve stimulation through intraoperative testing.
 |
Figure 4 Artist rendition of relevant anatomy prior to lead placement (A) and after lead placement (B). |
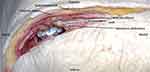 |
Figure 5 Cadaveric dissection revealing lead location after percutaneous lead place. |
The deep circumflex iliac artery (DCIA) branches from the external iliac artery and travels posterior to the inguinal ligament toward ASIS. The ascending branch of DCIA branches 2.69 cm from the origin and ascends in the TAP on the deep side of the fascia in close proximity to the thoracoabdominal nerves.11 Since needle placement is deep to the internal oblique, but superficial to the fascia plane, this artery and the segmental nerves remain undamaged.
The utilization of an 8 contact non-tined lead for medial to lateral placement has several theoretical benefits. The nerves in the TAP are difficult to visualize, and often individual nerves are not clearly identified. Placing leads in a fashion parallel to the fascial plane allows for greater success in capturing the target nerves. This provides the ability to test various combinations of contact usage for stimulation coverage intraoperatively to ensure optimal lead placement. Furthermore, by being located between the muscles, the chance for lead migration is theoretically lower given that the lead itself is not in the muscle belly. The lead being superficial to the fascial layer rather than within it also reduces the likelihood of internal migration.
Though the recommended trajectory is from a medial to lateral approach, it is feasible to place the lead in a more caudal to cephalad orientation if the pain presents primarily in the lower abdominal quadrants. By starting from a caudal to cephalad orientation below the umbilicus with a medial to lateral approach, it is possible to capture the iliohypogastric nerve and ilioinguinal nerve as it pierces from the transverse abdominis through the internal oblique. With lead placement in this position, it potentially would allow for stimulation of all 3 nerves to provide a more targeted treatment of lower abdominal quadrant pain. Important considerations for this approach would include being proficient with visualizing the intended fascial plane with the ultrasound probe in a perpendicular orientation, adjusting for changes in thickness of layers as the lead traverses caudally, and being aware of the potential increased risk of lead migration and pistoning in this orientation with flexion and extension of the abdomen during the course of normal movement and physical activity.
A non-tined lead would theoretically allow for easier revisions if revisions are needed, without extensive abdominal wall dissection secondary to scar tissue development. This is particularly pertinent in the subset of CAP patients who have thinning of the muscles in the abdominal wall secondary to surgery or nutritional issues related to their gastrointestinal pathology. An additional consideration is that current tined leads are mostly four contact leads, decreasing potential nerve catchment with stimulation. A tined lead could be considered for use with the potential benefit of greater lead securement with less incidence of lead migration though it would make lead revisions more challenging. However, this would be a risk benefit decision individualized per patient made by the implanting physician.
CAP is a common and difficult to treat entity that affects many individuals globally. Treatment depends on the diagnosis, and often the etiology of the pain cannot be defined. Even with a diagnosis and optimal management of the disease state, there may not be an effective treatment for the pain. When conservative medical management fails, interventions may be an option to address the patient’s pain. However, the efficacy of these interventions varies and may carry a risk profile that may preclude their use in some patients. In these patient populations with neuropathic characteristics, thoracoabdominal nerve peripheral neuromodulation via a TAP approach may represent an option.
Conclusions
Thoracoabdominal nerves have been targeted with great effect for the management of CAP and may provide long-term success in managing CAP in select patients. Currently, there are no available published guides or reports regarding percutaneous placement of thoracoabdominal nerve PNS leads via a TAP approach for the management of CAP. To our knowledge, this is the initial manuscript to propose a methodology and document the implantation technique for thoracoabdominal nerve PNS via a TAP approach. Thus, this guide is limited by the number of real-world cases for this technique. The approach noted within allows for secure access to avoid neurovascular structures by adopting a technique already currently used for TAP nerve blocks, thus increasing the safety for PNS lead placement. Empirical investigation is needed to determine the long-term efficacy and safety of this technique, and we are confident that it may provide an option for treatment for this challenging chronic condition.
Acknowledgment
Nalu Medical paid the artist used for our illustration for this study.
Funding
Nalu Medical provided an unrestricted grant for funding for the artist anatomic drawings used within.
Disclosure
Dr Dawood Sayed reports stock options from SPR, Painteq, Surgentec, Neuralace, Vertos; personal fees from Abbott, Saluda, outside the submitted work. Dr Alaa Abd-Elsayed reports consultant for Curonix. Dr Amitabh Gulati reports consulting for Medtronic, AIS HealthCare, Neurovasis, SPR Therapeutics, Tersera Medical, Hinge Health, Nalu Medical, Bausch health, outside the submitted work. Dr Michael E Schatman is a research consultant for Modoscript and advisory committee for Syneos Health, outside the submitted work. Dr Timothy Deer reports personal fees for consultant, Advisory Board, Research from SPR Therapeutics, during the conduct of the study; personal fees for consultant, advisory board, and/or research from Abbott, Vertos, SpineThera, Saluda, Mainstay, Cornerloc, Boston Scientific, PainTeq, Spinal Simplicity, and Biotronik, outside the submitted work. In addition, Dr Timothy Deer has a patent pending to Abbott. Dr Usman Latif reports personal fees from Nalu Medical, SPR Therapeutics, Nevro, Vertos Medical, Omnia Medical, Hydrocision, InFormed Consent, Spinal Simplicity; grants from Mainstay Medical, outside the submitted work. The authors report no other conflicts of interest in this work.
References
1. Sabo CM, Grad S, Dumitrascu DL. Chronic abdominal pain in general practice. Dig Dis. 2021;39(6):606–614. doi:10.1159/000515433
2. Shian B, Larson ST. Abdominal wall pain: clinical evaluation, differential diagnosis, and treatment. Am Fam Physician. 2018;98(7):429–436.
3. Peery AF, Crockett SD, Murphy CC, et al. Burden and cost of gastrointestinal, liver, and pancreatic diseases in the United States: update 2018. Gastroenterology. 2019;156(1):254–272 e211. doi:10.1053/j.gastro.2018.08.063
4. Lakhoo K, Almario CV, Khalil C, Spiegel BMR. Prevalence and characteristics of abdominal pain in the United States. Clin Gastroenterol Hepatol. 2021;19(9):1864–1872 e1865. doi:10.1016/j.cgh.2020.06.065
5. Wie C, Ghanavatian S, Pew S, et al. Interventional treatment modalities for chronic abdominal and pelvic visceral pain. Curr Pain Headache Rep. 2022;26(9):683–691. doi:10.1007/s11916-022-01072-4
6. Nizamuddin SL, Koury KM, Lau ME, Watt LD, Gulur P. Use of targeted transversus abdominus plane blocks in pediatric patients with anterior cutaneous nerve entrapment syndrome. Pain Physician. 2014;17(5):E623–627.
7. Sellam S, Nguyen AT, Pogu M, et al. Transversus abdominis plane block in the treatment of chronic postsurgical abdominal wall pain improves patient quality of life: a retrospective study and literature review. Pain Physician. 2023;26(2):E91–E100.
8. Abd-Elsayed A, Luo S, Falls C. Transversus abdominis plane block as a treatment modality for chronic abdominal pain. Pain Physician. 2020;23(4):405–412. doi:10.36076/ppj.2020/23/405
9. Abd-Elsayed A, Malyuk D. Efficacy of transversus abdominis plane steroid injection for treating chronic abdominal pain. Pain Pract. 2018;18(1):48–52. doi:10.1111/papr.12580
10. Standring S. Gray’s Anatomy: The Anatomical Basis of Clinical Practice. Edinburgh: Churchill Livingstone Elsevier; 2021.
11. Rozen WM, Tran TM, Ashton MW, et al. Refining the course of the thoracolumbar nerves: a new understanding of the innervation of the anterior abdominal wall. Clin Anat. 2008;21(4):325–333. doi:10.1002/ca.20621
12. Fuse Y, Yoshimatsu H, Karakawa R, Yano T. Novel classification of the branching patterns of the superficial branch and the deep branch of the superficial circumflex iliac artery and the superficial inferior epigastric artery on computed tomographic angiography. J Reconstr Microsurg. 2022;38(4):335–342. doi:10.1055/s-0041-1733976
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.