Back to Journals » Cancer Management and Research » Volume 12
Not All Hepatocellular Carcinoma Patients with Microvascular Invasion After R0 Resection Could Be Benefited from Prophylactic Transarterial Chemoembolization: A Propensity Score Matching Study
Authors Wang L , Ke Q, Lin K , Chen J, Wang R, Xiao C , Liu X, Liu J
Received 27 February 2020
Accepted for publication 6 May 2020
Published 22 May 2020 Volume 2020:12 Pages 3815—3825
DOI https://doi.org/10.2147/CMAR.S251605
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Xueqiong Zhu
Lei Wang,1,* Qiao Ke,2,* Kongying Lin,2,* Jingbo Chen,3 Ren Wang,4 Chunhong Xiao,5 Xiaolong Liu,6 Jingfeng Liu2,6
1Department of Radiation Oncology, Mengchao Hepatobiliary Hospital of Fujian Medical University, Fuzhou, People’s Republic of China; 2Department of Hepatopancreatobiliary Surgery, Mengchao Hepatobiliary Hospital of Fujian Medical University, Fuzhou, People’s Republic of China; 3Department of Oncology, Shengli Clinical Medical College of Fujian Medical University, Fujian Provincial Hospital, Fuzhou, People’s Republic of China; 4Department of Pediatric Surgery, Huai’an Women and Children’s Hospital, Huaian, People’s Republic of China; 5Department of General Surgery, 900th Hospital of PLA, Fuzhou, People’s Republic of China; 6The United Innovation of Mengchao Hepatobiliary Technology Key of Fujian Province, Mengchao Hepatobiliary Hospital of Fujian Medical University, Fuzhou, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Jingfeng Liu
Tel +86 139 0502 9580
Fax +86 591 8370 2529
Email [email protected]
Background: Prophylactic transarterial chemoembolization (p-TACE) is strongly recommended for hepatocellular carcinoma (HCC) patients with microvascular invasion (MVI), but the potential beneficiaries remain controversial.
Methods: Data of HCC patients with MVI who underwent R0 resection between December 2013 and December 2015 were identified through the primary liver cancer big data. Disease-free survival (DFS) and overall survival (OS) were compared between patients who received p-TACE or not using Kaplan–Meier survival curves before and after propensity scoring match (PSM).
Results: A total of 695 patients were eligible for this study, including 199 patients (28.6%) receiving p-TACE and 496 patients (71.4%) receiving resection alone. In the crude cohort, median DFS and OS were longer in the p-TACE group than those in the non-TACE group without significant differences (25.0 months vs 24.2 months, P=0.100; 48.0 months vs 46.5 months, P=0.150; respectively), but significant differences were observed both in DFS and OS (both P< 0.05) after 1:1 PSM. p-TACE was identified as one of the independent risk factors of both DFS and OS using multivariate analysis in the matched cohort (HR=0.69, 95% CI=0.54– 0.88; HR=0.66, 95% CI=0.50– 0.88; respectively). Subgroup analysis showed that p-TACE could beneficiate patients if they were male, aged ≥ 50 years old, had HBV infection, preoperative AFP level ≥ 400 ng/mL, Child-Pugh grading A, no transfusion, single tumor, tumor diameter ≥ 5cm, Edmondson–Steiner grading I/II, capsule, or BCLC stage A, CNLC stage Ib, AJCC stage II both in DFS and OS (all P< 0.05).
Conclusion: With the current data, we concluded that not all HCC patients with MVI would be benefited from p-TACE, and p-TACE could benefit patients with “middle risk” according to the current staging systems.
Keywords: hepatocellular carcinoma, microvascular invasion, R0 resection, transarterial chemoembolization
Introduction
In the recent decade, microvascular invasion (MVI) has been proposed as an aggressive biomarker of hepatocellular carcinoma (HCC).1,2 The incidence of MVI is reported to be approximately 15% to 57%,3 which would be higher after the advocation of MVI detecting in pathology. Patients with MVI are much more likely to endure recurrence and suffer a worse prognosis,4,5 hence strategies intended to prevent the recurrence and improve the prognosis are well concerned in the heads of hepatobiliary surgeons.
Sorafenib, transarterial chemoembolization (TACE), radiotherapy and hepatic arterial infusion chemotherapy (HAIC) have been tried prevalently as adjuvant treatments in clinic,6–9 among of which TACE is preferred in China. Patients with “high risk” are strongly recommended to receive p-TACE,10,11 although it has been rarely identified in the current guidelines. Recently, patients with MVI were found to be benefited from the p-TACE,12–14 which was also confirmed in our previous meta-analysis.15 However, who could be benefited most from the p-TACE remains controversial.
In the present study, data of patients with HCC and MVI were extracted from the primary liver cancer big data (PLCBD) in China as reported previously.16 The efficacy of p-TACE on patients with MVI was re-evaluated, and the potential beneficiaries were furtherly identified.
Materials and Methods
Ethics Approval and Consent to Participate
This study was conducted under the guideline of the 1975 Declaration of Helsinki (revised in 2013) and was approved by Mengchao Hepatobiliary Hospital of Fujian medical University’s Ethics Committee (No. 2019_039_01). Considering that patient medical data were analyzed retrospectively, all informed consents were waived by the ethics committee. Of note, no patients-identifiable information was utilized.
Patient Selection
HCC patients underwent R0 resection from December 2013 to December 2015 were identified. Data including baseline characteristics (age, gender, HBV infection, preoperative AFP level, Child-pugh grading, cirrhosis), operation parameters (transfusion), tumor characteristics (tumor number, tumor size, differentiation, capsule, satellite), and follow-up were extracted from PLCBD by an IT engineer, and then were verified by three independent researchers (Lei Wang, Qiao Ke, and Kongying Lin). Patients who met the following criterial were enrolled if they had 1) underwent an R0 resection, 2) pathological diagnosis of HCC and MVI. Patients who had 1) hepatectomy for recurrent HCC, 2) extrahepatic metastasis, 3) macrovascular or bile duct invasion, 4) lymph node metastasis, 5) mortality within one month after surgery, 6) patients receiving TACE within four weeks or beyond eight weeks after resection, 7) incomplete clinical data were excluded in this study.
Definitions
MVI was defined as the presence of tumor cells in a portal vein, hepatic vein, or large capsule vessel of the liver tissue adjacent to the tumor edge, which was only detected under microscopy.3,17
R0 resection was defined as no tumor residual in surgical margin under microscopy.18
Interventions
p-TACE was defined as TACE conducted within four to eight weeks following resection as depicted previously.16 Briefly, TACE was conducted through the femoral artery using Seldinger technique, and 5-F catheter or microcatheter was selectively inserted into the appropriate hepatic artery under the guide of digital subtraction angiography (DSA). And then, chemotherapeutic agents including cisplatin (10–30 mg), doxorubicin hydrochloride (10 mg) and pharmorubicin (20–40 mg) were injected slowly followed by an emulsion of lipiodol (2–5 mL).
Perioperative management of TACE is conducted according to the Chinese guideline,18 which typically includes routine items such as blood, liver function, and coagulation. Patients are strongly not recommended to receive TACE if they have jaundice (total bilirubin >52 µmol/L), Cr>176.8µmol/L, white blood cell counting <3.0×109/L, plate counting <50×109/L.
Follow-Up and Definition of Endpoints
All patients were periodically followed up according to the recommendation of the Chinese guideline.18 Briefly, follow-up was conducted once every 2–3 months in the first 2 years, once every 6 months from 2 to 5 years, and once every year after 5 years. AFP, and abdominal ultrasound were the routine follow-up items, and computed tomography (CT) or magnetic resonance imaging (MRI) was warranted if recurrence was clinically suspected. Recurrence or metastasis was defined as new lesions with radiologic characteristics of HCC, and further treatment was immediately started once recurrence was confirmed.
The primary endpoint was disease-free survival (DFS), which was defined as from the data of resection to the data of recurrence (either intrahepatic or extrahepatic) or the date of the latest follow-up. The secondary endpoint was overall survival (OS), which was calculated from the data of resection to either the data of death or the latest follow-up. Follow-up data for all patients were summarized in October 2018, with a median observation time of 30 months.
Adverse events (AE) of p-TACE were recorded within two months after p-TACE, which were evaluated according to the Clavien-Dindo standardized classification.19
Clinicopathological Variables
Potential variables associated with the prognosis of HCC patients were determined according to previous studies.16,20 Tumor size, number, differentiation, satellite, and capsule were extracted from histopathological examination. Tumor size (<5 cm vs. ≥5cm), and tumor number (single vs multiple) were categorized according to the American Joint of Cancer Committee system (AJCC),21 and differentiation was determined based on the Edmondson–Steiner grading system, which was categorized as I/II vs III/IV according to the previous report.22 HBV infection was defined as history of HBV infection, regardless of status of HBsAg and HBV-DNA. The data of blood transfusion were extracted from anesthesia records, which typically included intraoperative transfusion of red blood cell and plasma. Age (<50 years old vs.≥50 years old) was categorized as previously reported,16 and preoperative AFP levels (<400 ng/mL vs.≥400 ng/mL) were categorized using cutoff value of previous studies.23 In addition, all patients were categorized according to the newly Barcelona Clinic Liver Cancer staging system (BCLC, 0, A, B),24 China Liver cancer staging system (CNLC, Ia, Ib, IIa, IIb),18 and AJCC staging system (Ia, II, IIIa).21
Statistics
Propensity score matching (PSM) was adopted to minify the selection bias.25 Potentially confounding factors either unbalanced in the baseline Table or independent in the multivariable cox model were matched by a one-to-one ratio using the nearest neighbor method with a caliber of 0.2.
Clinicopathological variables were evaluated by the chi-square test or Fisher’s exact test between the two groups before and after PSM. Medians DFS and OS with hazard ratio and confidence interference (CI) 95% between groups of p-TACE and non-TACE were determined using the Kaplan–Meier method before and after PSM between the TACE group and non-TACE group. Risk factors associated with DFS and OS were identified by univariate analysis, and then risk factors with P<0.05 were selected to identify corresponding independent risk factors using the enter method of the multivariate Cox regression model.
Subgroup analysis for age (<50 years vs ≥50 years), gender (female vs male), HBV infection (no vs yes), cirrhosis (no vs yes), AFP (<400ng/mL vs ≥400ng/mL), Child-pugh grading (A vs B), transfusion (no vs yes), tumor number (single vs multiple), tumor size (<5 cm vs.≥5 cm), capsule (no vs yes), differentiation (I/IIvs. III/IV), satellite (no vs yes), and BCLC (0 vs A vs B), CNLC (Ia vs Ibvs. IIa vs IIb), and AJCC (Ia vs II vs IIIa) staging systems were performed using the Kaplan–Meier method, and then forest plot of subgroup analysis was depicted with each estimated HRs and 95% CI.
All the data were analyzed via R-studio using “Table 1”, “MatchIt”, “survminer”, “survival”, “forestplot” packages. Statistically significance was determined by two-sided significant levels of 0.05.
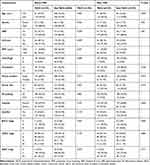 |
Table 1 Clinicopathological Characteristics Before and After PSM |
Results
Initially, 1046 HCC patients were confirmed with MVI by pathology, 38 patients (3.6%) were excluded for recurrent HCC, 33 patients (3.2%) were combined with extrahepatic or lymph metastasis, 169 patients (16.2%) were involved with macrovascular or bile duct invasion, and 21 patients (2.0%) with perioperative mortality. During the period of follow-up (2.0–67.0 months), 39 patients (3.7%) lost to follow-up. Finally, 695 patients were enrolled in this study, and 199 patients (28.6%) received p-TACE four to eight weeks after surgery (Figure 1).
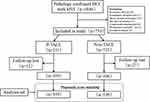 |
Figure 1 Flow chart of patients’ enrollment from the primary liver cancer big data. |
Patients’ Characteristics
The baseline characteristics of the eligible patients are shown in Table 1. Of note, the incidence of patients with multiple tumors was significantly higher in the p-TACE group than that in the non-TACE group (31.7% vs 22.2%, P=0.01, Table 1), and patients with advanced stages were much more likely to receive p-TACE (Table 1). After 1:1 PSM, the baseline characteristics between the two groups were well balanced (all P>0.05).
Primary and Secondary Endpoint
In the crude cohort, no difference was observed between groups of p-TACE and non-TACE in the term of median DFS (25.0 months vs 24.2 months, P=0.100, Figure 2A). The 1-, 3-, and 5-year DFS in the groups of p-TACE and non-TACE were 70.4% vs 65.3%, 44.2% vs 38.7%, and 39.2% vs 34.9%, respectively. Similar result was observed in the median OS between groups of p-TACE and non-TACE (48.0 months vs 46.5 months, P=0.150, Figure 2B) with equivalent 1-, 3-, and 5-year survival rates (91.5% vs 81.6%, 62.8% vs 57.3%, 53.8% vs 51.9%, respectively).
After 1:1 PSM, the median DFS in the TACE was significantly longer than that in the non-TACE groups (25.0 months vs 22.1 months, P=0.006, Figure 2C), and the 1-, 3-, and 5-year DFS in the two groups were 70.4% vs 61.8%, 44.2% vs 33.7%, and 39.2% vs 30.7%, respectively. Significant difference was also observed in the median OS between groups of p-TACE and non-TACE (48.0 months vs 30.0 months, P=0.013, Figure 2D), and the 1-, 3-, and 5-year SR in the two groups were 91.5% vs 78.9%, 62.8% vs 51.3%, and 53.8% vs 48.2%, respectively.
Risk Factors Associated with DFS and OS in the Matched Cohort
Results of univariate and multivariate analysis of 398 patients with MVI receiving R0 resection for DFS and OS are depicted in Table 2. Briefly, AFP level (HR=1.37, 95% CI=1.07–1.75), tumor number (HR=1.63, 95% CI=1.26–2.10), tumor size (HR=1.57, 95% CI=1.22–2.02), and p-TACE (HR=0.69, 95% CI=0.54–0.88) were identified as independent risk factors of DFS, and AFP level (HR=1.59, 95% CI=1.20–2.11), tumor number (HR=1.40, 95% CI=1.04–1.87), tumor size (HR=2.28, 95% CI=1.66–3.13), capsule (HR=1.50, 95% CI=1.11–2.01), and p-TACE (HR=0.66, 95% CI=0.50–0.88) were identified as independent risk factors of OS.
 |
Table 2 Univariate and Multivariate Analysis of Disease-Free Survival and Overall Survival After PSM |
Subgroup Analysis Stratified by Each Clinicopathological Variable in the Matched Cohort
Subgroup analysis showed that patients with the following characteristics benefited from AT in terms of DFS: male, aged ≥50 years old, HBV infection, preoperative AFP level ≥400 ng/mL, Child-Pugh grading A, no transfusion, single tumor, tumor diameter ≥5cm, cirrhosis, ES grading I/II, capsule, or BCLC stage A, CNLC stage Ib and IIa, and AJCC stage II (all P<0.05, Figure 3).
 |
Figure 3 Subgroup analysis of disease-free survival (DFS) stratified by clinicopathological variables related to the prognosis in the matched cohort. |
Subgroup analysis showed that patients with the following characteristics benefited from AT in terms of OS: male, aged ≥50 years old, HBV infection, preoperative AFP level ≥400 ng/mL, Child-Pugh grading A, no transfusion, single tumor, tumor diameter ≥5cm, ES grading I/II, capsule, satellite, or BCLC stage A, CNLC stage Ib, and AJCC stage II (all P<0.05, Figure 4).
 |
Figure 4 Subgroup analysis of overall survival (OS) stratified by clinicopathological variables related to the prognosis in the matched cohort. |
AEs Related to p-TACE
The overall incidence of AEs was 41.7% (83/199), but no severe AEs were observed following p-TACE. Majority of AEs were Clavien-Dindo grade I (62/199, 31.1%), including slight nausea or vomiting, fever and pain, and then grade II (21/199, 10.6%), including mild liver function abnormalities, pancreatitis and leukopenia, which were cured by pharmacologic treatments.
Discussion
The prognosis of patients with MVI following R0 resection remains far from promising,1,26 but the application of p-TACE has always been questioned in clinic.14,27,28 In this study, only 28.6% of patients with MVI received p-TACE, which was similar to previous report (34.9%).16 In addition, the efficacy of p-TACE was only confirmed in the matched cohort, but not in the crude cohort, which indicated that p-TACE could not benefit all patients with MVI, and the candidates should be selected prudently.
MVI, as one of the important origin of intrahepatic recurrence or metastasis,29,30 is deserved more and more attentions of HPB surgeons, and patients with MVI typically suffer a poor prognosis. In addition, wide surgical resection (≥1cm) is often hard to realize constrained by anatomic location, residual liver function and surgical techniques.31 Hence, strategies intended to prevent the recurrence and prolong the survival of patients with MVI such as sorafenib, TACE, HAIC, external beam radiotherapy including IMRT and SBRT have been tried worldwide,6,7,9,32 but none has been clearly recommended in the current guidelines.
As is known to all, TACE is often the first-line treatment of unresectable or recurrent HCC patients,24,33,34 but in the recent decade TACE has also been conducted for patients received resection especially in China.20,35,36 The clinical efficacy of p-TACE was identified by previous studies including one RCT,13 which was also confirmed by our recent meta-analysis.15 However, who would be the best candidates of p-TACE remains controversial. For example, patients with tumor size ≥5cm were found to be benefited from p-TACE in the RCT,13 but it was on the contrary in a large series after a well-designed PSM;14 p-TACE was reported to benefit patients with multiple tumors by Qi et al,8 but was inconsistent with Wang et al report.12 Hence, selection of appropriate candidates in such adjuvant settings are the key.
In the crude cohort, median DFS and OS were longer in the p-TACE group than those in the non-TACE group without significant differences (both P>0.05), but significant differences were observed after a well-designed PSM. The main reason causing this divergence was that patients with advanced stages were much more likely receive the p-TACE as depicted in Table 1, who often suffered a worse prognosis. Hence, we hypothesized that not all patients with MVI would be benefited from p-TACE. And then, subgroup analysis stratified by any variables related to the prognosis was furtherly analyzed. Results showed that p-TACE could beneficiate patients if they were male, aged ≥50 years old, had HBV infection, preoperative AFP level ≥400 ng/mL, Child-Pugh grading A, no transfusion, single tumor, tumor diameter ≥5cm, ES grading I/II, or capsule both in DFS and OS (all P<0.05).
However, to select patients according to the risk factors above is unfeasible, and comprehensive system including performance status, liver function and tumor burden is much more practical in clinic. Hence, three most widely used staging systems including BCLC, CNLC and AJCC were introduced to identify the potential beneficiaries of p-TACE in this study.18,21,24 Results showed that only patients with BCLC stage A, CNLC stage Ib, and AJCC stage II both (all P<0.05) were found to be benefited more from p-TACE, but not patients with BCLC stage 0/B, CNLC stage IIb, and AJCC stage IIIa (all P>0.05). This was coincident with our hypothesis that p-TACE could only benefit patients with “middle risk”, but not patients with either “high risk” or “low risk”.
Of note, p-TACE in this study was conducted only once following R0 resection. Ye et al28 found that three cycles of p-TACE could improve the prognosis, but more cycles often brought disadvantages. Repeated TACE was reported to be associated with liver damage and potential metastasis,37 although the mechanisms were still unknown. From the other hand, one cycle of p-TACE was not inferior to more cycles in the improvement of prognosis, which was also confirmed by previous meta-analysis.15 Hence, p-TACE was strongly recommended only once following R0 resection.
There were several restrictions in this study. First, selection and recalling bias were inevasible in retrospective studies, although a well-designed PSM and subgroup analysis stratified by any variables related to the prognosis were conducted. Second, the definition of MVI was a little from different centers,3,30 because all the pathological reports were retrospectively extracted before 2016 when the corresponding guideline was published. Third, MVI classification including M1 and M2 was reported to be associated with prognosis and should be as a guide for postoperative management,2 but relevant data were unavailable especially before 2016. Fourth, the epidemiology of HCC between the West and the East,38 which meant that the conclusion would not apply to the western series.
Conclusion
With the current data, we concluded that p-TACE was efficient and safe for the patients with patients with MVI following R0 resection. And, patients with “middle risk” according to the current staging systems would be benefited more from p-TACE.
Abbreviations
HCC, hepatocellular carcinoma; MVI, microvascular invasion; PSM, propensity scoring match; DFS, disease-free survival; OS, overall survival; HR, hazard ratio; CI, confidence interval; SR, surgical resection; TACE, transarterial chemoembolizations; HAIC, hepatic artery infusion chemotherapy; BCLC, Barcelona Clinic Liver Cancer staging system; CNLC, China Liver cancer; AJCC, the American Joint of Cancer Committee system.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Wang H, Wu MC, Cong WM. Microvascular invasion predicts a poor prognosis of solitary hepatocellular carcinoma up to 2 cm based on propensity score matching analysis. Hepatol Res. 2019;49(3):344–354. doi:10.1111/hepr.13241
2. Zhao H, Chen C, Fu X, et al. Prognostic value of a novel risk classification of microvascular invasion in patients with hepatocellular carcinoma after resection. Oncotarget. 2017;8(3):5474–5486. doi:10.18632/oncotarget.12547
3. Rodriguez-Peralvarez M, Luong TV, Andreana L, Meyer T, Dhillon AP, Burroughs AK. A systematic review of microvascular invasion in hepatocellular carcinoma: diagnostic and prognostic variability. Ann Surg Oncol. 2013;20(1):325–339. doi:10.1245/s10434-012-2513-1
4. Nitta H, Allard MA, Sebagh M, et al. Prognostic value and prediction of extratumoral microvascular invasion for hepatocellular carcinoma. Ann Surg Oncol. 2019;26(8):2568–2576. doi:10.1245/s10434-019-07365-0
5. Zhang X, Li J, Shen F, Lau WY. Significance of presence of microvascular invasion in specimens obtained after surgical treatment of hepatocellular carcinoma. J Gastroenterol Hepatol. 2018;33(2):347–354. doi:10.1111/jgh.13843
6. Wang L, Chen B, Li Z, et al. Optimal postoperative adjuvant treatment strategy for HBV-related hepatocellular carcinoma with microvascular invasion: a propensity score analysis. Onco Targets Ther. 2019;12:1237–1247. doi:10.2147/OTT.S179247
7. Zhang XP, Chai ZT, Gao YZ, et al. Postoperative adjuvant sorafenib improves survival outcomes in hepatocellular carcinoma patients with microvascular invasion after R0 liver resection: a propensity score matching analysis. HPB (Oxford). 2019;21(12):1687–1696. doi:10.1016/j.hpb.2019.04.014
8. Qi YP, Zhong JH, Liang ZY, et al. Adjuvant transarterial chemoembolization for patients with hepatocellular carcinoma involving microvascular invasion. Am J Surg. 2019;217(4):739–744. doi:10.1016/j.amjsurg.2018.07.054
9. Wang L, Wang W, Yao X, et al. Postoperative adjuvant radiotherapy is associated with improved survival in hepatocellular carcinoma with microvascular invasion. Oncotarget. 2017;8(45):79971–79981. doi:10.18632/oncotarget.20402
10. Qi X, Liu L, Wang D, Li H, Su C, Guo X. Hepatic resection alone versus in combination with pre- and post-operative transarterial chemoembolization for the treatment of hepatocellular carcinoma: a systematic review and meta-analysis. Oncotarget. 2015;6(34):36838–36859. doi:10.18632/oncotarget.5426
11. Liao M, Zhu Z, Wang H, Huang J. Adjuvant transarterial chemoembolization for patients after curative resection of hepatocellular carcinoma: a meta-analysis. Scand J Gastroenterol. 2017;52(6–7):624–634. doi:10.1080/00365521.2017.1292365
12. Wang YY, Wang LJ, Xu D, et al. Postoperative adjuvant transcatheter arterial chemoembolization should be considered selectively in patients who have hepatocellular carcinoma with microvascular invasion. HPB (Oxford). 2019;21(4):425–433. doi:10.1016/j.hpb.2018.08.001
13. Wei W, Jian PE, Li SH, et al. Adjuvant transcatheter arterial chemoembolization after curative resection for hepatocellular carcinoma patients with solitary tumor and microvascular invasion: a randomized clinical trial of efficacy and safety. Cancer Commun (Lond). 2018;38(1):61. doi:10.1186/s40880-018-0331-y
14. Liu S, Li H, Guo L, et al. Tumor size affects efficacy of adjuvant transarterial chemoembolization in patients with hepatocellular carcinoma and microvascular invasion. Oncologist. 2019;24(4):513–520. doi:10.1634/theoncologist.2018-0305
15. Wang L, Ke Q, Lin N, Zeng Y, Liu J. Does postoperative adjuvant transarterial chemoembolization benefit for all patients with hepatocellular carcinoma combined with microvascular invasion: a meta-analysis. Scand J Gastroenterol. 2019;54(5):528–537. doi:10.1080/00365521.2019.1610794
16. Wang L, Ke Q, Deng M, et al. Adjuvant transarterial chemoembolization for patients with hepatocellular carcinoma after radical hepatectomy: a real world study. Scand J Gastroenterol. 2019;54(11):1403–1411. doi:10.1080/00365521.2019.1684986
17. Sumie S, Nakashima O, Okuda K, et al. The significance of classifying microvascular invasion in patients with hepatocellular carcinoma. Ann Surg Oncol. 2014;21(3):1002–1009. doi:10.1245/s10434-013-3376-9
18. Zhou J, Sun HC, Wang Z, et al. Guidelines for diagnosis and treatment of primary liver cancer in China (2017 edition). Liver Cancer. 2018;7(3):235–260. doi:10.1159/000488035
19. Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg. 2004;240(2):205–213. doi:10.1097/01.sla.0000133083.54934.ae
20. Wang Z, Ren Z, Chen Y, et al. Adjuvant transarterial chemoembolization for HBV-related hepatocellular carcinoma after resection: a randomized controlled study. Clin Cancer Res. 2018;24(9):2074–2081. doi:10.1158/1078-0432.CCR-17-2899
21. Benson AB, D’Angelica MI, Abbott DE, et al. Guidelines insights: hepatobiliary cancers, version 2. 2019. J Natl Compr Canc Netw. 2019;17(4):302–310. doi:10.6004/jnccn.2019.0019
22. Wang H, Du PC, Wu MC, Cong WM. Postoperative adjuvant transarterial chemoembolization for multinodular hepatocellular carcinoma within the Barcelona clinic liver cancer early stage and microvascular invasion. Hepatobiliary Surg Nutr. 2018;7(6):418–428. doi:10.21037/hbsn.2018.09.05
23. Yang B, Zheng B, Yang M, et al. Liver resection versus transarterial chemoembolization for the initial treatment of Barcelona clinic liver cancer stage B hepatocellular carcinoma. Hepatol Int. 2018;12(5):417–428. doi:10.1007/s12072-018-9888-4
24. Galle PR, Forner A, Llovet JM. EASL clinical practice guidelines: management of hepatocellular carcinoma. J Hepatol. 2018;69(1):182–236. doi:10.1016/j.jhep.2018.03.019
25. Yao XI, Wang X, Speicher PJ, et al. Reporting and guidelines in propensity score analysis: a systematic review of cancer and cancer surgical studies. J Natl Cancer Inst. 2017;109(8). doi:10.1093/jnci/djw323.
26. Liu J, Zhu Q, Li Y, et al. Microvascular invasion and positive HB e antigen are associated with poorer survival after hepatectomy of early hepatocellular carcinoma: a retrospective cohort study. Clin Res Hepatol Gastroenterol. 2018;42(4):330–338. doi:10.1016/j.clinre.2018.02.003
27. Ke-Wei L, Tian-Fu W, Xi L, et al. The effect of postoperative TACE on prognosis of HCC with microscopic venous invasion. Hepatogastroenterology. 2012;59(118):1944–1946. doi:10.5754/hge11845
28. Ye JZ, Chen JZ, Li ZH, et al. Efficacy of postoperative adjuvant transcatheter arterial chemoembolization in hepatocellular carcinoma patients with microvascular invasion. World J Gastroenterol. 2017;23(41):7415–7424. doi:10.3748/wjg.v23.i41.7415
29. Lim KC, Chow PK, Allen JC, et al. Microvascular invasion is a better predictor of tumor recurrence and overall survival following surgical resection for hepatocellular carcinoma compared to the Milan criteria. Ann Surg. 2011;254(1):108–113. doi:10.1097/SLA.0b013e31821ad884
30. Sumie S, Kuromatsu R, Okuda K, et al. Microvascular invasion in patients with hepatocellular carcinoma and its predictable clinicopathological factors. Ann Surg Oncol. 2008;15(5):1375–1382. doi:10.1245/s10434-008-9846-9
31. Yang P, Si A, Yang J, et al. A wide-margin liver resection improves long-term outcomes for patients with HBV-related hepatocellular carcinoma with microvascular invasion. Surgery. 2019;165(4):721–730. doi:10.1016/j.surg.2018.09.016
32. Hsiao JH, Tsai CC, Liang TJ, et al. Adjuvant hepatic arterial infusion chemotherapy is beneficial for selective patients with Hepatocellular carcinoma undergoing surgical treatment. Int J Surg. 2017;45:35–41. doi:10.1016/j.ijsu.2017.07.071
33. Chen RX, Gan YH, Ge NL, et al. Comparison of transarterial chemoembolization with radiofrequency ablation for unresectable Barcelona clinic liver cancer stage 0/A hepatocellular carcinoma: a propensity score matching. J Gastroenterol Hepatol. 2016;31(2):442–449. doi:10.1111/jgh.13077
34. Shao W, Li C, Tang J, et al. Efficacy and safety of raltitrexed plus oxaliplatin-based transarterial chemoembolization in patients with unresectable hepatocellular carcinoma. Cancer Manag Res. 2019;11:9863–9869. doi:10.2147/CMAR.S217524
35. Hatano E, Uemoto S, Yamaue H, Yamamoto M. Significance of hepatic resection and adjuvant hepatic arterial infusion chemotherapy for hepatocellular carcinoma with portal vein tumor thrombus in the first branch of portal vein and the main portal trunk: a project study for hepatic surgery of the Japanese Society of Hepato-Biliary-Pancreatic Surgery. J Hepatobiliary Pancreat Sci. 2018;25(9):395–402. doi:10.1002/jhbp.574
36. Tong Y, Li Z, Liang Y, et al. Postoperative adjuvant TACE for patients of hepatocellular carcinoma in AJCC stage I: friend or foe? A propensity score analysis. Oncotarget. 2017;8(16):26671–26678. doi:10.18632/oncotarget.15793
37. Gao Z, Du G, Pang Y, et al. Adjuvant transarterial chemoembolization after radical resection contributed to the outcomes of hepatocellular carcinoma patients with high-risk factors. Medicine (Baltimore). 2017;96(33):e7426. doi:10.1097/MD.0000000000007426
38. Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394–424. doi:10.3322/caac.21492
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

