Back to Journals » Infection and Drug Resistance » Volume 16
Non-Typhoidal Salmonella Infections Among Children in Fuzhou, Fujian, China: A 10-Year Retrospective Review from 2012 to 2021
Authors Chen H, Qiu H, Zhong H , Cheng F, Wu Z , Shi T
Received 23 February 2023
Accepted for publication 22 April 2023
Published 6 May 2023 Volume 2023:16 Pages 2737—2749
DOI https://doi.org/10.2147/IDR.S408152
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Suresh Antony
Huiyu Chen,1 Huahong Qiu,1 Hui Zhong,1 Feng Cheng,2 Zhihui Wu,1 Tengfei Shi3
1Department of Clinical Laboratory, Fujian Maternity and Child Health Hospital, College of Clinical Medicine for Obstetrics & Gynecology and Pediatrics, Fujian Medical University, Fuzhou, Fujian, People’s Republic of China; 2Department of Laboratory Medicine, Fujian Children’s Hospital, Fujian Provincial Maternity and Children’s Hospital, Fuzhou, Fujian, People’s Republic of China; 3Department of Clinical Laboratory, Fuzhou Second Hospital Affiliated to Xiamen University, Fuzhou, Fujian, People’s Republic of China
Correspondence: Tengfei Shi, Department of Clinical Laboratory, Fuzhou Second Hospital Affiliated to Xiamen University, Shang Teng Road No. 47 Cang’shan District, Fuzhou, Fujian, People’s Republic of China, Tel +86-591-22169098, Email [email protected]
Purpose: Non-typhoidal salmonella (NTS) infection is a leading cause of acute gastroenteritis in children. Recently, NTS infections have increased, especially those associated with Salmonella Typhimurium, which has become a global problem because of its high level of drug resistance. Diseases caused by NTS serotypes vary considerably. We summarised NTS infections among children in Fuzhou, Fujian, China, from 2012 to 2021, and synthesised studies indicating the clinical symptoms, laboratory test results, and drug resistance associated with S. Typhimurium and non-S. Typhimurium to enhance the knowledge of these infections and improve their diagnoses and treatment.
Patients and Methods: Between January 2012 and December 2021, 691 children with NTS infections confirmed by positive culture test results were recruited from Fujian Children’s Hospital and Fujian Maternity and Child Health Hospital. Clinical demographic data of each case were collected from the electronic medical records and analysed.
Results: A total of 691 isolates were identified. The number of NTS infections increased significantly in 2017 and increased sharply during 2020 and 2021, especially S. Typhimurium greatly increased and was the dominant serotype (58.3%). S. Typhimurium infection was commonly occurred in children younger than 3 years and most of them were gastrointestinal infection, while non-S. Typhimurium more often observed in older children and associated with extra-intestinal infection. The rate of multidrug-resistant S. Typhimurium was significantly higher than that of non-S. Typhimurium, especially during the last 2 years of this study (2020 and 2021).
Conclusion: S. Typhimurium was the dominant serotype and greatly increased among children in Fuzhou city. There are significant differences in clinical symptoms, laboratory test results, and drug resistance between S. Typhimurium and non-S. Typhimurium. More attention should be paid on S. Typhimurium. Long-term high-quality surveillance and control measures should be conducted to prevent salmonella infections and drug resistance.
Keywords: S. Typhimurium, non-S. Typhimurium, clinical symptoms, laboratory test results, drug resistance
Introduction
Salmonella enterica constitutes a major public health concern and it is estimated to cause more than 300,000 annual deaths, mostly in developing countries.1,2 This highly ubiquitous species consists of more than 2600 different serotypes that can be divided into typhoidal and non-typhoidal salmonella (NTS) types.3,4 Despite their genetic similarity, these two groups elicit very different diseases and distinct immune responses in humans; therefore, they are often discussed separately.5 NTS infections are an important cause of illness in children. A study performed in the United States, including children younger than 5 years of age with laboratory-confirmed bacterial enteritis reported that NTS was the most commonly isolated (42%) bacterial enteric pathogen, followed by Campylobacter (28%), Escherichia coli O157, Shigella, and Yersinia enterocolitica.6 In Taiwan, NTS is the most common pathogen associated with childhood bacterial enterocolitis requiring hospitalization.7 In China, 70% to 80% of food poisoning incidents were caused by NTS.8 Therefore, NTS infections among children remains a significant burden on health care and are global public health concern.9 Despite extensive government and industry efforts to reduce the incidence of NTS, not much progress has been made in the reduction of the number of salmonellosis cases each year.10,11 Limited information on NTS infections in children is available in China. In recent years, NTS increased notably especially S. Typhimurium (one of serotypes in NTS) raised sharply, being one of the dominant serotypes in many countries.12–14 Meanwhile, increasing numbers of multidrug-resistant strains, including those resistant to first-line treatment such as cephalosporin and quinolones, have been observed for S. Typhimurium in many countries,15–17 and most infection outbreaks have been associated with S. Typhimurium.18–21 With the rapid growth and high drug resistance rate of S. Typhimurium in children,14,22 to enhance the knowledge of these infections and improve their diagnoses and treatment, pediatricians have paid great attention to the differences between S. Typhimurium and non-S. Typhimurium. Indeed, relevant studies have reported the genetic differences between serotypes suggest that several mechanisms are serotype-specific. Diseases caused by different serotypes vary significantly with the infection site and severity of illness. Some serotypes are more likely to cause gastrointestinal symptoms, and others are more likely to cause extra-intestinal infection especially invasive disease even death.23 Therefore, the use of specific serotypes should be considered when studying the clinical pathogenicity of NTS. Most reviews have compared the host response to both NTS and typhoidal salmonella.5,24 However, a better understanding of the diversity of NTS serotypes and clinical symptoms is lacking, and few published studies have reported the differences between S. Typhimurium and non-S. Typhimurium based on the combination of clinical symptoms, laboratory test results, and drug resistance. Therefore, this study summarised NTS infections among children in Fuzhou, Fujian, China, from 2012 to 2021, and synthesised studies indicating the diversity of clinical symptoms, laboratory test results, and drug resistance associated with S. Typhimurium and non-S. Typhimurium to enhance the understanding of these infections and improve their diagnoses and treatment in clinical settings.
Materials and Methods
Sample Collection
This retrospective study was conducted at Fujian Children’s Hospital and Fujian Maternity and Child Health Hospital between January 2012 and December 2021. Fujian Children’s Hospital is a comprehensive, domestic, first-class children’s medical center and the Fujian branch of Shanghai Children’s Medical Center. Fujian Maternity and Child Health Hospital, which is affiliated with Fujian Medical University, is the first tertiary maternal and child health hospital and one of the first neonate-specific hospitals in China. The annual number of inpatient visits is approximately 70,000, and the annual number of outpatient visits is nearly 2 million. A total of 691 children younger than 14 years of age with positive NTS culture test results were enrolled in this study. Clinical demographic data of each case were consecutively collected from the electronic medical records, including the unique sample number, name, sex, age, specimen type, serotypes, clinical symptoms, laboratory tests and results, bacterial antibiotic resistance, date of disease onset, and date of sample collection. Isolates were confirmed to be infected with Salmonella using VITEK Gram-negative identification cards (bioMerieux, Inc., Marcy-l’Étoile, France) and serotyped with salmonella diagnostic serum (Ningbo Tianrun Bio-Pharmaceutical Co., Ltd., Zhejiang Sheng, China). The isolates were stored in Baso preservative tubes (Baso Diagnostics Inc., Zhuhai, China) at −80°C until analysis.
Definitions
We defined “S. Typhimurium” infection confirmed by positive culture test results with “S. Typhimurium” concluding typical strains and monophasic variants. “non-S. Typhimurium” as NTS infection except for “S. Typhimurium” infection concluding typical strains and monophasic variants.
We defined “non-invasive” cases as those in which Salmonella had been isolated from stool, urine and vaginal secretions specimens and “invasive” cases as those in which Salmonella had been isolated from specimens, such as blood, cerebrospinal fluid, other normally sterile fluids, pus and urine, which from sterile sites.
Multidrug resistance (MDR) was defined as acquired non-susceptibility to at least one agent in three or more antimicrobial categories (classes), such as aminopenicillins (ampicillin), β-lactam combination agents (ampicillin-sulbactam, piperacillin-tazobactam), cephalosporins (ceftriaxone, ceftazidime, cefepime), monobactams (aztreonam), carbapenems (imipenem), dihydrofolate reductase inhibitors (trimethoprim-sulfamethoxazole), and fluoroquinolones (ciprofloxacin, levofloxacin).25
Antimicrobial Susceptibility Testing
Antimicrobial susceptibility was evaluated using VITEK Gram-negative drug susceptibility cards (GN09) for the following nine antimicrobial agents: amoxicillin, ampicillin-sulbactam, ceftriaxone, ceftazidime, cefepime, piperacillin-tazobactam, imipenem, meropenem, and trimethoprim/sulfamethoxazole. Antimicrobial susceptibility was evaluated using the disk diffusion test for the antimicrobial agents ciprofloxacin (5 μg) and levofloxacin (5 μg).26 All disks were obtained from Oxide Ltd. (Cambridge, UK). The results were interpreted according to the Clinical and Laboratory Standards Institute guidelines.26 E. coli ATCC25922 was used for routine quality control. Multidrug-resistance (MDR) was defined as the resistance to more than three types of antimicrobials.
Statistical Analysis
Statistical analyses were performed using GraphPad Prism 7.04 data editor. Data were described using frequencies and their proportions, and the chi-squared (X2) test was used for comparisons. Measurement data were described as the median (± standard deviation) and compared using the unpaired t-test. For all analyses, statistical significance was set at P<0.05.
Results
Prevalence
The number of NTS infections in children was stable and low during 2012 to 2016; however, infections increased significantly in 2017, and increased sharply during 2020 and 2021 (Figure 1). Serogroup B (68.2%) was the predominant serogroup isolate, followed by serogroups C (15.2%), D (9.3%), E (5.4%), F (0.1%), and ungrouped (0.3%). Among serogroup B, S. Typhimurium was the most predominant serotype, accounting for 54.7% (378/691) of cases comprising more than half of all isolates. Notably, the average rate of S. Typhimurium infections was 27.7% (2012–2015) before 2015; however, this rate increased to 58.3% after 2015 (2016–2021) (Figure 1). The next most common serotypes were Salmonella Enteritidis (8.4%; 58/691), Salmonella Stanley (4.1%; 28/691), Salmonella London (3.8%; 26/691), and Salmonella Derby (3.3%; 23/691). The distribution of 691 NTS isolates, serogroups, and serotypes are presented in Table 1.
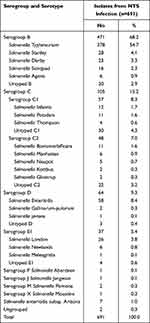 |
Table 1 Distribution of 691 NTS Isolates of Different Serotypes, Fuzhou, China, 2012–2021 |
 |
Figure 1 Distribution of 691 isolates of different serotypes and the ratio of S. Typhimurium/NTS in children <14 years old with NTS infection by year, Fuzhou, China, 2012–2021. |
NTS infections occurred throughout the year, but they were more frequently observed during summer and autumn (May–October), accounting for 80.9% of cases; most cases occurred in July (13.5%), August (17.8%), and September (21.4%). During each month, the proportion of S. Typhimurium infections was approximately 50% (Figure 2).
 |
Figure 2 Distribution of 691 isolates of different serotypes in children <14 years old with NTS infection by month, Fuzhou, China, 2012–2021. |
Demographic and Clinical Manifestations
During the 10-year study period, 105,326 blood, 6327 urine, 6781 cerebrospinal fluid and 18215 stool (1861 in 2012, 1255 in 2013, 1362 in 2014, 1127 in 2015, 1200 in 2016, 1847 in 2017, 1959 in 2018, 2032 in 2019, 2772 in 2020, 2800 in 2021), 53 vaginal secretions culture, 10 Joint fluid culture samples were screened in total; 691 cases of NTS infections were confirmed (among which 656 (94.9%) were only positive in faeces culture, 21 (3.0%) were only positive in blood culture, 4 (0.6%) were only positive in joint fluid culture and 1 (0.1%) was only positive in vaginal secretions culture, urine culture, abdominal culture and cerebrospinal fluid culture, respectively. Both faecal and blood culture were positive in 6 cases (0.9%) (Table 2).
 |
Table 2 Demographic and Clinical Manifestations of Children with NTS Infection, Fuzhou, China, 2012–2021 |
Most of the NTS infections confirmed by blood samples were observed in children younger than 2 years of age (26 cases; 96.3%); however, 21 cases (77.8%) were observed in children younger than 1 year of age and 2 cases were observed in newborns. Among these infections, only one was observed in a child older than 2 years of age. Among the 691 NTS infections, the male-to-female ratio was 1.4:1. NTS infections commonly occurred in children 1 month to 3 years of age, accounting for 91.9% of the isolates (635/691). Laboratory test results indicated white blood cell, neutrophil, and C-reactive protein levels of 11.23±0.1871, 52.43±0.7153, and 30.36±1.611, respectively (Table 2). Among these patients, 91.5% (617/674) had diarrhea (mostly watery or sticky stools). Pus was detected in 68.1%, erythrocytes were detected in 40.1%, and occult blood was detected in 78.7% of the stool samples. A total of 65.6% of the patients had fever. Respiratory symptoms, such as cough, expectoration, and shortness of breath, were relatively common in children with NTS infections (25.8%).
We compared the demographics and clinical manifestations of S. Typhimurium infections and non-S. Typhimurium infections. S. Typhimurium infections commonly occurred in children 1 month to 3 years of age, accounting for 92.6% of isolates (350/378), which was significantly higher than the rate of non-S. Typhimurium infections (87.6%; 274/313). However, the opposite was observed in those older than 3 years. Significantly more stool samples were positive for S. Typhimurium infections than for non-S. Typhimurium infections; furthermore, invasive infections, such as blood infections, were significantly more frequently observed with non-S. Typhimurium than with S. Typhimurium. Among the 371 patients with S. Typhimurium infections, 352 (94.9%) had diarrhea. However, 265 patients (87.5%) in the non-S. Typhimurium group had diarrhea. The rate of diarrhea of patients with S. Typhimurium infections was significantly higher than that of patients with non-S. Typhimurium infections. Among children with diarrhea, 74.3% with S. Typhimurium infections had mucus in their stool samples; this rate was significantly higher than that of children with non-S. Typhimurium infections (60.5%) (Table 2).
Antimicrobial Resistance
During this 10-year study period, 691 NTS isolates were identified and 415 infections (60.1%) were MDR. The MDR rate during the last 5 years (2017–2021) was significantly higher than that during the first 5 years (2012–2016) of the study. The drug resistance of almost all drugs and MDR increased during the last 5 years of the study, mainly the resistance to trimethoprim/sulfamethoxazole increased significantly (Figure 3). The NTS isolates were highly resistant to amoxicillin (69.3%) and ampicillin sulbactam (59.0%). Notably, resistance to third-generation cephalosporins, such as ceftriaxone and ceftazidime, was detected in 24.5% and 14.6% of isolates, respectively, whereas resistance to fluoroquinolones, such as ciprofloxacin, was observed in 15.8% of samples and 53.3% of isolates presented reduced susceptibility to ciprofloxacin (n=368). Only 13 isolates were resistant to piperacillin/tazobactam, and only four isolates were resistant to imipenem (Table 3).
 |
Table 3 Antimicrobial Resistance Among S. Typhimurium and Non-S. Typhimurium Isolates from Children <14 Years Old, Fuzhou China, 2012–2021 |
We compared the antimicrobial resistance of S. Typhimurium with that of non-S. Typhimurium and found that the resistance of S. Typhimurium to all antibiotics, including amoxicillin and ampicillin sulbactam, ceftriaxone, ciprofloxacin and trimethoprim/sulfamethoxazole, was significantly higher than that of non-S. Typhimurium. More S. Typhimurium isolates compared to non-S. Typhimurium isolates were resistant to first-line agents (Table 3). The rate of MDR associated with S. Typhimurium isolates was higher than that associated with non-S. Typhimurium isolates, particularly during the last 2 years of this study (2020 and 2021) (Figure 4).
Discussion
During our study, we summarized NTS infections among children <14 years old in Fuzhou, Fujian, China, during 2012 to 2021 and found that S. Typhimurium was the dominant serotype and increased sharply. Meanwhile, our data indicated the difference of clinical symptoms, laboratory test results, and drug resistance between S. Typhimurium infection and non-S. Typhimurium infection. We found that S. Typhimurium infection was commonly occurred in children younger than 3 years and most of them are gastrointestinal infection while non-S. Typhimurium infection more often observed in older children and associated with extra-intestinal infection. The rate of multidrug-resistant with S. Typhimurium isolates was significantly higher than that of non-S. Typhimurium isolates, especially during the last 2 years of this study (2020 and 2021).
Fuzhou is located on the southeast edge of Eurasia and southeast coast of China (25°15′–26°39′N, 118°08′–120°31′E); It has a typical subtropical monsoon climate, with an average annual temperature of 20°C to 25°C, the hottest temperatures occur from July to September, with an average temperature of 33°C to 37°C. The annual relative humidity is approximately 77%. In this study, Salmonella infection increased in the warm seasons, especially in summer. Stronger research evidence indicated that Salmonella infections are elevated in warm climates.27,28
In our study, S. Typhimurium was the most predominant serotype in children <14 years old and greatly increased annually. Other studies performed in China also showed that S. Typhimurium was the most common serotype in children.14,22,29 However, some studies have shown that S. Enteritidis was the most common serotype in China.30,31 According to our results, the next most common serotypes were S. Enteritidis and S. Stanley. In some countries, most human clinical cases are dominated by one serotype, such as S. Typhimurium in Australia or S. Enteritidis in Brazil and Europe.32–34 However, other countries have a more balanced distribution of cases associated with each serotype, such as S. Enteritidis, Salmonella Newport, and S. Typhimurium in the United States, and S. Enteritidis, S. Stanley, and Salmonella Weltevreden in Thailand.35,36 Despite the discrepancy in the rates of illness in each geographic region, S. Typhimurium and S. Enteritidis have been consistently reported as the serotypes that contribute to the highest rates of clinical human salmonellosis worldwide.22,29–32,37 The variation in serotype distribution in a region may be associated with the presence of a local animal reservoir especially pets like turtles38 or food source mainly come from eggs and poultry.21 Meanwhile, the cause of the increase in S. Typhimurium infections with children annually is not clear. This trend was also happened in other areas in China like Hangzhou,39 Chongqing,14 Ningbo22 and so on. Data from the European Food Safety Authority (EFSA) showed that outbreaks caused by S. Typhimurium were mainly attributed to pork.21 Also a study investigating meat derived from livestock revealed that S. Typhimurium was the dominant serotype in China from 2018 to 2020.40 Another investigation in China revealed the most prevalent Salmonella serotypes were S. Typhimurium in retail meats and eggs.41 S. Typhimurium has had the main ecological role in regional food production systems, such as poultry and egg, which has been successfully occupied by S. Typhimurium and has become a reservoir for its further transmission through contaminated food products. To prioritise interventions, it is important to evaluate the sources and trends of salmonellosis outbreaks. We recommend monitoring food safety to reduce the risk of the infection of S. Typhimurium.
In our study, S. Typhimurium infection was commonly occurred in children younger than 3 years and most of them are gastrointestinal infection, while non-S. Typhimurium more often observed in older children and associated with extra-intestinal infection. This could be because it is customary for Chinese parents to feed congee mixed with cooked minced pork and steamed egg during the weaning of infants, and S. Typhimurium infections are mostly attributed to pork and egg.21,41 The result was similar to the other study.14,22 Other studies has showed that nontyphoidal Salmonella serotypes are diverse in their host range and epidemiology and vary in their propensity to cause bloodstream infection and severe human disease.23 In our study, a significantly higher number of patients with S. Typhimurium infections had diarrhea than non-S. Typhimurium. Our findings were not entirely consistent with investigation from a large survey conducted at the same time in Zhejiang province, which may result from differences in the selection of population.42 The group we had choose was children confirmed by positive culture test results including medical and surgical patients while they choose all groups with bacterial foodborne diseases. However, invasive infections, especially blood infections, were observed significantly more often with non-S. Typhimurium than with S. Typhimurium. This phenomenon was similar to that reported by others both here and abroad.23,43 It has been reported that Salmonella Typhimurium is considered to have a modest likelihood of causing NTS infections, while other less common serotypes, such as Heidelberg, Choleraesuis, and Dublin, are more likely to cause invasive disease.43 We found that Salmonella Saintpaul could easily cause invasive infections; therefore, further research will be performed in the future. Virulence mechanisms, genetic differences in serotypes, and the host immune status contribute to the diversity of clinical symptoms.
Gastroenteritis and fever were the most common clinical symptoms of infected patients. Among the 691 cases of salmonella infections, a significantly higher number of patients with S. Typhimurium infections had diarrhea. S. Typhimurium is an invasive bacterium than invades intestinal mucosal epithelial cells, causes mucus, pus, and blood in the stool, and produces enterotoxin, thereby enhancing intestinal mucosal secretion and leading to diarrhea.44 Routine fecal analyses were often performed to assess the functional status of the gastrointestinal tract. Mucus, pus, and red blood cells were commonly observed with bacterial enteritis. Among those with diarrhea, significantly more children with S. Typhimurium had feces containing mucus compared to those with non-S. Typhimurium. S. Typhimurium results in more efficient host cell invasion and is likely to contribute to a strong inflammatory response and higher infiltration of neutrophils and other inflammatory cells.45 Fever is also a common clinical symptom of infection. In general, the rates of vomiting and bloody stools were not high. Because the age of onset was approximately 3 years, the symptoms of abdominal pain could not be accurately evaluated clinically. In addition to digestive tract symptoms, respiratory tract infections were relatively common in children infected with salmonella, with an incidence close to that of children infected with S. Typhimurium in the Xiamen area.13 Another concomitant symptom was convulsions. Convulsions were complicated by high fevers, and their rates are similar to those of febrile convulsions.18 It is considered that convulsions are secondary to high fevers. Salmonella infections some cause myocardial damage. Laboratory examinations of white blood cells, neutrophils, and C-reactive protein levels were commonly performed to evaluate the degree of the inflammatory response. For children with diarrhea and bloody stools accompanied by fever and increased white blood cells and C-reactive protein levels, it is necessary to perform a fecal culture test as soon as possible to clarify the diagnosis and guide treatment.
The resistance to the most antibiotics and the rate of MDR of S. Typhimurium was significantly higher than that of non-S. Typhimurium, consistent with domestic report in China.46,47 Our findings demonstrated a high rate of ampicillin resistance among S. Typhimurium isolates. The result was similar to the nationwide average.48 These results suggest that ampicillin is not appropriate for the treatment of S. Typhimurium in the region where this study was performed. Additionally, fluoroquinolones have not been approved by the Food and Drug Administration for those younger than 18 years of age, and they are not recommended unless the benefits of therapy outweigh the potential risks of drug administration.49 Therefore, third-generation cephalosporins are considered alternative drugs for the treatment of salmonellosis in infants.50 But our study revealed a high rate of resistance to third-generation cephalosporins, such as ceftriaxone that was similar to the nationwide average.48 Therefore, the rapid increase in the resistance to third-generation cephalosporins in infants is considered a warning signal. The proportion of multidrug-resistant isolates reached 70.1%, thus surpassing that of Salmonella spp. This finding is consistent with that of Ke et al,51 who found that the rate of MDR associated with S. Typhimurium was very high and increased yearly, with even higher rates observed for infants. Therefore, the increase in multidrug-resistant salmonella isolates, particularly those resistant to ciprofloxacin and third-generation cephalosporins, may lead to the failure of human infection treatments. Colistin is a last-line antibiotic used for the clinical treatment of multidrug-resistant Enterobacteriaceae infections. Unfortunately, a 10-year study involving continuous surveillance and a genomic study performed in Guangdong, Southern China, revealed that the emergence of and relatively high prevalence of the mobilised colistin resistance (mcr-1) gene have spread throughout the entire human health system, thereby threatening the usage of colistin in the clinical setting.52 During our study, most isolates were sensitive to piperacillin/tazobactam, indicating that it can be considered an alternative for treating infections caused by third-generation cephalosporin-resistant strains or multidrug-resistant salmonella isolates in infants, thus reducing the use of carbapenem antibiotics. Careful surveillance of salmonella and its microbial resistance patterns is important to the prevention and control of the increase of multidrug-resistant clones.53
Limitations
This retrospective study had some limitations. First of all, the case data were collected through the electronic medical records, some information was either missing or incomplete such as the etiology and food categories, settings, etc. because the physicians focus on the diagnosis and treatment of the disease, the etiology often is ignored, so the conclusions might not be representative. Second, lack of cognition and limited detection capacity, we did not carry out the detection of monophasic isolates clinically. Due to the absence of the strain, we were unable to retest to differentiate S. Typhimurium typical strains and monophasic variants. Further case surveillance should focus on the etiology and food, and make efforts to carry out the test to differentiate S. Typhimurium typical strains and monophasic variants clinically.
Conclusion
In summary, our current data described the epidemiology characteristics of NTS infections among children in Fuzhou city for the first time over the past 10 years. S. Typhimurium was the dominant serotype and greatly increased. There are some significant differences in clinical symptoms, laboratory test results, and drug resistance between S. Typhimurium and non-S. Typhimurium. Understanding the differences which might benefit the prevention and treatment of NTS infection. Meanwhile, the rapid increase in the resistance of S. Typhimurium is considered a warning signal. It is necessary to carry out drug resistance analysis and whole genome sequencing of Salmonella cases, and further explore its biological mechanism. An overall assessment of Salmonella infection in residents by strengthening surveillance, source attribution and burden estimation is a matter of great urgency and more efforts should be directed toward conducting comprehensive assessments for specific public health policy formulation.
Abbreviations
MDR, S. Typhimurium, non-S. Typhimurium.
Ethics Approval and Consent to Participate
This study was approved by the Ethics Committee of the Fujian Maternity and Child Care Hospital (No. 2023KY001). Informed consent was obtained from all the participants prior to enrollment. Our study was carried out followed the principles outlined in the Declaration of Helsinki.
Acknowledgments
We would like to thank Editage (www.editage.cn) for English language editing.
Funding
This work was supported by the Fuzhou Science and Technology Bureau [grant number 2021-S-163].
Disclosure
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
1. Seif Y, Kavvas E, Lachance JC, et al. Genome-scale metabolic reconstructions of multiple Salmonella strains reveal serovar-specific metabolic traits. Nat Commun. 2018;9(1):3771. doi:10.1038/s41467-018-06112-5
2. Majowicz SE, Musto J, Scallan E, et al. The global burden of nontyphoidal Salmonella gastroenteritis. Clin Infect Dis. 2010;50(6):882–889. doi:10.1086/650733
3. Zhang J, Wei L, Kelly P, et al. Detection of Salmonella spp. using a generic and differential FRET-PCR. PLoS One. 2013;8(10):e76053. doi:10.1371/journal.pone.0076053
4. Issenhuth-Jeanjean S, Roggentin P, Mikoleit M, et al. Supplement 2008–2010 (no. 48) to the White-Kauffmann-Le Minor scheme. Res Microbiol. 2014;165(7):526–530. doi:10.1016/j.resmic.2014.07.004
5. Gal-Mor O, Boyle EC, Grassl GA. Same species, different diseases: how and why typhoidal and non-typhoidal Salmonella enterica serotypes differ. Front Microbiol. 2014;5:391. doi:10.3389/fmicb.2014.00391
6. Scallan E, Mahon BE, Hoekstra RM, Griffin PM. Estimates of illnesses, hospitalizations and deaths caused by major bacterial enteric pathogens in young children in the United States. Pediatr Infect Dis J. 2013;32(3):217–221. doi:10.1097/INF.0b013e31827ca763
7. Su LH, Chiu CH. Salmonella: clinical importance and evolution of nomenclature. Chang Gung Med J. 2007;30(3):210–219.
8. Scallan E, Hoekstra RM, Angulo FJ, et al. Foodborne illness acquired in the United States--major pathogens. Emerg Infect Dis. 2011;17(1):7–15. doi:10.3201/eid1701.P11101
9. Magwedere K, Rauff D, De Klerk G, Keddy KH, Dziva F. Incidence of nontyphoidal Salmonella in food-producing animals, animal feed, and the associated environment in South Africa, 2012–2014. Clin Infect Dis. 2015;61(Suppl 4):S283–S289. doi:10.1093/cid/civ663
10. Boore AL, Hoekstra RM, Iwamoto M, Fields PI, Bishop RD, Swerdlow DL. Salmonella enterica infections in the United States and assessment of coefficients of variation: a novel approach to identify epidemiologic characteristics of individual serotypes, 1996–2011. PLoS One. 2015;10(12):e0145416. doi:10.1371/journal.pone.0145416
11. Ford L, Glass K, Veitch M, et al. Increasing incidence of Salmonella in Australia, 2000–2013. PLoS One. 2016;11(10):e0163989. doi:10.1371/journal.pone.0163989
12. Centers for Disease Control and Prevention. Vital signs: incidence and trends of infection with pathogens transmitted commonly through food--foodborne diseases active surveillance network, 10 US sites, 1996–2010. MMWR Morb Mortal Wkly Rep. 2011;60(22):749–755.
13. Maia DSV, Haubert L, Würfel SFR, et al. Listeria monocytogenes in sliced cheese and ham from retail markets in southern Brazil. FEMS Microbiol Lett. 2019;366(22). doi:10.1093/femsle/fnz249
14. Wu LJ, Luo Y, Shi GL, Li ZY. Prevalence, clinical characteristics and changes of antibiotic resistance in children with nontyphoidal Salmonella infections from 2009–2018 in Chongqing, China. Infect Drug Resist. 2021;14:1403–1413. doi:10.2147/IDR.S301318
15. Neuert S, Nair S, Day MR, et al. Prediction of phenotypic antimicrobial resistance profiles from whole genome sequences of non-typhoidal Salmonella enterica. Front Microbiol. 2018;9:592. doi:10.3389/fmicb.2018.00592
16. Kuang D, Zhang J, Xu X, et al. Emerging high-level ciprofloxacin resistance and molecular basis of resistance in Salmonella enterica from humans, food and animals. Int J Food Microbiol. 2018;280:1–9. doi:10.1016/j.ijfoodmicro.2018.05.001
17. Hong YP, Wang YW, Huang IH, et al. Genetic relationships among multidrug-resistant Salmonella enterica Serovar Typhimurium strains from humans and animals. Antimicrob Agents Chemother. 2018;62(5). doi:10.1128/AAC.00213-18
18. Russini V, Corradini C, Rasile E, et al. A familiar outbreak of monophasic Salmonella serovar Typhimurium (ST34) involving three dogs and their owner’s children. Pathogens. 2022;11(12):12. doi:10.3390/pathogens11121500
19. Bai L, Wang J, Liu LS, et al. 单相鼠伤寒沙门菌污染巧克力产品所致多国暴发事件 对我国食源性致病菌污染风险管理的启示 [Implications for risk management of foodborne pathogens in China from the outbreak of monophasic salmonella enterica serovar Typhimurium contaminated chocolate products]. Zhonghua Yu Fang Yi Xue Za Zhi. 2022;56(11):1648–1656. Chinese. doi:10.3760/cma.j.cn112150-20220712-00711
20. Laidlow TA, Stafford R, Jennison AV, et al. A multi-jurisdictional outbreak of Salmonella Typhimurium infections linked to backyard poultry-Australia, 2020. Zoonoses Public Health. 2022;69(7):835–842. doi:10.1111/zph.12973
21. Chanamé Pinedo L, Mughini-Gras L, Franz E, Hald T, Pires SM. Sources and trends of human salmonellosis in Europe, 2015–2019: an analysis of outbreak data. Int J Food Microbiol. 2022;379:109850. doi:10.1016/j.ijfoodmicro.2022.109850
22. Ke Y, Lu W, Liu W, Zhu P, Chen Q, Zhu Z. Non-typhoidal Salmonella infections among children in a tertiary hospital in Ningbo, Zhejiang, China, 2012–2019. PLoS Negl Trop Dis. 2020;14(10):e0008732. doi:10.1371/journal.pntd.0008732
23. Jones TF, Ingram LA, Cieslak PR, et al. Salmonellosis outcomes differ substantially by serotype. J Infect Dis. 2008;198(1):109–114. doi:10.1086/588823
24. Gilchrist JJ, MacLennan CA, Hill AV. Genetic susceptibility to invasive Salmonella disease. Nat Rev Immunol. 2015;15(7):452–463. doi:10.1038/nri3858
25. Magiorakos AP, Srinivasan A, Carey RB, et al. Multidrug-resistant, extensively drug-resistant and pandrug-resistant bacteria: an international expert proposal for interim standard definitions for acquired resistance. Clin Microbiol Infect. 2012;18(3):268–281. doi:10.1111/j.1469-0691.2011.03570.x
26. Institute. CaLS. Performance standards for antimicrobial susceptibility testing[s]. M100-S31 Wayne, PA: CLSI; 2021.
27. Lake IR. Food-borne disease and climate change in the United Kingdom. Environ Health. 2017;16(Suppl 1):117. doi:10.1186/s12940-017-0327-0
28. Naumova EN, Jagai JS, Matyas B, DeMaria A Jr, MacNeill IB, Griffiths JK. Seasonality in six enterically transmitted diseases and ambient temperature. Epidemiol Infect. 2007;135(2):281–292. doi:10.1017/S0950268806006698
29. Yue M, Li X, Liu D, Hu X. Serotypes, antibiotic resistance, and virulence genes of Salmonella in children with diarrhea. J Clin Lab Anal. 2020;34(12):e23525. doi:10.1002/jcla.23525
30. Zhang SX, Zhou YM, Tian LG, et al. Antibiotic resistance and molecular characterization of diarrheagenic Escherichia coli and non-typhoidal Salmonella strains isolated from infections in Southwest China. Infect Dis Poverty. 2018;7(1):53. doi:10.1186/s40249-018-0427-2
31. Li Y, Xie X, Xu X, et al. Nontyphoidal salmonella infection in children with acute gastroenteritis: prevalence, serotypes, and antimicrobial resistance in Shanghai, China. Foodborne Pathog Dis. 2014;11(3):200–206. doi:10.1089/fpd.2013.1629
32. Hendriksen RS, Vieira AR, Karlsmose S, et al. Global monitoring of Salmonella serovar distribution from the World Health Organization global foodborne infections network country data bank: results of quality assured laboratories from 2001 to 2007. Foodborne Pathog Dis. 2011;8(8):887–900. doi:10.1089/fpd.2010.0787
33. OzFoodNet Working Group. Monitoring the incidence and causes of diseases potentially transmitted by food in Australia: annual report of the OzFoodNet network, 2011. Commun Dis Intell Q Rep. 2015;39(2):E236–E264.
34. Authority EF. The European Union summary report on trends and sources of zoonoses, zoonotic agents and food-borne outbreaks in 2016. EFSA J. 2017;15(12):e05077. doi:10.2903/j.efsa.2017.5077
35. CfDCaPC. National Enteric Disease Surveillance: Salmonella Annual Report, 2016. Atlanta, GA: Centers for Disease Control and Prevention; 2016.
36. Hendriksen RS, Bangtrakulnonth A, Pulsrikarn C, et al. Risk factors and epidemiology of the ten most common Salmonella serotypes from patients in Thailand: 2002–2007. Foodborne Pathog Dis. 2009;6(8):1009–1019. doi:10.1089/fpd.2008.0245
37. OzFoodNet Working Group. Monitoring the incidence and causes of diseases potentially transmitted by food in Australia: annual report of the OzFoodNet network, 2010. Commun Dis Intell Q Rep. 2012;36(3):E213–E241.
38. Waltenburg MA, Perez A, Salah Z, et al. Multistate reptile- and amphibian-associated salmonellosis outbreaks in humans, United States, 2009–2018. Zoonoses Public Health. 2022;69(8):925–937. doi:10.1111/zph.12990
39. Yue M, Liu D, Li X, et al. Epidemiology, serotype and resistance of Salmonella isolates from a children’s hospital in Hangzhou, Zhejiang, China, 2006–2021. Infect Drug Resist. 2022;15:4735–4748. doi:10.2147/IDR.S374658
40. Weng R, Gu Y, Zhang W, et al. Whole-genome sequencing provides insight into antimicrobial resistance and molecular characteristics of Salmonella from livestock meat and diarrhea patient in Hanzhong, China. Front Microbiol. 2022;13:899024. doi:10.3389/fmicb.2022.899024
41. Li C, Gu X, Zhang L, et al. The occurrence and genomic characteristics of mcr-1-harboring Salmonella from retail meats and eggs in Qingdao, China. Foods. 2022;11(23):3854.
42. He Y, Wang J, Zhang R, et al. Epidemiology of foodborne diseases caused by Salmonella in Zhejiang Province, China, between 2010 and 2021. Front Public Health. 2023;11:1127925. doi:10.3389/fpubh.2023.1127925
43. Crump JA, Sjölund-Karlsson M, Gordon MA, Parry CM. Epidemiology, clinical presentation, laboratory diagnosis, antimicrobial resistance, and antimicrobial management of invasive Salmonella infections. Clin Microbiol Rev. 2015;28(4):901–937. doi:10.1128/CMR.00002-15
44. Galán JE. Salmonella Typhimurium and inflammation: a pathogen-centric affair. Nat Rev Microbiol. 2021;19(11):716–725. doi:10.1038/s41579-021-00561-4
45. Aviv G, Cornelius A, Davidovich M, et al. Differences in the expression of SPI-1 genes pathogenicity and epidemiology between the emerging Salmonella enterica serovar infantis and the model Salmonella enterica serovar Typhimurium. J Infect Dis. 2019;220(6):1071–1081. doi:10.1093/infdis/jiz235
46. Shen H, Chen H, Ou Y, et al. Prevalence, serotypes, and antimicrobial resistance of Salmonella isolates from patients with diarrhea in Shenzhen, China. BMC Microbiol. 2020;20(1):197. doi:10.1186/s12866-020-01886-5
47. Wei Z, Xu X, Yan M, et al. Salmonella Typhimurium and Salmonella enteritidis infections in sporadic diarrhea in children: source tracing and resistance to third-generation cephalosporins and ciprofloxacin. Foodborne Pathog Dis. 2019;16(4):244–255. doi:10.1089/fpd.2018.2557
48. Fupin HU, Guo Y, Zhu D, et al. CHINET surveillance of antimicrobial resistance among the bacterial isolates in 2021. Chin J Infect Chemother. 2022;22(5)::521–529.
49. A.A.O. Pediatrics. Pickering, red book: 2012 report of the committee on infectious diseases. J Clin Neurophysiol. 2012;11:128–132.
50. World Health Organization. WHO guidelines approved by the guidelines review committee. In: WHO Recommendations on the Management of Diarrhoea and Pneumonia in HIV-Infected Infants and Children: Integrated Management of Childhood Illness (IMCI). Geneva: Copyright © World Health Organization 2010; 2010.
51. Ke B, Sun J, He D, Li X, Liang Z, Ke CW. Serovar distribution, antimicrobial resistance profiles, and PFGE typing of Salmonella enterica strains isolated from 2007–2012 in Guangdong, China. BMC Infect Dis. 2014;14:338. doi:10.1186/1471-2334-14-338
52. Sun RY, Fang LX, Ke BX, et al. Carriage and transmission of mcr-1 in Salmonella typhimurium and its monophasic 1,4,[5],12: i:-variants from diarrheal outpatients: a 10-year genomic epidemiology in Guangdong, Southern China. Microbiol Spectr. 2023:e0311922. doi:10.1128/spectrum.03119-22
53. Wong MH, Yan M, Chan EW, Liu LZ, Kan B, Chen S. Expansion of Salmonella enterica serovar typhimurium ST34 clone carrying multiple resistance determinants in China. Antimicrob Agents Chemother. 2013;57(9):4599–4601. doi:10.1128/AAC.01174-13
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.


