Back to Journals » Journal of Pain Research » Volume 16
Nonpharmacological Interventions for Management of the Pain-Fatigue-Sleep Disturbance Symptom Cluster in Breast Cancer Patients: A Systematic Review and Network Meta-Analysis of Randomized Controlled Trials
Authors He CC, Lin DM, Liu HZ, Wang FF, Guo XF, Zhang XB, Ai YQ, Meng LM
Received 23 February 2023
Accepted for publication 9 July 2023
Published 7 August 2023 Volume 2023:16 Pages 2713—2728
DOI https://doi.org/10.2147/JPR.S409798
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Amitabh Gulati
Cong-Cong He, Dong-Mei Lin, Hui-Zhen Liu, Fei-Fei Wang, Xiu-Fang Guo, Xiao-Bo Zhang, Yi-Qin Ai, Li-Min Meng
School of Nursing, Gannan Medical University, Ganzhou City, Jiangxi Province, People’s Republic of China
Correspondence: Li-Min Meng, School of Nursing, Gannan Medical University, No. 1 Medical Road, Ganzhou, People’s Republic of China, Tel +86 15970987999, Email [email protected]
Background: The pain-fatigue-sleep disturbance symptom cluster is commonly experienced by breast cancer patients, and a variety of nonpharmacological interventions are used to treat this symptom cluster.
Objective: To compare the efficacy of nonpharmacological interventions in improving the symptoms of the pain-fatigue-sleep disturbance symptom cluster in breast cancer patients.
Methods: A comprehensive literature search was conducted in the PubMed, EMBASE, Cochrane Library, CINAHL, CNKI, and Wanfang databases to identify randomized controlled studies from database inception to May 2022. Two reviewers independently performed data retrieval and risk of bias assessments. The consistency model was used to conduct network meta-analyses (NMA) based on the frequentist framework to assess the interventions, which were ranked by the surface under the cumulative ranking curve (SUCRA). Finally, the CINeMA application was used to evaluate the results of the NMA and the evidence of quality. The results Twenty-three eligible studies assessing 14 interventions were included. According to SUCRA values, among the management effects of the three symptoms, the effect of progressive muscle relaxation (PMR) ranked first, followed by mindfulness-based stress reduction (MBSR). The overall evidence quality of our study ranges from very low to moderate.
Conclusion: PMR and MBSR were effective interventions for the pain-fatigue-sleep disturbance symptom cluster in breast cancer patients. Clinical recommendations prioritize PMR for symptom management, followed by MBSR. However, this should be interpreted cautiously, as the confidence in the evidence was not high.
Keywords: breast cancer, pain-fatigue-sleep disturbance symptom cluster, nonpharmacological intervention, systematic review, network meta-analysis
Introduction
According to the 2020 global cancer statistics published by CA: A Cancer Journal for Clinicians, breast cancer has the highest morbidity in the world among all cancers.1 With the development of health care technology in recent years, effective treatments have increased the 5-year survival rate of breast cancer patients to more than 80%.2 However, during the development and treatments of the disease, breast cancer patients experience various symptoms (such as fatigue and pain), which are correlated and clustered together.3 These clusters where two or more symptoms exist simultaneously and interact with each other are called symptom clusters, and the symptoms within the cluster may have the same etiology.4 They have a greater impact than a single symptom on the health status and quality of life of cancer patients.5
The pain-fatigue-sleep disturbance symptom cluster is one of the most common symptom clusters in breast cancer patients and is directly related to treatments such as surgery, radiation, and chemotherapy.6 More than half of all cancer patients undergoing treatments or with advanced metastases experience pain, fatigue, and sleep disturbances, which occur at all stages of treatment and interact with each other, severely affecting the patient’s life status.7,8 Studies have shown that fatigue is more severe when patients have poor sleep quality or sleep more during the day, and several studies have shown that when fatigue is not relieved, sleep disturbance and pain levels are exacerbated and mental status is affected.9,10 There are few studies on the mechanisms of symptom clusters, and previous studies have shown that there is a common biological mechanism for symptoms within the cluster that may be closely linked to inflammatory cytokines such as interleukin-1β and C-reactive protein.11,12
Brant13 comprehensively analysed the existing symptom management theories and pointed out that implementing an effective symptom intervention strategy is necessary for improving patient outcomes and that considering the characteristics of symptom clusters interactions in the intervention strategy can more efficiently manage patients’ symptoms, simplify the symptom intervention process, and improve the utilization rate of medical hospitals. Currently, nonpharmacological interventions are widely used in clinical practice for patient symptom management. As a supplement to conventional drug therapy, it has a positive effects on cancer patients’ symptom management while reducing the use of drugs (such as morphine) and causing fewer side effects.8,14
Current research on non-pharmacological interventions for the pain-fatigue-sleep disturbance symptom cluster in breast cancer patients is scattered, and these interventions can be broadly classified into four categories: psychological interventions, Chinese medicine, exercise therapy, and other types of interventions. These include progressive muscle relaxation, aerobic exercise, cognitive behavioral therapy, Qigong, Yoga, and so on. Up to now, there is no clear diagnostic threshold for this symptom cluster, and the severity of the symptom cluster is mainly assessed by various symptom assessment scales (including multidimensional scales and unidimensional scales), followed by targeted interventions.8 Some of these interventions were delivered directly by health care workers, while others were trained by health care professionals and then practiced by the subjects themselves.15 Most of the studies analyzed the effects of the interventions in comparison with usual care, and the length of the interventions ranged from 1 week to several months.16 There is no evidence of best practice for non-pharmacological interventions for symptom clusters in breast cancer patients.17 Some studies have found that interventions such as acupuncture and Qigong are significantly effective in relieving some individual symptoms in breast cancer patients by means of Meta-analysis, but there are no clear conclusions about the best non-pharmacological interventions for pain-fatigue-sleep disturbance symptom clusters,18,19 and these conventional Meta-analyses can only compare two groups of interventions and do not allow for a comprehensive analysis of multiple interventions in the study. Network meta-analysis (NMA) can solve this problem by combining direct and indirect evidence and comparing multiple treatments to guide decision-making in the clinical.20
Given the above, the purpose of the study was to systematically evaluate the intervention effects of different nonpharmacological interventions on pain-fatigue-sleep disturbance symptom cluster severity in breast cancer patients. The results provide evidence-based data that can guide medical staff in choosing the best intervention protocol.
Methods
The systematic review and NMA were conducted according to the Preferred Reporting Items for Systematic Reviews and Network Meta-Analyses (PRISMA-NMA).21 The research has been registered in the International Prospective Register of Systematic Reviews (PROSPERO) under registration number CRD42022332007.
Search Strategy
A preliminary search of PubMed was conducted to determine the keywords that could be used for a comprehensive search. Six databases were comprehensively searched, including PubMed (MEDLINE), the Cochrane Library, EMBASE, CINAHL, CNKI, and the Wanfang Database, from their inception through May 2022. Additionally, the reference lists of all relevant systematic reviews and meta-analyses were reviewed to avoid the omission of eligible studies. The PubMed database search strategy is shown in Table 1.
 |
Table 1 PubMed Search Strategy |
Studies That Met the Following Criteria Were Eligible for NMA
(1) Breast cancer patients aged 18 years and older receiving conventional treatments, such as radiotherapy, chemotherapy, surgery, or endocrine therapy; (2) interventions applied during or posttreatment (before and after treatment are collectively referred to as posttreatment); (3) nonpharmacological interventions; (4) pain, fatigue, and sleep disturbance severity scores exist simultaneously in a single study; and (5) randomized controlled trials (RCTs) published in English or Chinese.
The Exclusion Criteria Included the Following
(1) Duplicate publications; (2) missing data, such as sample size or standard deviation values; and (3) studies for which the full text was not available.
Data Extraction and Evaluation of the Risk of Bias
Two researchers independently extracted the data from each study, including the author, publication year, country, sample size, patient age, and characteristics of the intervention (intervention type, intervention duration, timing of outcome measures).
The assessment tool scores used to measure pain, fatigue, or sleep disturbance were extracted, and when multiple scales were used to measure the same symptoms, multidimensional, multi-item scales were prioritized. The data included in the studies were postintervention assessment scores, and if direct data could not be used, inferred data were assessed, or the original author was consulted to request the raw data. When data were impossible to analyse, the study was excluded.
Two researchers assessed the quality of each RCT using the Cochrane risk of bias tool, including seven domains, namely, random sequence generation, allocation concealment, blinding of participants and personnel, blinding of the outcome assessment, incomplete outcome data, selective reporting, and other bias domains. Each study domain was assigned a rating of low, high, or unclear risk of bias. Studies that received a literature quality grade of A had a low risk of bias for 4 or more items, and those that received a literature quality grade of B had a low risk of bias for 2 or 3 items. Those that received a literature quality grade of C had a low risk of bias for ≤ 1 item, and the study was excluded. In the case of disagreement, a third researcher evaluated the study, and a consensus was reached through discussion.22
Statistical Analysis
Stata 16.0 was used to make a network evidence map to analyse whether the included studies met the prerequisites for network meta-analysis. A network meta-analysis was conducted based on the frequency framework, and the intervention effects of different interventions on pain, fatigue, and sleep disturbance were compared and ranked.23 To estimate statistical consistency within every closed loop, we used the “node-splitting” technique to statistically analyse inconsistencies between direct and indirect evidence within the entire network framework. If P >0.05, the heterogeneity was considered not significant, and then we used the consistency model for statistical analysis.24 The ranking probabilities of all interventions were obtained by calculating surface under the cumulative ranking curve (SUCRA) values. A SUCRA value, as a percentage, is interpreted as the probability that the intervention is the most effective on the outcome, infinitely close to 1 when the intervention is considered to be the best intervention and infinitely close to zero when the intervention is considered to be the worst intervention.25 Publication bias was detected by comparison-correction funnel plots. Sensitivity analysis was conducted by excluding studies with fewer than 30 samples and recalculating the network results. Whether the results were stable was judged by whether there were significant changes in the SMD and 95% CI of the difference between the two comparison groups.
Assessment of Certainty of Evidence
The CINeMA application (http://cinema.ispm.ch) was used to evaluate the results of the NMA, and the evidence quality and recommendation of outcome indicators were graded.26 The evaluation items included six aspects: within-study bias (quality of methodology included in the study), reporting bias (comprehensiveness of inclusion of standard-compliant studies), indirectness (relevance and transmissibility with research questions), imprecision (accuracy of combined results of different studies), heterogeneity and incoherence (inconsistency between direct and indirect evidence). The severity of the above six aspects can be divided into no concern (no downgrade), some concern (one grade down), and major concern (two grades down). Finally, the quality level of evidence is obtained, which can be divided into high, moderate, low, and very low.
Results
Included Studies
The initial search yielded 2710 English and 931 Chinese studies. 558 duplicate studies were excluded by Note Express software, and 52 studies remained after reading the title and abstract to exclude those unrelated to the topic. 30 studies were excluded by reading the full text, and 1 study was obtained by manually searching the references of related articles, and 23 studies were finally included. The literature screening process is shown in Figure 1.
Baseline Characteristics
The 23 included studies included 2113 participants with 14 different interventions and provided sufficient data published between 2009 and 2021. A total of 1091 patients were randomly assigned to the intervention group, and the remaining 1022 patients were assigned to 3 control groups (usual care, waiting list, and placebo groups). Studies were conducted in China (=10), the United States (=5), South Korea (=2), Brazil (=1), Turkey (=2), the Netherlands (=1), France (n=1), and Iran (=1). The research participants were mainly middle-aged and elderly women who mostly underwent surgery and/or received chemotherapy, radiotherapy, endocrine therapy, or other treatments. The included studies all described the durations and frequencies of the interventions in detail, with four studies having a total intervention duration of within one month, 16 studies having a total duration of 1–3 months, and the remaining studies having a total intervention duration of more than 3 months; the frequency of the intervention was not consistent in most studies, with a single intervention duration ranging from 5–120 min. Most studies were conducted to assess the severity of symptoms before as well as immediately after the intervention. The basic features of the included literature are detailed in Table 2.
 |
Table 2 A Summary of the Characteristics of the Included Studies |
Quality of the Included Studies
The individual and overall levels of study quality are plotted in Figures 2 and 3, respectively. Random sequence generation was reported in 19 of the 23 included studies, but some studies did not specify the randomization protocols. Six studies described the allocation concealment methods. Most participants and people involved in the studies were not blinded to the methods, resulting in a high risk of bias. Four studies had a high risk of bias due to outcome assessment, two studies had a high risk of bias due to incomplete outcome data, one study had a high risk of selection bias, and five studies had an unclear risk of other bias due to small sample sizes and experimental deficiencies.
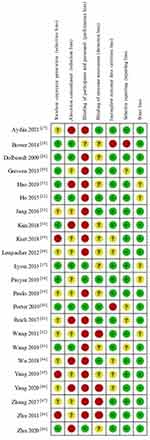 |
Figure 2 Risk of bias assessment by individual trials. |
 |
Figure 3 Overall risk of bias assessment using the Cochrane tool. |
Results of the Network Meta-Analysis
Evidence Network Diagram
Figure 4 shows the network evidence map of the 23 included studies with both arms, including 14 interventions, forming a closed loop. The thicker the line between two interventions is, the larger the number of studies comparing the two interventions. The larger the node is, the larger the study sample size. Except for one study that compared only nonpharmacological interventions, all the studies included a control group (usual care/placebo/waiting list groups).
The inconsistency test results for pain (P=0.81), fatigue (P=0.52), and sleep disturbance (P=0.92) all had P values >0.05, so the consistency model was used in the Bayesian network meta-analysis for fitting. The probability distributions of each intervention, sorted by the effects of interventions for pain, fatigue, and sleep disturbance symptoms, were based on the SUCRA values. A higher SUCRA value indicated that the intervention was more efficacious. The overall ranking of interventions was estimated by SUCRA values. The possible grade for each treatment (from the highest grade to the lowest grade depending on the outcome) is shown on the horizontal axis, and the cumulative probability that each treatment is the best choice among the treatments is shown on the vertical axis.
Results of the Network Meta-Analysis of Pain
Consistent model analysis showed that progressive muscle relaxation (PMR) had significant effects compared with control group (usual care/placebo/waiting list groups) (SMD=4.76, 95% CI (1.36, 8.17), P<0.05) and psychological education (PE) (SMD=4.39, 95% CI (0.22, 8.57), P<0.05). In the remaining interventions, there was no difference in the effect of pairwise comparisons (see Supplementary File S1). The SUCRA results (Figure 5) indicated that PMR was ranked best among nonpharmacological interventions in relieving pain symptoms.
Results of the Network Meta-Analysis of Fatigue
Consistent model analysis showed that PMR had significant effects compared with the control group (usual care/placebo/waiting list groups) (SMD=3.80, 95% CI (1.31, 6.29), P<0.05) and (SMD=3.26, 95% CI (0.20, 6.32), P<0.05). In the remaining interventions, there was no difference in the effect of pairwise comparisons (see Supplementary File S1). The SUCRA results (Figure 5) indicated that PMR was ranked best among nonpharmacological interventions in relieving fatigue symptoms.
Results of Network Meta-Analysis of Sleep Disturbance
Consistent model analysis showed that PMR had significant effects compared with control group (usual care/placebo/waiting list groups) (SMD=5.61, 95% CI (1.99, 9.22), P<0.05), PE (SMD=4.74, 95% CI (0.31, 9.17), P<0.05), and aerobic exercise and resistance exercise (AR) (SMD=5.10, 95% CI (0.54, 9.66), P<0.05), P<0.05). In the remaining interventions, there was no difference in the effect of pairwise comparisons (see Supplementary File S1). The SUCRA results (Figure 5) indicated that PMR was ranked best among nonpharmacological interventions in relieving sleep disturbance symptoms.
Publication Bias
Publication bias analyses were performed for three groups, and except for some small studies with distributions outside the inverted funnel, the control adjustment funnel was relatively symmetrical, indicating that there was no evidence of publication bias (see Supplementary File S2).
Sensitivity Analysis
Sensitivity analysis results showed that after excluding studies with a sample size of less than 30, the difference in the intervention effects of the interventions did not change significantly, suggesting that small sample studies had less impact on the results (see Supplementary File S3).
Certainty of Evidence
The CINeMA application was used to evaluate the quality of evidence for all outcome indicators in the study. The evidence certainty of the results of the NMA’s assessment of pain, fatigue, and sleep disturbance ranges from very low to moderate. The main degradation factors are as follows: First, the reports of random and blind methods and other relevant information in quite a few studies are incomplete, which leads to serious bias risk. Second, the sample size of some included studies was not large, inevitably resulting in a confidence interval of NMA results that was too wide, which led to low accuracy of the comprehensive results of different studies. Third, the heterogeneity of studies is high, which may be caused by the included randomized controlled trials being different in terms of intervention timing, intervention frequency, measurement tools, treatment schemes, etc., and some study control groups contained different control measures (See Supplementary File S4).
Discussion
The 14 interventions were ranked according to the network meta-analysis results and SUCRA values. We identified the intervention with a SUCRA value>50% as more likely to reduce the severity of symptoms. Our study’s results showed that the effect of most interventions to alleviate the burden of pain, fatigue, and sleep disturbance symptoms is inconsistent. Only PMR and MBSR ranked high in the three groups of symptoms at the same time, which indicates that these two interventions are more likely to alleviate the burden of the pain-fatigue-sleep disturbance symptom cluster than other interventions.
Our study shows that PMR may be the best intervention to alleviate the pain-fatigue-sleep disturbance symptom cluster of breast cancer patients. The results of the study were similar to a meta-analysis showing that PMR is effective in alleviating symptom burden as well as quality of life in cancer patients.50 Progressive muscle relaxation exercise is the most commonly used relaxation exercise at present. It guides patients to systematically contract and relax the skeletal muscle groups of the body with the self-concept of exercise as the core concept. This intervention can alleviate the resulting physical tension and fatigue through physical and mental concentration and deep relaxation of muscles. Moreover, the generated endorphins, enkephalins, and serotonin may also alleviate physical pain and fatigue and improve sleep quality.51 Previous reports indicate that PMR is effective in reducing the incidence and severity of pain, fatigue, and sleep disturbances in patients with other types of cancer, such as stomach and gynecological cancers.52,53 However, most of these studies have a general limitation: subjects in the intervention group were administered the same intensity and frequency of the intervention protocol, without taking into account individual patient variability. Due to the high age and physical requirements of PMR, it is more difficult for frail and elderly people (>60 years old) to exercise, compliance is not high, and the intervention effect cannot be equal to that of young adults.54 Therefore, it is suggested that clinical workers should evaluate the physical condition and age of patients when using PMR interventions. For patients with weak physiques, it is necessary to reduce the exercise intensity appropriately. Future research can further analyse the intervention effects of different PMR intervention intensities on the symptom cluster. In addition, none of these studies bothered to evaluate the effects of the intervention, and it is recommended that future studies be able to evaluate the effects during the intervention and continuously improve the intervention protocol to facilitate the development of best clinical practice evidence.
Our study shows that MBSR may be likely to reduce the burden of pain-fatigue-sleep disturbance symptom clusters in breast cancer patients, which is consistent with previous report.42 MBSR is one of the most common complementary treatment currently used in cancer patients (eg, breast, lung, colorectal cancer patients, etc.).55 MBSR is a systematic stress management technology based on “mindfulness”, which emphasizes conscious awareness, focuses on the present, and does not judge all current concepts to achieve internal balance.56 The main mechanism of alleviating the burden of the pain-fatigue-sleep disturbance symptom cluster is to help patients recognize the current state and events and establish positive emotions and stress management, thus alleviating pain, fatigue, sleep disturbance, and other symptoms. At the same time, it can guide individuals to consciously expose their thoughts and emotions, enhance individual attention, empathy, and tolerance, and promote individual tolerance to symptoms.56 A meta-analysis has confirmed that MBSR also has a positive effect on psychological symptoms such as anxiety and depression in lung cancer patients, which can effectively reduce their physical and psychological symptoms and improve their quality of life. However, at present, there has been controversy about the best time to implement MBSR.57 Bisseling showed that providing MBSR during the treatment of breast cancer patients can allow them to quickly apply what they have learned to deal with the adverse reactions caused by cancer treatment, but intensive treatment and intervention plans will consume much of their time and energy.58 Patients who were treated after treatment, although their physical condition improved after the end of treatment, often experienced the fear and anxiety caused by treatment during their treatment. This negative emotion will lead to a great reduction in the follow-up intervention effect. Therefore, it is very important to choose the appropriate intervention time when conducting MBSR for breast cancer patients clinically. Patients should be given full autonomy so that they can assess their psychological vulnerability and choose when to intervene.
Our research results also show that AR and aerobic exercise are likely to alleviate two symptoms in the pain-fatigue-sleep disturbance symptom cluster, while yoga, PE and Qigong are likely to alleviate only one symptom. The other interventions (mindfulness-based cognitive therapy, Cognitive behavioural therapy, comprehensive psychological intervention, dance movement therapy, internet-based continuous rehabilitation nursing support, cranial electron therapy stimulation) ranked lower in the three groups of symptoms (SUCRA values≤50%) and were considered to be less likely to reduce the burden of the pain-fatigue-sleep disturbance symptom cluster. In the future, more studies are needed to verify the intervention effects of these interventions.
Limitations
Our NMA has several limitations. First, this study conducted only an NMA of the effects of nonpharmacological interventions on the pain-fatigue-sleep disturbance symptom cluster. Since some studies measured only symptom scores immediately after the application of the intervention, the long-term effects during different periods after application of the intervention were not evaluated. Second, because pain, fatigue, and sleep disturbance were not the primary outcome measures in some studies, they may have been missed during the screening process. Third, the overall evidence quality of our study is not high. Our sensitivity analysis shows that small sample studies have little impact on the results. However, we still need to carefully explain and promote the conclusions. In the future, more high-quality and large-sample RCTs are needed to increase the evident quality of nonpharmacological interventions to manage breast cancer symptom clusters.
Implications for Practice
In general, this study provides evidence for symptom management of breast cancer patients and proves that PMR and MBSR have a positive role in reducing the burden of symptoms of breast cancer patients. Clinical recommendations prioritize PMR for symptom management, followed by MBSR. After relevant training, nurses can integrate these interventions into the clinical setting according to the patient’s physiological status. Individualized non-pharmacological interventions are developed for psychological characteristics and age. The patient’s feedback is noted at the time of intervention, the effectiveness of the intervention is evaluated in a timely manner, and the intervention program is continuously improved in order to develop best practice evidence for the pain-fatigue-sleep disturbance symptom cluster in breast cancer patients.
Conclusions
The NMA results of this study show that PMR may be the best intervention to reduce the severity of the pain-fatigue-sleep disturbance symptom cluster in breast cancer patients. To optimize the quality of patient care, the study suggests that PMR involvement should be incorporated into daily care in breast patients. However, the findings should be treated with caution due to very low-to-moderate certainty of evidence. In the future, it is necessary to further verify the conclusions of this study through high-quality, large-sample, multicentre randomized double-blind trials. In addition, it is suggested to further explore the effect of combined nonpharmacological and pharmacological interventions on reducing the burden of symptoms of breast cancer patients to improve the clinical application prospects.
Acknowledgments
Thank Professor Jinhui Tian for his help in the statistical analysis of this study, thanks for AJE language editing company’s professional polishing.
Disclosure
The authors have no conflicts of interest to disclose for this work.
References
1. Sung H, Ferlay J, Siegel RL, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71(3):209–249. doi:10.3322/caac.21660
2. American Cancer Society. Breast Cancer Facts & Figures 2021. Atlanta: American Cancer Society, Inc; 2021.
3. Bjerkeset E, Röhrl K, Schou-Bredal I. Symptom cluster of pain, fatigue, and psychological distress in breast cancer survivors: prevalence and characteristics. Breast Cancer Res Treat. 2020;180(1):63–71. doi:10.1007/s10549-020-05522-8
4. Dodd MJ, Miaskowski C, Paul SM. Symptom clusters and their effect on the functional status of patients with cancer. Oncol Nurs Forum. 2001;28(3):465–470.
5. Al Qadire M, Alsaraireh M, Alomari K, et al. Symptom clusters predictive of quality of life among Jordanian women with breast cancer. Semin Oncol Nurs. 2021;37(2):151144. doi:10.1016/j.soncn.2021.151144
6. So WK, Law BM, Ng MS, et al. Symptom clusters experienced by breast cancer patients at various treatment stages: a systematic review. Cancer Med. 2021;10(8):2531–2565. doi:10.1002/cam4.3794
7. Beck S, Dudley WN, Barsevick AM. Using a mediation model to test a symptom cluster: pain, sleep disturbance, and fatigue in cancer patients. Oncol. Nurs Forum. 2005;32:E48–E55. doi:10.1188/05.ONF.E48-E55
8. So WK, Law BMH, Chan DNS, et al. The effect of nonpharmacological interventions on managing symptom clusters among cancer patients: a systematic review. Cancer Nurs. 2020;43(6):E304–E327. doi:10.1097/NCC.0000000000000730
9. Iwase S, Kawaguchi T, Tokoro A, et al. Assessment of cancer-related fatigue, pain, and quality of life in cancer patients at palliative care team referral: amulticenter observational study (JORTC PAL-09). PLoS One. 2015;10(8):e134022. doi:10.1371/journal.pone.0134022
10. Luciani A, Jacobsen P, Extermann M, et al. Fatigue and functional dependence in older cancer patients.Am. J Clin Oncol. 2008;31(5):424–430.
11. Su XY, Wang QQ, Liu FX. Advances in the study of biological mechanisms of symptom clusters in tumor patients. Oncol Prog. 2022;20(06):555–558+572.
12. Lynch Kelly D, Dickinson K, Hsiao CP, et al. Biological basis for the clustering of symptoms. Semin Oncol Nurs. 2016;32(4):351–360. doi:10.1016/j.soncn.2016.08.002
13. Brant JM, Beck S, Miaskowski C. Building dynamic models and theories to advance the science of symptom management research. J Adv Nurs. 2010;66(1):228–240. doi:10.1111/j.1365-2648.2009.05179.x
14. He CC, Meng LM, Liu HZ, et al. Effectiveness of non-pharmacological interventions on symptom clusters in cancer patients: a network meta-analysis. Chin Gen Pract. 2022;25(19):2414–2420.
15. Wang YY, Wu J, Li NN, et al. Research progress of intervention strategies on symptom clusters of cancer patients. Chin Nurs Res. 2020;34(02):273–278.
16. Sheikh-Wu SF, Downs CA, Anglade D. Interventions for managing a symptom cluster of pain, fatigue, and sleep disturbances during cancer survivorship: a systematic review. Oncol Nurs Forum. 2020;47(4):E107–E119. doi:10.1188/20.ONF.E107-E119
17. Tan JB, Zhai J, Wang T, et al. Self-managed non-pharmacological interventions for breast cancer survivors: systematic quality appraisal and content analysis of clinical practice guidelines. Front Oncol. 2022;12:866284. doi:10.3389/fonc.2022.866284
18. Wang L, Chen X, Peng Y, et al. Effect of a 4-week internet-delivered mindfulness-based cancer recovery intervention on the symptom burden and quality of life of patients with breast cancer: randomized controlled trial. J Med Internet Res. 2022;24(11):e40059. doi:10.2196/40059
19. Li H, Schlaeger JM, Jang MK, et al. Acupuncture improves multiple treatment-related symptoms in breast cancer survivors: a systematic review and meta-analysis. J Altern Complement Med. 2021;27(12):1084–1097. doi:10.1089/acm.2021.0133
20. Salanti G. Indirect and mixed-treatment comparison, network, or multiple-treatments meta-analysis: many names, many benefits, many concerns for the next generation evidence synthesis tool. Res Synth Methods. 2012;3(2):80–97. doi:10.1002/jrsm.1037
21. Page MJ, McKenzie JE, Bossuyt PM, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. PLoS Med. 2021;18(3):e1003583. doi:10.1371/journal.pmed.1003583
22. Higgins JPT, Green S. Cochrane handbook for systematic reviews of interventions version 5.1.0 [EB/OL]; 2011. Available from: http://handbook.cochrand/org/pdf.
23. Chaimani A, Higgins JP, Mavridis D, et al. Graphical tools for network meta-analysis in STATA. PLoS One. 2013;8(10):e76654. doi:10.1371/journal.pone.0076654
24. van Valkenhoef G, Dias S, Ades AE, et al. Automated generation of node-splitting models for assessment of inconsistency in network meta-analysis. Res Synth Methods. 2016;7(1):80–93. doi:10.1002/jrsm.1167
25. Salanti G, Ades AE, Ioannidis JP. Graphical methods and numerical summaries for presenting results from multiple-treatment meta-analysis: an overview and tutorial. J Clin Epidemiol. 2011;64(2):163–171. doi:10.1016/j.jclinepi.2010.03.016
26. Nikolakopoulou A, Higgins JPT, Papakonstantinou T, et al. CINeMA: an approach for assessing confidence in the results of a network meta-analysis. PLoS Med. 2020;17(4):e1003082. doi:10.1371/journal.pmed.1003082
27. Aydin M, Kose E, Odabas I, et al. The effect of exercise on life quality and depression levels of breast cancer patients. Asian Pac J Cancer Prev. 2021;22(3):725–732. doi:10.31557/APJCP.2021.22.3.725
28. Bower JE, Crosswell AD, Stanton AL, et al. Mindfulness meditation for younger breast cancer survivors: a randomized controlled trial. Cancer. 2015;121(8):1231–1240. doi:10.1002/cncr.29194
29. Dolbeault S, Cayrou S, Brédart A, et al. The effectiveness of a psycho-educational group after early-stage breast cancer treatment: results of a randomized French study. Psychooncology. 2009;18(6):647–656. doi:10.1002/pon.1440
30. Garssen B, Boomsma MF, Meezenbroek Ede J, et al. Stress management training for breast cancer surgery patients. Psychooncology. 2013;22(3):572–580. doi:10.1002/pon.3034
31. Hao M, Tan MY, Wu Q, et al. Effect of group mindfulness cognitive therapy on the depression, anxiety and quality of life in patients with breast cancer during chemotherapy. J Chengdu Med Coll. 2019;14(4):485–489. Chinese.
32. Ho RT, Fong TC, Cheung IK, et al. Effects of a short-term dance movement therapy program on symptoms and stress in patients with breast cancer undergoing radiotherapy: a randomized, controlled, single-blind trial. J Pain Symptom Manage. 2016;51(5):824–831. doi:10.1016/j.jpainsymman.2015.12.332
33. Jang SH, Kang SY, Lee HJ, et al. Beneficial effect of mindfulness-based art therapy in patients with breast cancer-A randomized controlled trial. Explore. 2016;12(5):333–340. doi:10.1016/j.explore.2016.06.003
34. Kim YH, Choi KS, Han K, et al. A psychological intervention programme for patients with breast cancer under chemotherapy and at a high risk of depression: a randomised clinical trial. J Clin Nurs. 2018;27(3–4):572–581. doi:10.1111/jocn.13910
35. Kurt B, Kapucu S. The effect of relaxation exercises on symptom severity in patients with breast cancer undergoing adjuvant chemotherapy: an open label non-randomized controlled clinical trial. Eur J Integr Med. 2018;22:54–61. doi:10.1016/j.eujim.2018.08.002
36. Lengacher CA, Reich RR, Post-White J, et al. Mindfulness based stress reduction in post-treatment breast cancer patients: an examination of symptoms and symptom clusters. J Behav Med. 2012;35(1):86–94. doi:10.1007/s10865-011-9346-4
37. Lyon D, Kelly D, Walter J, et al. Randomized sham controlled trial of cranial microcurrent stimulation for symptoms of depression, anxiety, pain, fatigue and sleep disturbances in women receiving chemotherapy for early-stage breast cancer. Springerplus. 2015;4:369. doi:10.1186/s40064-015-1151-z
38. Pasyar N, Barshan TN, Mansouri P, et al. Effect of yoga exercise on the quality of life and upper extremity volume among women with breast cancer related lymphedema: a pilot study. Eur J Oncol Nurs. 2019;42:103–109. doi:10.1016/j.ejon.2019.08.008
39. Paulo TRS, Rossi FE, Viezel J, et al. The impact of an exercise program on quality of life in older breast cancer survivors undergoing aromatase inhibitor therapy: a randomized controlled trial. Health Qual Life Outcomes. 2019;17(1):17. doi:10.1186/s12955-019-1090-4
40. Porter LS, Carson JW, Olsen M, et al. Feasibility of a mindful yoga program for women with metastatic breast cancer: results of a randomized pilot study. Support Care Cancer. 2019;27(11):4307–4316. doi:10.1007/s00520-019-04710-7
41. Reich RR, Lengacher CA, Alinat CB, et al. Mindfulness-based stress reduction in post-treatment breast cancer patients: immediate and sustained effects across multiple symptom clusters. J Pain Symptom Manage. 2017;53(1):85–95. doi:10.1016/j.jpainsymman.2016.08.005
42. Wang WQ, Lu WH. The effect of humanistic nursing care on quality of life for breast cancer patients undergoing chemotherapy. China Mod Doctor. 2011;49(33):100–102. Chinese.
43. Wang W, Zhou KN, Zhao WQ, et al. Effect of internet-based continuous rehabilitation nursing support on health-related quality of life in postoperative chemotherapy patients with breast cancer. Chin Nurs Res. 2019;33(11):1821–1826. Chinese.
44. Wu L. The Influence of the Baduanjin on the Quality of Life of Patients with Breast Cancer Endocrine Treatment [dissertation]. Guangzhou Sport University; 2018.
45. Yang YL, Liang XY, Liu Q, et al. Effects of moderate intensity walking exercise program on breast cancer patients with cancer-related fatigue. J Ningxia Medl Univ. 2019;41(10):1063–1068. Chinese.
46. Yang ML. Application and Evaluation of Progressive Muscle Relaxation Training in Breast Cancer Patients Receiving Chemotherapy [dissertation]. University of South China; 2020.
47. Zhang FY. Effect of Psychological Intervention on Mental State and Quality of Life of Patients with Advanced Chemotherapy [dissertation]. University of Jinan; 2017.
48. Zhu SW. Analysis of Related Factors and Intervention about Memory Recession in Breast Cancer patients with adjuvant chemotherapy [dissertation]. Nanjing Medical University; 2011.
49. Zhu KL, Wang LL, Ji QC. Effect of aerobic exercise combined with resistance training on quality of life in postmenopausal breast cancer patients treated with aromatase inhibitors. J Xinjiang Med Univ. 2020;43(8):2767–2768. Chinese.
50. Tan L, Fang P, Cui J, et al. Effects of progressive muscle relaxation on health-related outcomes in cancer patients: a systematic review and meta-analysis of randomized controlled trials. Complement Ther Clin Pract. 2022;49:101676. doi:10.1016/j.ctcp.2022.101676
51. Demiralp M, Oflaz F, Komurcu S. Effects of relaxation training on sleep quality and fatigue in patients with breast cancer undergoing adjuvant chemotherapy. J Clin Nurs. 2010;19(7–8):1073–1083. doi:10.1111/j.1365-2702.2009.03037.x
52. Song YQ, Sun GH, Wu SQ, et al. Application of progressive muscle relaxation exercise in the symptom cluster of pain, fatigue, and somnipathy disorder in patients with first-time chemotherapy after gastric cancer surgery. Oncol Prog. 2021;19(03):313–316.
53. Dikmen HA, Terzioglu F. Effects of reflexology and progressive muscle relaxation on pain, fatigue, and quality of life during chemotherapy in gynecologic cancer patients. Pain Manag Nurs. 2019;20(1):47–53. doi:10.1016/j.pmn.2018.03.001
54. Yoo HJ, Ahn SH, Kim SB, et al. Efficacy of progressive muscle relaxation training and guided imagery in reducing chemotherapy side effects in patients with breast cancer and in improving their quality of life. Support Care Cancer. 2005;13(10):826–833. doi:10.1007/s00520-005-0806-7
55. Wong KU, Palladino L, Langhan ML. Exploring the effect of mindfulness on burnout in a pediatric emergency department. Workplace Health Saf. 2021;69(10):467–473. doi:10.1177/21650799211004423
56. Chiesa A, Anselmi R, Serretti A. Psychological mechanisms of mindfulness-based interventions: what do we know? Holist Nurs Pract. 2014;28(2):124–148. doi:10.1097/HNP.0000000000000017
57. Tian X, Yi LJ, Liang CS, et al. The impact of Mindfulness-Based Stress Reduction (MBSR) on psychological outcomes and quality of life in patients with lung cancer: a meta-analysis. Front Psychol. 2022;13:901247. doi:10.3389/fpsyg.2022.901247
58. Bisseling EM, Schellekens MPJ, Jansen ETM, et al. Mindfulness-based stress reduction for breast cancer patients: a mixed method study on what patients experience as a suitable stage to participate. Support Care Cancer. 2017;25(10):3067–3074. doi:10.1007/s00520-017-3714-8
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.



