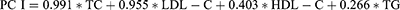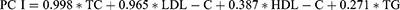Back to Journals » Diabetes, Metabolic Syndrome and Obesity » Volume 16
Novel Associations of Dyslipidaemia with Vitamin D and Bone Metabolism in Elderly Patients with Diabetes: A Cross-Sectional Study
Authors Zha K, Wang N, Zhou Y, Ying R, Gu T, Zhao Y, Guo H, An Z, Lu Y
Received 28 May 2023
Accepted for publication 4 September 2023
Published 22 September 2023 Volume 2023:16 Pages 2939—2950
DOI https://doi.org/10.2147/DMSO.S423287
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Prof. Dr. Antonio Brunetti
Kexi Zha, Ningjian Wang, Ying Zhou, Rong Ying, Tao Gu, Yan Zhao, Hui Guo, Zengmei An, Yingli Lu
Department of Endocrinology and Metabolism, Huangpu Branch of Shanghai Ninth People’s Hospital, Shanghai, People’s Republic of China
Correspondence: Yingli Lu; Zengmei An, Department of Endocrinology and Metabolism, Huangpu Branch of Shanghai Ninth People’s Hospital, Shanghai, 200011, People’s Republic of China, Tel +86-13636352507 ; +86-15921537791, Fax +86-21-63136856, Email [email protected]; [email protected]
Objective: Little is known about whether diabetic dyslipidaemia contributes to increased bone fragility in patients with diabetes. This study aimed to explore the potential effects of dyslipidaemia on vitamin D and bone metabolism in elderly subjects with type 2 diabetes (T2D).
Methods: A total of 1479 male patients and 1356 female patients 50 years or older with T2D were included in Shanghai, China. Lipid profiles, 25-hydroxyvitamin D (25(OH)D), serum procollagen type I N-terminal propeptide (P1NP), β-C-terminal telopeptide (β-CTX) and other parameters were measured. Principal component regression (PCR) and mediation analysis were used to estimate the associations of lipid profile, 25(OH)D and bone turnover levels.
Results: Female patients presented with higher blood lipids, lower 25(OH)D, and higher P1NP and β-CTX levels than male patients with T2D. TC was associated with P1NP in males and females (β=0.056, P< 0.05; β=0.095, P< 0.01, respectively), and 25(OH)D fully mediated the associations in males and mediated approximately 17.89% of the effects in females. LDL-C was associated with P1NP in males and females (β=0.072 and 0.105 respectively, all P< 0.01), and 25(OH)D mediated the relationships approximately 20.83% in males and 14.29% in females. TG was negatively associated with P1NP (in males, β= − 0.063, P< 0.05; in females, β= − 0.100, P< 0.01) and β-CTX (in males, β= − 0.108; in females, β= − 0.128, all P< 0.01) independent of 25(OH)D, while HDL-C was not associated with P1NP or β-CTX in diabetic patients.
Conclusion: Hypercholesterolemia and hypertriglyceridaemia might affect bone metabolism by distinguishing pathways in diabetes patients. Ameliorating lipid control in elderly diabetes patients, especially female patients, will benefit both vitamin D and bone metabolism.
Plain Language Summary: Diabetic dyslipidaemia may be one of the factors involved in increased bone fragility in diabetes. Currently, there have been few studies regarding the effects of dyslipidaemia on vitamin D and bone metabolism in diabetes. We aimed to explore the potential effects of dyslipidaemia on vitamin D and bone metabolism in elderly subjects with T2D. Thus, we conducted a cross-sectional study of 2835 adult T2D patients 50 years or older in Shanghai, China. Our study revealed that high levels of cholesterol and triglycerides might have detrimental effects on vitamin D metabolism and bone turnover by distinguishing pathways in elderly patients with T2D, and discrepancies exist between male and female patients. Ameliorating lipid control in elderly diabetes patients, especially female patients, will benefit both vitamin D and bone metabolism.
Keywords: type 2 diabetes, elderly patients, lipid profile, vitamin D, bone turnover markers
Introduction
Type 2 diabetes mellitus (T2D) is a chronic metabolic disease and a powerful risk factor for the development of atherosclerotic cardiovascular disease (ASCVD).1 Furthermore, diabetes is well known to affect bone health and contributes to a heightened risk of fracture.2 Clinical studies have linked osteoporosis with atherosclerotic vascular diseases,3,4 suggesting the possibility of a common pathogenetic factor underlying these diseases. Hypercholesterolemia or dyslipidaemia has been proposed as one of the most likely candidates.5 Currently, little is known about the precise nature of the potential relationship between hypercholesterolemia and osteoporosis. In particular, few studies have examined the probable influences of hypertriglyceridaemia, which is more common in T2D,6,7 on bone metabolism. Recently Cai et al8 reported that high-fat-diets (HFD) resulted in elevated blood lipids, increased adipose differentiation at the bone marrow level, and delayed bone regeneration in rats.
Vitamin D is important for the maintenance of good bone and skeletal health as well as for calcium homeostasis.9 The prevalence of vitamin D deficiency is higher in T2D and hyperlipidaemia patients than in people without those conditions.10,11 One retrospective analysis showed that almost all T2D patients had vitamin D insufficiency or deficiency, and high levels of triglycerides (TG) and LDL-C increased the risk of vitamin D deficiency in T2D patients.12 Animal model studies also found that HFD-fed mice showed elevated serum concentrations of cholesterol, TG, fatty acids, glucose and insulin and lower serum 25(OH)D3 and 1,25(OH)2D3 sequentially.13 Therefore, it is plausible that vitamin D dysmetabolism may mediate the associations between dyslipidaemia and aberrant bone metabolism in patients with T2D.
To the best of our knowledge, there have been few studies regarding the associations among diabetic dyslipidaemia, vitamin D dysmetabolism and aberrant bone metabolism or bone turnover in T2D patients thus far. In addition, differences in lipid profiles exist between male and female subjects;14 hence, the effects of blood lipids on vitamin D and bone metabolism may differ to a certain extent between male and female patients with diabetes. Thus, we conducted a cross-sectional study of 2835 adult T2D patients 50 years or older in Shanghai, China. We attempted herein to elucidate the following hypothesis: hypercholesterolemia or dyslipidaemia may contribute to abnormal bone turnover in patients with T2D, and the development of vitamin D deficiency might play a novel mediation role between dyslipidaemia and aberrant bone turnover.
Materials and Methods
Study Population
A cross-sectional study, named the METAL study (Environmental Pollutant Exposure and Metabolic Diseases in Shanghai, ChiCTR1800017573, www.chictr.org.cn), was conducted from April to June 2018. Participants were from seven communities in the Pudong and Huangpu districts, Shanghai, China. More detailed sampling information was described in previous articles.15 A total of 5827 people participated in the investigation (2563 males and 3264 females). To accurately investigate the associations among lipid profiles, vitamin D status, and bone turnover levels in patients with T2D, patients were excluded if they (1) were younger than 50 years old; (2) had endocrine disorders such as type 1 diabetes, Cushing’s syndrome, hyperthyroidism, hypothyroidism, primary hyperparathyroidism, or hypogonadism; (3) had other diseases, including serious chronic liver, renal (eGFR<60 mL•min−1•1.73 m−2), or lung disease; rheumatoid arthritis; malignancy; or serious cerebrovascular disease (4) received medications affecting bone metabolism (eg, oestrogen, vitamin D, alfacalcidol, calcium, calcitonin, bisphosphonates, glucocorticoids, thiazolidinedione, SGLT-2 inhibitor) for more than 6 months or within the previous 12 months; (5) had a fracture history within the previous 12 months; and (6) had incomplete laboratory or questionnaire data. Finally, 2835 patients with T2D were included, comprising 1479 men and 1356 women (Supplemental Figure 1). Informed consent was obtained from all participants included in the study.
Biochemical and Anthropometric Measurements
A questionnaire was adopted to assess sociodemographic characteristics, medical history, family history, and lifestyle factors during the interview. A well trained and experienced staff conducted the interviews and clinical examinations, which included participant weight, height and blood pressure according to a standard protocol.16 Blood samples were obtained between 6:00 and 9:00 a.m. from fasting participants. The blood samples were refrigerated immediately after being collected. It was then centrifuged in two hours and the serum was aliquoted and frozen in a central laboratory.
Plasma glucose and lipid profiles, including total cholesterol (TC), TG, high-density lipoprotein cholesterol (HDL-C), and low-density lipoprotein cholesterol (LDL-C), were performed using Beckman Coulter AU680 (Brea, USA). Glycated hemoglobin (HbA1c) was measured using high-performance liquid chromatography (MQ-2000PT, China). C peptide was measured using chemiluminescence equipment (Abbott i2000 SR, USA). Bone turnover markers (BTMs) including Procollagen type I N-terminal propeptide (P1NP) and β-C-terminal telopeptide of type I collagen (β-CTX) were measured using a chemiluminescence instrument (Roche E602, Switzerland). The 25-hydroxyvitamin D [25(OH)D] was detected using a chemiluminescence assay (ADVIA Centaur XP, Siemens, Germany).
Definition of Variables
Body mass index (BMI) was defined as weight in kilograms divided by height in metres squared. An updated homeostasis model (HOMA2 IR) was used to estimate insulin resistance using C peptide, which can be calculated by the HOMA2 Calculator (http://www.dtu.ox.ac.uk/homacalculator). The estimated glomerular filtration rate (eGFR) was calculated according to the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) equation for “Asian origin”.17 Diabetes was defined as having a previous diagnosis by health care professionals, fasting plasma glucose (FPG) of 7.0 mmol/L or higher, or HbA1c of 6.5% or higher.
Statistical Analysis
Data analyses were performed using IBM SPSS Statistics 19 (IBM Corporation, Armonk, NY, USA). Normally distributed variables are summarized as the mean ± SD, whereas skewed-distribution variables are presented as the median (first to third quartiles). Group differences were compared with analysis of variance (ANOVA) for normally distributed variables, while the nonparametric Mann‒Whitney U-test was performed for skewed parameters. Categorical variables presented as numbers and proportions were compared using the chi-square test. p < 0.05 (2-tailed) indicated statistical significance.
Spearman’s bivariate correlation tests were employed to study the associations between 25(OH)D and anthropometric indices, FPG, HbA1c, serum biochemical parameters, BTMs (P1NP and β-CTX), HOMA2-IR, free triiodothyronine (FT3), free thyroxine (FT4), thyrotropin (TSH), estradiol (E2), total testosterone (T), follicle-stimulating hormone (FSH), luteinizing hormone (LH), use of statins, current smoking status and milk intake. Principal component analysis (PCA) of the blood lipid profile (TC, HDL-C, LDL-C and TG) was conducted to create continuous variables representing individual lipid levels. An eigenvalue ≥ 1 was considered as a cut-off for factor retention. Variables showing significant relationships with 25(OH)D or P1NP/β-CTX in Spearman correlations were used as independent variables in the principal component regression (PCR) models to test the combined effect of these independent factors on the dependent variable 25(OH)D or P1NP/β-CTX. The concentrations of 25(OH)D, P1NP and β-CTX were log transformed to achieve a normal distribution if needed in the analyses.
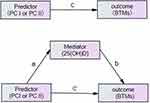
Mediation analysis was performed to estimate whether 25(OH)D mediated the association of lipid profile with BTMs and then was assessed individually to ascertain which component of the lipid profile was affected. The mediation analysis was conducted using SPSS statistics AMOS 24 in a bootstrap approach. We used 5000 bootstrap samples in the current study and determined the mediating effect of the 95% confidence interval (CI). In this study, the principal component I (PC I) and principal component II (PC II), which represent the lipid profile, as well as TC, LDL-C, HDL-C and TG, were separately used as independent variables. BTMs were regarded as the outcome, and 25(OH)D was used as a mediating variable. We predicted that the lipid profile affects 25(OH)D, with 25(OH)D acting as a mediator variable and affects BTMs further. We adjusted for confounders of age, BMI, HbA1c, HOMA2-IR, E2 or total T, FSH, eGFR, use of statins, smoking status and milk intake.
Results
General Characteristics of Participants
The general demographic and laboratory characteristics of the participants are presented in Table 1. In total, this study enrolled 2835 subjects with type 2 diabetes mellitus. Among them, 1479 (52.2%) were males, and 1356 (47.8%) were females. The mean age was comparable between the male and female groups. Compared with the male group, the levels of BMI, HbA1c, FT3, FT4, E2, total T and 25(OH)D were lower, while total cholesterol, TG, HDL-C, LDL-C, eGFR, TSH, FSH, LH levels were significantly higher in the female group with T2D. Strikingly, 25(OH)D levels were lower, while both P1NP and β-CTX levels were significantly higher in the female group than in the male group with T2D (all p<0.001).
 |
Table 1 General Characteristics of Elderly Male and Female Patients with T2D |
Associations of Serum 25(OH)D with Lipid Profiles and Bone Turnover Markers in Subjects with T2D
The simple correlations between serum 25(OH)D levels and other anthropometric and serum biochemical indices, the use of statins, current smoking status and milk intake were analysed. In male patients with T2D, serum 25(OH)D was positively associated with milk intake and FT3 (r=0.053 and 0.094, p=0.040 and <0.001, respectively) and was inversely associated with age, HbA1c, TC, TG, HDL-C, LDL-C, P1NP, β-CTX and current smoking (r= −0.053 to −0.186, all p=0.040 -<0.001). The correlation coefficient between 25(OH)D and TC was the highest (r= −0.186). In the female group, serum 25(OH)D was positively associated with eGFR, FT3, and FT4 (r=0.061 to 0.096, p=0.025 - <0.001), but was negatively associated with age, HOMA2-IR, TC, TG, LDL-C, P1NP, and β-CTX (r= −0.061 to −0.169, all p=0.025-<0.001) (Figure 1, Supplemental Figure 2). Serum 25(OH)D was not correlated with BMI in either male or female participants with T2D (r=−0.020 and −0.049, p=0.444 and 0.072, respectively).
Comparisons of Lipid Levels in All Patients with T2D with Different Levels of BTMs
To explore the potential effects of serum lipids on bone turnover, we first compared serum TC, LDL-C, HDL-C and TG levels according to P1NP and β-CTX quartiles in all patients. It was shown that with the increase in P1NP from the lowest to the highest quartiles, there was a steady increase in TC, LDL-C and HDL-C but a steady decrease in TG (p for trend from 0.021 to <0.001). When all covariates, such as age, sex, BMI and HbA1c, were considered, the differences for TC, LDL-C and TG persisted (p = 0.027 to 0.001); however, the difference for HDL-C was lost (p = 0.145).
Similar results were observed in β-CTX quartiles, with the highest TC, LDL-C and HDL-C, but the lowest TG levels were observed in subjects in the highest β-CTX quartile (p for trend from 0.005 to <0.001). Only the difference in TG persisted when such covariates were considered (p = 0.006) (Supplemental Figure 3).
Factors Determining the Levels of 25(OH)D and Bone Turnover Markers in Subjects with T2D
PCA of the blood lipid profile (TC, HDL-C, LDL-C and TG) was conducted, and generated two continuous variables: PC I, characterized by TC and LDL-C; PC II, characterized by TG and HDL-C. The cumulative contribution rate accounted for 86.43% of the total variance for males and 88.92% for females.
Multivariate PCR analyses were performed to assess the association between serum 25(OH)D concentration (the dependent variable) and lipid parameters (the independent variable). Our data showed that PC I had an inverse association with serum levels of 25(OH)D in both male and female patients with T2D after adjusting for age, BMI, HbA1c, eGFR, HOMA2-IR, milk intake and current smoking status (Model I, all p<0.001). Further adjustment for the use of statins did not obviously attenuate the association (Model II, all p<0.001). PC II was also negatively associated with serum levels of 25(OH)D in male participants after adjusting for confounding variables (p=0.038 and 0.027, respectively), while it was not significantly associated with 25(OH)D in female patients (Table S1).
To investigate whether serum lipid profiles and 25(OH)D levels were independently related to serum BTMs (P1NP and β-CTX), multivariate PCR analyses using BTMs as the dependent variable were performed, and the results are shown in Table 2. In summary, PC II was negatively correlated with Lnβ-CTX in male patients with T2D (p=0.003). In female patients with T2D, PC I was positively correlated with LnP1NP but not Lnβ-CTX (p=0.015 and 0.821). Meanwhile, PC II was negatively correlated with both LnP1NP and Lnβ-CTX (p=0.013 and 0.006). In addition, serum 25(OH)D levels were negatively correlated with both LnP1NP and Lnβ-CTX in male and female participants with T2D (all p<0.05).
 |
Table 2 Principal Component Regression Analysis of Serum Bone Turnover Markers as Dependent Variables and Parameters of Lipid Profile and 25(OH)D in Subjects with T2D |
Mediation Analysis
We developed a path diagram to study the associations between lipid profiles, 25(OH)D and BTMs in subjects with T2D (Figure 2). The mediation analysis demonstrated that 25(OH)D was a potential mediator of the association between PC I and BTMs (P1NP and β-CTX) in males and females. In brief, PC I had effects on P1NP through 25(OH)D in both male and female patients. 25(OH)D mediated approximately 33.33% and 18.28% of the effects between PC I and P1NP in males and females, respectively. As the direct effects between PC I and β-CTX were not significant after adjusting for confounding factors, 25(OH)D had a complete mediation effect on the associations between PC I and β-CTX in both males and females. In contrast, PC II was negatively associated with P1NP or β-CTX in both male and female patients, independent of 25(OH)D (Figure 3).
Four lipid components (including TC, TG, HDL-C, and LDL-C) may have effects on P1NP or β-CTX directly or through 25(OH)D, and the results are shown in Table S2. 25(OH)D fully mediated the relationship between TC and P1NP in males and mediated approximately 17.89% of the effects of TC on P1NP in females. 25(OH)D mediated the relationships between LDL-C and P1NP, and the proportions explained were 20.83% in males and 14.29% in females. TG was directly negatively associated with P1NP or β-CTX in both male and female patients, while HDL-C was not associated with P1NP or β-CTX in diabetic patients.
Discussion
The pathophysiological mechanisms underlying bone fragility in diabetes mellitus are complex. Currently, there is scarce information in the literature regarding the effects of dyslipidaemia on vitamin D and bone metabolism in diabetes. In the present study, we found that female elderly patients with T2D exhibited different blood lipid, vitamin D and bone turnover profiles than male elderly patients with T2D. In addition, hypercholesterolemia and hypertriglyceridaemia may influence bone metabolism through distinct pathways in diabetes patients.
Previous studies have declared that lipid levels are higher in women than in men. Our results were in accordance with the findings. Liu et al18 reported that TC, TG, and LDL-C levels were higher at ages greater than 50 years, while HDL-C levels were higher over the entire age range in women than men in a total of 39,207 participants aged 18–79 years in a rural Chinese population. Russo et al19 reported that women with T2D had significantly higher mean TC, LDL-C and HDL-C levels than men, while serum TG levels were not significantly different between groups in a total of 415,294 patients with T2D. The underlying physiological mechanisms contributing to the sex discrepancies in lipid metabolism are complicated and remain to be fully elucidated. Gonadal hormones and sex chromosome complement might be two major determinants; other factors may include X chromosome imprinting and inactivation, environmental stimulants, and the gut microbiome.20–22
Contrary to lipid levels, female patients with T2D showed reduced 25(OH)D levels compared to male patients in our study, which probably meant that higher lipid levels were associated with lower 25(OH)D levels. Indeed, in our study, lipid profiles showed a significantly inverse linear correlation with the levels of 25(OH)D after adjusting for confounding variables. Previously, several studies reported that 25(OH)D levels had an inverse association with dyslipidaemia, and deficient 25(OH)D is associated with an atherogenic lipid profile.23,24 However, the relationship between 25(OH)D status and lipid metabolism may be bidirectional. There is increasing evidence showing that dyslipidaemia can also result in vitamin D dysmetabolism. In vivo and in vitro studies revealed that elevation of circulating cholesterol, glucose and insulin could reduce 25(OH)D levels by downregulating hepatic 25-hydroxylase (Cyp2r1) in HFD- and HCD-fed mice.13 Yavuz et al25 found an increase of 25-hydroxyvitamin D and 1,25-dihydroxyvitamin D in blood levels after 8 weeks of treatment with rosuvastatin in 91 hyperlipidaemia patients. In the current study, our results also suggested that blood lipids might be important determining factors of serum 25(OH)D levels. Ameliorating lipid control may be beneficial for vitamin D metabolism.
Vitamin D is important for normal development and maintenance of the skeleton. In our study, female patients with T2D presented with lower 25(OH)D but higher P1NP and β-CTX levels than male patients, and 25(OH)D showed negative associations with P1NP and β-CTX in both male and female patients with T2D. Previously, Kuchuk et al26 reported that serum 25(OH)D was significantly higher in men than in women, whereas BTMs, serum osteocalcin and urinary deoxypyridinoline/creatinine were higher in women than in men, in a total of 1319 subjects between the ages of 65 and 88 yr, which is supportive of our findings.
The detrimental effects of dyslipidaemia on bone metabolism have attracted increasing attention recently. Substantial data showed a negative association between serum TC and LDL-C and BMD.27–29 In our study, mediation analysis indicated that TC or LDL-C had positive effects on P1NP, directly or partly mediated by 25(OH)D, both in men and women with T2D. Similar mediation effects were also observed between LDL-C and β-CTX in female patients. Previous studies showed controversial results between dyslipidaemia and bone turnover levels. Matins et al30 reported that elderly type 2 diabetic patients with dyslipidaemia presented higher P1NP, which is supportive of our study’s findings. Majima et al31 also reported increased bone turnover in hypercholesterolaemic or dyslipidaemic patients regardless of sex. Zhou et al32 reported that men with hypercholesterolemia displayed increased β‑CTX and PINP levels and a lower BMD compared to those of control subjects, and similar results were observed in ApoE−/− male rats. Cholesterol directly increased osteoblast functional gene expression in vitro. In contrast, Yamauchi et al33 reported that serum LDL-C levels were not significantly correlated with serum levels of P1NP or CTX in a total of 211 healthy postmenopausal women (age range, 46–80 years). The underlying mechanisms between these controversial results are not yet clear, and a more rigorous study is needed.
Paradoxically, we observed that TG, but not HDL-C, had significantly negative effects on P1NP or β-CTX, independent of 25(OH)D, both in men and women with T2D. A series of studies have reported inverse associations between TG levels and bone turnover markers.34–36 Li et al34 found that hypertriglyceridaemia was negatively associated with N-MID osteocalcin, β-CTX and P1NP after adjusting for potential confounders, but no significant association was found between serum BTMs and HDL-C in patients with T2D, supporting our conclusion. Liu et al35 reported that TG levels were negatively correlated with OC or β-CTX levels in young and middle-aged male patients with T2D. Currently, mounting evidences suggest that diabetes mellitus is a state of low bone turnover,37,38 and the underlying mechanism may be complicated. The most common pattern of dyslipidaemia in patients with T2D is elevated triglyceride levels and decreased HDL-C levels, while the mean concentration of LDL-C is not significantly different from individuals without diabetes.6,39 We proposed that the negative effect of TG would outweigh the positive effect of TC or LDL-C on BTMs and might contribute to the inhibition of bone turnover in diabetic patients.
Interestingly, positive effects of TC and LDL-C on P1NP but negative effects of TG on P1NP and β-CTX were observed in our study. The underlying mechanism of the distinct performances is not yet clear. In our previous study, we demonstrated that HOMA-IR was negatively associated with β-CTX, P1NP and N-MID osteocalcin in dysglycaemia patients.40 Similarly, in men with metabolic syndrome, impaired insulin sensitivity was associated with lower bone turnover.36 Hypertriglyceridaemia and low HDL-C have been proved to be independent and long-term predictors of insulin sensitivity in longitudinal studies, and controlling hypertriglyceridaemia and low HDL-C can delay the onset of insulin resistance in some intervention studies. Meanwhile, LDL-C has been excluded as a relevant factor since there is no evidence to support a causal relationship between LDL-C and insulin resistance.41 Thus, it is plausible that insulin resistance may mediate the inhibitory effect of TG on β-CTX or P1NP, different from the effect of TC or LDL-C. A longitudinal and rigorous study is needed to verify the association between dyslipidaemia, insulin resistance and bone turnover levels.
In this study, we confirmed the differences in lipid profiles, 25(OH)D concentration and bone turnover levels between male and female elderly patients with T2D. Female patients with T2D exhibited higher serum cholesterol and TG levels, reduced 25(OH)D levels and higher bone turnover levels than male subjects. In addition, we explored the effects of dyslipidaemia on bone turnover in diabetic patients. Our results indicated that TC and LDL-C were positively correlated with P1NP levels, which was partly mediated by 25(OH)D. In contrast, TG showed a significantly negative association with P1NP or β-CTX, independent of 25(OH)D. For a patient with predominantly hypercholesterolemia, it will increase the risk of high-turnover osteoporosis.32 For a patient with hypertriglyceridaemia predominantly, it is inclined to be in a low bone turnover state, which in turn may lead to more fragile bone.37 For the first time, we raised the idea that hypercholesterolemia and hypertriglyceridaemia might damage bone health through distinct pathways in diabetes patients, which merits further in vivo and in vitro studies for confirmation.
As far as we know, this is the first Chinese cross-sectional study which investigates the potential detrimental effects of dyslipidaemia on Vitamin D and bone metabolism in elderly patients with T2D. However, our study has some limitations. First, this is a cross-sectional study. Thus, this study is insufficient to establish a causal relationship between BTMs and determinant factors such as lipid profiles and 25(OH)D levels. Second, bone metabolism status could be more comprehensively described with both BTMs and BMD. However, we did not measure BMD in our study. Third, some correlation coefficients were inclined to be very low in our study. A statistically significant finding might not necessarily be clinically significant; thus, it should be analysed and interpreted with caution.
In conclusion, our study revealed that dyslipidaemia might have detrimental effects on vitamin D metabolism and bone turnover in elderly patients with T2D, although discrepancies exist between male and female patients. Hypercholesterolemia and hypertriglyceridaemia may be detrimental to bone health by distinguishing pathways in diabetes patients. We emphasize the importance of ameliorating lipid control in elderly diabetes patients, especially female patients, which may be beneficial for both vitamin D and bone metabolism.
Data Sharing Statement
The data that support the findings of this study are openly available in the METAL study Environmental Pollutant Exposure and Metabolic at http://www.chictr.org.cn, reference number ChiCTR1800017573.
Ethics Statement
The study protocol was approved by the Ethics Committee of Shanghai Ninth People’s Hospital, Shanghai JiaoTong University School of Medicine. All procedures followed were in accordance with the principles of the Declaration of Helsinki.
Informed Consent
Informed consent was obtained from all individual participants included in the study.
Acknowledgments
The authors thank all team members and the participants in the METAL study. This study was supported by National Natural Science Foundation of China (91857117); Science and Technology Commission of Shanghai Municipality (19140902400, 18410722300). We also thank Prof. Jiajun Zhao from the Department of Endocrinology, Shandong Provincial Hospital Affiliated to Shandong First University, for the critical comments and revisions of the manuscript.
Funding
This work was supported by Science and Technology Commission Foundation of Huangpu District (No. HKM201909).
Disclosure
The authors declare that they have no conflicts of interest in this work.
References
1. Raggi P. Screening for atherosclerotic cardiovascular disease in patients with type 2 diabetes mellitus: controversies and guidelines. Can J Diabetes. 2020;44(1):86–92. doi:10.1016/j.jcjd.2019.08.009
2. Walsh JS, Vilaca T. Obesity, type 2 diabetes and bone in adults. Calcif Tissue Int. 2017;100(5):528–535. doi:10.1007/s00223-016-0229-0
3. Farhat GN, Cauley JA, Matthews KA, et al. Volumetric BMD and vascular calcification in middle-aged women: the study of women’s health across the nation. J Bone Miner Res. 2006;21(12):1839–1846. doi:10.1359/jbmr.060903
4. Tankò LB, Bagger YZ, Christiansen C. Low bone mineral density in the Hip as a marker of advanced atherosclerosis in elderly women. Calcif Tissue Int. 2003;73(1):15–20. doi:10.1007/s00223-002-2070-x
5. Tankó LB, Christiansen C, Cox DA, et al. Relationship between osteoporosis and cardiovascular disease in postmenopausal women. J Bone Miner Res. 2005;20(11):1912–1920. doi:10.1359/jbmr.050711
6. Bahiru E, Hsiao R, Phillipson D, Watson KE. Mechanisms and treatment of dyslipidemia in diabetes. Curr Cardiol Rep. 2021;23(4):26. doi:10.1007/s11886-021-01455-w
7. Kaze AD, Santhanam P, Musani SK, Ahima R, Echouffo-Tcheugui JB. Metabolic dyslipidemia and cardiovascular outcomes in type 2 diabetes mellitus: findings from the look AHEAD study. J Am Heart Assoc. 2021;10(7):e016947. doi:10.1161/jaha.120.016947
8. Cai F, Yusufu A, Liu K, et al. High-fat diet causes undesirable bone regeneration by altering the bone marrow environment in rats. Front Endocrinol. 2023;14:1088508. doi:10.3389/fendo.2023.1088508
9. van Driel M, Van Leeuwen J. Vitamin D and bone: a story of endocrine and auto/paracrine action in osteoblasts. Nutrients. 2023;15(3):480. doi:10.3390/nu15030480
10. Lips P, Eekhoff M, van Schoor N, et al. Vitamin D and type 2 diabetes. J Steroid Biochem Mol Biol. 2017;173:280–285. doi:10.1016/j.jsbmb.2016.11.021
11. Sacerdote A, Dave P, Lokshin V, Bahtiyar G. Type 2 diabetes mellitus, insulin resistance, and vitamin D. Curr Diab Rep. 2019;19(10):101. doi:10.1007/s11892-019-1201-y
12. Yu JR, Lee SA, Lee JG, et al. Serum vitamin d status and its relationship to metabolic parameters in patients with type 2 diabetes mellitus. Chonnam Med J. 2012;48(2):108–115. doi:10.4068/cmj.2012.48.2.108
13. Zhu T, Zhao J, Zhuo S, et al. High fat diet and high cholesterol diet reduce hepatic vitamin D-25-hydroxylase expression and serum 25-hydroxyvitamin D(3) level through elevating circulating cholesterol, glucose, and insulin levels. Mol Nutr Food Res. 2021;65(21):e2100220. doi:10.1002/mnfr.202100220
14. Wu LY, Yang TC, Kuo SW, et al. Correlation between bone mineral density and plasma lipids in Taiwan. Endocr Res. 2003;29(3):317–325. doi:10.1081/erc-120025039
15. Wan H, Wang Y, Zhang K, et al. Associations between Vitamin D and microvascular complications in middle-aged and elderly diabetic patients. Endocr Pract. 2019;25(8):809–816. doi:10.4158/ep-2019-0015
16. Wang N, Wang X, Li Q, et al. The famine exposure in early life and metabolic syndrome in adulthood. Clin Nutr. 2017;36(1):253–259. doi:10.1016/j.clnu.2015.11.010
17. Stevens LA, Claybon MA, Schmid CH, et al. Evaluation of the chronic kidney disease epidemiology collaboration equation for estimating the glomerular filtration rate in multiple ethnicities. Kidney Int. 2011;79(5):555–562. doi:10.1038/ki.2010.462
18. Liu X, Yu S, Mao Z, et al. Dyslipidemia prevalence, awareness, treatment, control, and risk factors in Chinese rural population: the Henan rural cohort study. Lipids Health Dis. 2018;17(1):119. doi:10.1186/s12944-018-0768-7
19. Russo G, Pintaudi B, Giorda C, et al. Age- and gender-related differences in LDL-cholesterol management in outpatients with type 2 diabetes mellitus. Int J Endocrinol. 2015;2015:957105. doi:10.1155/2015/957105
20. Wang X, Magkos F, Mittendorfer B. Sex differences in lipid and lipoprotein metabolism: it’s not just about sex hormones. J Clin Endocrinol Metab. 2011;96(4):885–893. doi:10.1210/jc.2010-2061
21. Link JC, Reue K. Genetic basis for sex differences in obesity and lipid metabolism. Annu Rev Nutr. 2017;37:225–245. doi:10.1146/annurev-nutr-071816-064827
22. Baars A, Oosting A, Lohuis M, et al. Sex differences in lipid metabolism are affected by presence of the gut microbiota. Sci Rep. 2018;8(1):13426. doi:10.1038/s41598-018-31695-w
23. Lupton JR, Faridi KF, Martin SS, et al. Deficient serum 25-hydroxyvitamin D is associated with an atherogenic lipid profile: the Very Large Database of Lipids (VLDL-3) study. J Clin Lipidol. 2016;10(1):72–81.e71. doi:10.1016/j.jacl.2015.09.006
24. Bahadorpour S, Hajhashemy Z, Saneei P. Serum 25-hydroxyvitamin D levels and dyslipidemia: a systematic review and dose-response meta-analysis of epidemiologic studies. Nutr Rev. 2022;81:1–25. doi:10.1093/nutrit/nuac038
25. Yavuz B, Ertugrul DT, Cil H, et al. Increased levels of 25 hydroxyvitamin D and 1,25-dihydroxyvitamin D after rosuvastatin treatment: a novel pleiotropic effect of statins? Cardiovasc Drugs Ther. 2009;23(4):295–299. doi:10.1007/s10557-009-6181-8
26. Kuchuk NO, Pluijm SM, van Schoor NM, et al. Relationships of serum 25-hydroxyvitamin D to bone mineral density and serum parathyroid hormone and markers of bone turnover in older persons. J Clin Endocrinol Metab. 2009;94(4):1244–1250. doi:10.1210/jc.2008-1832
27. Yang Y, Liu G, Zhang Y, et al. Association between bone mineral density, bone turnover markers, and serum cholesterol levels in type 2 diabetes. Front Endocrinol. 2018;9:646. doi:10.3389/fendo.2018.00646
28. Makovey J, Chen JS, Hayward C, Williams FM, Sambrook PN. Association between serum cholesterol and bone mineral density. Bone. 2009;44(2):208–213. doi:10.1016/j.bone.2008.09.020
29. Cui LH, Shin MH, Chung EK, et al. Association between bone mineral densities and serum lipid profiles of pre- and post-menopausal rural women in South Korea. Osteoporos Int. 2005;16(12):1975–1981. doi:10.1007/s00198-005-1977-2
30. Martins JM, Aranha P. Bone turnover and bone mineral density in old persons with type 2 diabetes. J Clin Transl Endocrinol. 2018;14:12–18. doi:10.1016/j.jcte.2018.09.002
31. Majima T, Shimatsu A, Komatsu Y, et al. Increased bone turnover in patients with hypercholesterolemia. Endocr J. 2008;55(1):143–151. doi:10.1507/endocrj.k07e-004
32. Zhou Y, Deng T, Zhang H, et al. Hypercholesterolaemia increases the risk of high‑turnover osteoporosis in men. Mol Med Rep. 2019;19(6):4603–4612. doi:10.3892/mmr.2019.10131
33. Yamauchi M, Yamaguchi T, Nawata K, et al. Increased low-density lipoprotein cholesterol level is associated with non-vertebral fractures in postmenopausal women. Endocrine. 2015;48(1):279–286. doi:10.1007/s12020-014-0292-0
34. Li W, Liu X, Liu L, et al. Relationships of serum bone turnover markers with metabolic syndrome components and carotid atherosclerosis in patients with type 2 diabetes mellitus. Front Cardiovasc Med. 2022;9:824561. doi:10.3389/fcvm.2022.824561
35. Liu XX, Jiang L, Liu Q, et al. Low bone turnover markers in young and middle-aged male patients with type 2 diabetes mellitus. J Diabetes Res. 2020;2020:6191468. doi:10.1155/2020/6191468
36. Laurent MR, Cook MJ, Gielen E, et al. Lower bone turnover and relative bone deficits in men with metabolic syndrome: a matter of insulin sensitivity? The European Male Ageing Study. Osteoporos Int. 2016;27(11):3227–3237. doi:10.1007/s00198-016-3656-x
37. Hygum K, Starup-Linde J, Harsløf T, Vestergaard P, Langdahl BL. Mechanisms in Endocrinology: diabetes mellitus, a state of low bone turnover - A systematic review and meta-analysis. Eur J Endocrinol. 2017;176(3):R137–r157. doi:10.1530/eje-16-0652
38. Zha KX, An ZM, Ge SH, et al. FSH may mediate the association between HbA1c and bone turnover markers in postmenopausal women with type 2 diabetes. J Bone Miner Metab. 2022;40(3):468–477. doi:10.1007/s00774-021-01301-7
39. Haffner SM. Dyslipidemia management in adults with diabetes. Diabetes Care. 2004;27(Suppl 1):S68–71. doi:10.2337/diacare.27.2007.s68
40. Guo H, Wang C, Jiang B, et al. Association of insulin resistance and β-cell function with bone turnover biomarkers in dysglycemia patients. Front Endocrinol. 2021;12:554604. doi:10.3389/fendo.2021.554604
41. Li N, Fu J, Koonen DP, et al. Are hypertriglyceridemia and low HDL causal factors in the development of insulin resistance? Atherosclerosis. 2014;233(1):130–138. doi:10.1016/j.atherosclerosis.2013.12.013
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.