Back to Journals » International Journal of General Medicine » Volume 14
Nomogram Incorporating the WNT/β-Catenin Signaling Pathway for Predicting the Survival of Cutaneous Melanoma
Authors Zhou YX, Wang X, Pang DQ , Wang YM, Bai J, Tian F, Han D, Shi S, Hu L
Received 5 March 2021
Accepted for publication 14 June 2021
Published 23 June 2021 Volume 2021:14 Pages 2751—2761
DOI https://doi.org/10.2147/IJGM.S309616
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Scott Fraser
Yu-Xin Zhou,1 Xin Wang,2 De-Quan Pang,1 Ying-Man Wang,3 Jing Bai,4 Fei Tian,1 Duo Han,1 Shuwei Shi,1 Lei Hu1
1Department of Radiation Oncology, North China University of Science and Technology Affiliated Hospital, Tangshan, Hebei, People’s Republic of China; 2Department of Radiation Oncology, Tianjin Medical University Cancer Institute & Hospital, National Clinical Research Center for Cancer, Key Laboratory of Cancer Prevention & Therapy, Tianjin, Tianjin’s Clinical Research Center for Cancer, Tianjin, People’s Republic of China; 3Department of Radiation and Chemotherapy Oncology, North China University of Science and Technology Affiliated Hospital, Tangshan, Hebei, People’s Republic of China; 4Department of Pharmacology, North China University of Science and Technology, Tangshan, Hebei, People’s Republic of China
Correspondence: De-Quan Pang
Department of Radiation Oncology, North China University of Science and Technology Affiliated Hospital No. 73, Jianshe South Road, Lubei District, Tangshan, Hebei, 063000, People’s Republic of China
Tel +86-18713899515
Email [email protected]
Background: Accurate prediction of the survival of cutaneous melanoma (CM) permits the selection of the optimal treatment. Currently, the TNM stage has limitations in predicting the survival of CM. There is evidence that the WNT/β-catenin signaling pathway has the potential to predict the CM prognosis. However, it still needs further investigation.
Objective: This study aims to establish a nomogram incorporating the WNT/β-catenin signaling pathway to improve the predicted accuracy of the overall survival (OS) of CM.
Methods: Two hundred and eighty CM patients were recruited and followed up. The clinicopathological characteristics and the key genes of the WNT/β-catenin signaling pathway (VEGF, β-catenin, and DKK1) were chosen as potential variables associated with the OS. In the training cohort (n = 190), a nomogram was built to estimate the 1-, 3-, and 5-year OS, and its discriminations and calibrations were valid by the verification cohort (n = 90). The predicted accuracies of the nomogram with or without the Wnt/β-catenin pathway and TNM stage were compared.
Results: A nomogram integrating independent risk factors (ulceration, lymph node metastasis, distant metastasis, Breslow thickness, dermal mitoses, β-catenin, VEGF, and DKK1), which were evaluated by a multivariate analysis, was constructed to predict the 1-, 3-, and 5-year OS of CM patients. Good discrimination and calibration were obtained regardless of the training or validation datasets. The nomogram incorporating the Wnt/β-catenin signaling pathway showed the highest accuracy [area under the curve (AUC)=0.914, 0.852, 0.785] compared with the nomogram without the Wnt/β-catenin signaling pathway (AUC=0.693, 0.640, 0.615) and the TNM stage (AUC=0.726, 0.693, 0.673).
Conclusion: The prognostic value of the established nomogram incorporating the WNT/β-catenin signaling pathway was better than it without WNT/β-catenin signaling pathway and TNM stage, which might be beneficial in the development of optimal treatment options.
Keywords: cutaneous melanoma, WNT/β-catenin signaling pathway, TNM, overall survial, nomogram
Introduction
Cutaneous melanoma (CM) is a highly aggressive malignant tumor with increasing morbidity and mortality rates worldwide.1 The use of the American Joint Committee on Cancer (AJCC) staging, which usually determines the prognosis of CM patient based on tumor thickness, ulceration, mitotic rate, lymph-node metastasis, and distant metastasis, has limitations in predicting the survival of CM since the survival outcomes vary widely even within the same stage.2–5 It has been hypothesized that the prognostic prediction of CM will be improved by adding measurements of molecular features to the current staging. New biomarkers to address the inconsistencies of an imperfect staging are needed.6 Evidence from melanocyte development indicate the possibility that WNT/β-catenin signaling orchestrates melanoma progression by regulating cell proliferation and invasion and modulating the immune microenvironment.7,8 An increasing level of nuclear β-catenin has been shown to play a critical role in melanoma during disease progression.9,10 In actively proliferating melanoma cells, nuclear β-catenin triggers the expression of microphthalmia-associated transcription factor that in turn activates the transcription of several downstream genes including VEGF.11–13 Besides, aberrant expression of WNT/β-catenin antagonists (eg, DKKs) is common in melanoma and is associated with elevated β-catenin level.14 Hence, it is not surprising that the WNT/β-catenin signaling pathway may play an important role in the initiation and progression of CM.15–17 Growing research has shown that key genes of the WNT/β-catenin signaling pathway (β-catenin, VEGF, and DKK1) have the potential to act as new biomarkers to predict the prognosis of CM patients.18–21 However, an interstudy variability exists despite extensive studies.22
This study aims to establish a nomogram associated with the clinicopathological characteristics and the WNT/β-catenin signaling pathway and assess whether the incorporation of the WNT/β-catenin signaling pathway increased the accuracy in the prediction of the CM prognosis.
Patients and Methods
Population
This prospective study was conducted following the declaration of Helsinki. All procedures were approved by the ethical committee of North China University of Science and Technology Affiliated Hospital. Written informed consents were obtained from all participants.
A total of 280 CM patients (53.03±16.82 years) who underwent complete surgical resection between January 2010 and December 2014 were recruited in this study. The pathologic diagnoses of CM were evaluated from postsurgical pathology. None of the patients received preoperative chemotherapy or radiation therapy. Patients with other malignancies and incomplete follow-up data were excluded.
Collection of Clinical and Pathological Indicators
Drawing on the literature and the available evidence, the following clinical and pathological characteristics were chosen as potential variables. The continuous variables were transformed into dichotomous variables at median values. Clinical characteristics including gender, age (<55 and ≥55 years), surgical resection range, lymph node metastasis, distant metastasis, dermal mitoses (<2/mm2 and ≥2/mm2),23 history of misdiagnosis, and postoperative interferon alfa-2b therapy. Pathological characteristics including Breslow thickness (<1 mm, 1–2 mm, and >2 mm),24 ulceration, Clark level (I–III vs IV–V),25 histologic subtype, and TNM stage (AJCC, seventh ed.).26
Immunohistochemistry
Formalin-fixed paraffin-embedded tissue specimens were first deparaffinized and rehydrated with xylene and graded alcohol, and then incubated in boiled citrate buffer (pH=6.0) for optimal antigen retrieval. Primary antibody of VEGF (Cat No. TA802346, Zhongshan Golden Bridge Biotechnology, China), β-catenin (Cat No. ZM-0442, Zhongshan Golden Bridge Biotechnology, China), and DKK1 (21112-1-AP, Proteintech, Wuhan, China) at 1:200 dilution was used to incubate the specimens overnight at 4°C. After rinsing with phosphate-buffered solution, the secondary antibody (Zhongshan Golden Bridge Biotechnology, Beijing, China) labeled with streptavidin-biotin-peroxidase reagent was used to incubate the slides for 2 h at room temperature and the 3,3ʹ-diaminobenzidine solution (Zhongshan Golden Bridge Biotechnology, Beijing, China) was applied for antigen visualization. Finally, the slides were counterstained with hematoxylin (Zhongshan Golden Bridge Biotechnology, Beijing, China).
The slides were scanned by Olympus Dot-slide microscope digital system and evaluated in 10 randomly selected fields in the core of the tumor. The results of immunohistochemistry were evaluated by two senior pathologists to select the densest tumor area. The expressions of β-catenin, VEGF, and DKK1 were scored based on the percentage of immunohistochemical staining in tumor cells (Table 1). Examples of different staining intensities are shown in Figure 1.
 |
Table 1 Criteria for Immunohistochemistry Score |
Study Protocol
The CM patients were followed up every 3 months for the first 3 years and then every 6 months for the fourth and fifth years. Overall survival (OS) rates were analyzed from the date of operation to the date of death or last follow-up. The recruited patients were randomly divided into two cohorts [(training cohort (n = 190) and verification cohort (n = 90)]. In the training cohort, univariate and multivariate analyses were used to determine the independent prognosis factors in CM patients. A nomogram was built based on the independent prognosis factors to estimate the 1-, 3-, and 5-year OS. The accuracies of the nomogram with or without the Wnt/β-catenin pathway and TNM stage in predicting the 1-, 3-, and 5-year OS were compared.
Statistical Analysis
The follow-up was analyzed by Kaplan–Meier (KM) method. Log rank tests were used to determine univariate prognostic factors and those with P < 0.05 were entered into a multivariate Cox regression model, which estimated the hazard ratio (HR) and 95% confidence interval (CI) for parameters associated with the prognosis of CM. The nomogram was verified internally and externally in the training and validation cohorts. The discrimination of the nomogram was evaluated by the area under the curve (AUC) of the receiver operating characteristic (ROC) curve, while the calibration was evaluated by the calibration curve and Hosmer-Lemeshow (HL) test. The AUCs of ROC were used for the comparison of the accuracies of different prediction models. IBM SPSS Statistics for Windows version 22.0 (IBM Corp., Armonk, NY, United States), R package in R version 3.6.2. and MedCalc Statistical Software version 22.0.1 (MedCalc Software Ltd, Ostend, Belgium) were used to analyse the data.
Results
Characteristics and OS of CM Patients
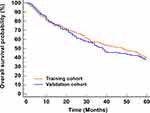
There is no obvious difference in the data between the training and validation cohorts, which demonstrated that it was reasonable to be used as the training and validation datasets. No significant difference was observed in the 1-, 3-, and 5-year OS rates between the training (78.4%, 56.3%, 43.9%) and validation datasets (77.8%, 51.6%, 41.0%) (Figure 2).
Independent Prognostic Factors
In the training cohort, the presence of ulceration, lymph node metastasis, distant metastasis, high levels of Breslow thickness, Clark levels IV–V, dermal mitoses ≥2/mm2, positive staining of β-catenin, VEGF, and negative staining of DKK1 were associated with shorter OS (all P < 0.05) (Figure 3 and Table 2). Multivariate Cox analysis showed that ulceration, lymph node metastasis, distant metastasis, Breslow thickness, dermal mitoses, β-catenin, VEGF, and DKK1 were independently associated with OS of CM patients (Figure 4).
 |
Table 2 Association of Survival with Clinical and Pathological Parameters in CM Patients Within 5 Years |
Nomogram Establishment and Validation
A nomogram integrating these independent risk factors was constructed to predict the 1-, 3-, and 5-year OS of CM patients (Figure 5). It assigned a score to each variable (based on HR of COX regression) and estimated OS by the sum of each score.
Good discriminations of 1-, 3-, and 5-year OS were demonstrated according to the AUC values (0.914, 0.852, 0.785; all >0.75) (Figure 6A–C) in the internal validation. The calibration plots indicated that the 1-, 3-, and 5-year OS rates predicted by the nomogram were consistent with the actual prognosis (Figure 6D–F). Good calibrations were found because of no significant difference in the HL test (P = 0.424, 0.477, 0.358). For the external validation, the ROC curves and calibration plots indicated good discriminations and calibrations as well (Figure 6G–I; AUC= 0.904, 0.840, 0.773) (Figure 6J–L; HL test, P = 0.278, 0.347, 0.473).
Accuracy Comparison of Different Prediction Models
The nomogram incorporating the Wnt/β-catenin signaling pathway showed the highest accuracy in predicting the 1-, 3-, and 5-year OS (AUC=0.914, 0.852, 0.785) compared with the nomogram without the Wnt/β-catenin signaling pathway (AUC=0.693, 0.640, 0.615) and the TNM stage (AUC=0.726, 0.693, 0.673). No obvious difference was observed between the accuracy of the nomogram without the Wnt/β-catenin signaling pathway and the TNM stage (Figure 7).
Discussion
Accurate prediction of the prognosis is critical to the selection of the optimal treatment and improving the survival of CM patients. In this study, the multivariate COX regression revealed that except for the components of TNM staging (Breslow thickness, ulceration, dermal mitoses, lymph-node metastasis, and distant metastasis), the key genes of the WNT/β-catenin signaling pathway (β-catenin, VEGF, and DKK1) were associated with the survival of CM. The developed nomogram incorporating the WNT/β-catenin signaling pathway showed good discrimination and calibration through internal and external validation. Its prognostic value was better than the nomogram without WNT/β- catenin signaling pathway and TNM staging system.
The understanding of the prognostic factors of CM is improving with the refinements of diagnostic techniques and treatment strategies. This updated understanding can in turn promote the diagnosis and treatment of CM. For example, the T1 stage was perfected based on mitosis in the seventh edition AJCC, while the threshold of T1a and T1b stage was improved regarding tumor thickness = 0.8 mm and the presence of ulceration in the eighth edition AJCC.27 It suggests that pathologists should pay more attention to tumor thickness and the presence of epidermal defects, and clinicians should focus on the symptoms of ulcer. These examples indicate that the research on the prognosis of CM is constantly evolving and complementary to clinical diagnosis and treatment.
VEGF and DKK1 are downstream molecules of the Wnt/β-catenin signaling pathway. Research of VEGF were earlier, and Endostar, a potent endogenous antiangiogenic factor, has entered clinical application for melanoma and achieved encouraging results,28,29 while the research on β-catenin or DKK1 genes are still in the stage of mechanism exploration. Survival analyses in the present study showed that VEGF, β-catenin, and DKK1 could affect the prognosis of CMM. Further multivariate analysis demonstrated that β-catenin, VEGF, and DKK1 expression levels were independent factors of the CM prognosis although their use is not currently mandated in CM reporting. Most studies demonstrated that the ectopic expression of β-catenin in the cytoplasm and nucleus activates the transcription of downstream genes to promote tumor cell differentiation, proliferation, and metastasis.30,31 DKK1 blocks the nuclear translocation of β-catenin, downregulation of its downstream targets VEGF resulting in reducing perivascular coverage in melanoma tumors.32–34 Although our results revealed that the Wnt/β-catenin signaling pathway was valuable prognostic markers for CM patients, they were unfeasible for predicting the prognosis of CM independently. Part of the reason for this is that the Wnt/β-catenin signaling pathway has also been reported to be upregulated in cutaneous inflammation and benign cutaneous neoplasm.35,36
Despite the strong evidence-based predictive capacity of the current AJCC melanoma staging system, it has long been observed that the prediction of CM prognosis remains a difficult one to perform accurately and consistently.37,38 Therefore, accurate prediction of survival for CM patients would be difficult only to rely on TNM stage. In the present study, we established a nomogram based on conventional clinical characteristics, which have been recognized as prognostic factors for CM,39–41 and the WNT/β-catenin signaling pathway. Its accuracy was significantly improved compared to the TNM stage and the nomogram without the Wnt/β-catenin signaling pathway. It suggested that the nomogram could assist physicians in performing individualized survival predictions, which would facilitate the selection of better treatment options and surveillance strategies. For example, if the overall score of a CM patient was about 259 [Breslow thickness 1–2 mm (48), ulceration (28), no lymph node metastasis (0), and distant metastasis (0), dermal mitosis <2/mm2 (0), positive VEGF (68), positive β-catenin (50), and negative DKK1 (65)], it indicated that the 1-, 3-, and 5-year OS rates were about 88%, 60%, and 15%, respectively. For these stage IIA patients, surveillance strategies could be intensified, or adjuvant interferon alfa-2b therapy could be considered, because most of them will not receive adjuvant therapy according to the current AJCC stage.
There are some limitations in this study. First, the TNM stage for the present study was based on the seventh AJCC staging manual. The most recent revision of the AJCC staging manual (eighth edition) was released in 2016 and implemented in 2018. With the revision of staging criteria, the accuracy in predicting the prognosis of CM may be improved. Second, potential selection bias might be inevitable in our single-center study with a limited sample size. Third, we could not exclude the biases from genetic susceptibility and specific genetic polymorphisms, considering that about 5–12% of melanomas are hereditary.38 Therefore, we plan to conduct a larger prospective study according to the eighth AJCC melanoma staging system to improve the constructed nomogram.
In conclusion, the established nomogram incorporating the WNT/β-catenin signaling pathway might be useful in predicting OS of CM patients. It delivered a pragmatic model, which might be useful in helping clinicians to choose, with greater accuracy, the best clinical therapy for CM patients.
Ethical Approval
Ethical approval for the study was obtained from the ethics committee of North China University of Science and Technology Affiliated Hospital.
Informed Consent
Written informed consent was obtained from all patients.
Acknowledgments
The authors wish to thank all the study participants, research staff, and students who participated in this work.
Author Contributions
Yu-xin Zhou and Xin Wang contributed equally and are co-first authors.
Study design: Yu-xin Zhou, Xin Wang, and De-quan Pang.
Data collection and analysis: Yu-xin Zhou, Xin Wang, Ying-Man Wang, Jing Bai, Fei Tian, Duo Han, Shu-wei Shi, and Lei Hu.
Supervision: De-quan Pang.
Statistics: Yu-xin Zhou, Xin Wang, Duo Han, Shu-wei Shi, and Lei Hu.
Manuscript writing: Yu-xin Zhou, Xin Wang, De-quan Pang, Ying-Man Wang, Jing Bai, and Fei Tian.
Manuscript revision: Yu-xin Zhou, Xin Wang, and De-quan Pang.
Approval of the manuscript: all authors.
All authors contributed to data analysis, drafting or revising the article, gave final approval for the version to be published, agreed to the submitted journal, and agree to be accountable for all aspects of the work.
Funding
Science and Technology Planning Project of Hebei Province (NO. 112761175)Science and Technology Planning Project of Tangshan city (NO. 12140209A-30).
Disclosure
The authors declare that they have no conflicts of interest.
References
1. Geller AC, Swetter SM, Brooks K, Demierre MF, Yaroch AL. Screening, early detection, and trends for melanoma: current status (2000–2006) and future directions. J Am Acad Dermatol. 2007;57(4):
2. Blateau P, Coyaud E, Laurent E, et al. Promoter mutation as an independent prognostic marker for poor prognosis MAPK inhibitors-treated melanoma. Cancers. 2020;12(8):2224. doi:10.3390/cancers12082224.
3. El Sharouni M-A, Aivazian K, Witkamp AJ, et al. Association of histologic regression with a favorable outcome in patients with stage 1 and stage 2 cutaneous melanoma. JAMA Dermatol. 2020. doi:10.1001/jamadermatol.2020.5032.
4. Liao M, Zeng F, Li Y, et al. A novel predictive model incorporating immune-related gene signatures for overall survival in melanoma patients. Sci Rep. 2020;10(1):12462. doi:10.1038/s41598-020-69330-2.
5. Yang J, Pan Z, Zhao F, et al. A nomogram for predicting survival in patients with nodular melanoma: a population-based study. Medicine. 2019;98(24):e16059. doi:10.1097/md.0000000000016059.
6. Weiss SA, Hanniford D, Hernando E, Osman I. Revisiting determinants of prognosis in cutaneous melanoma. Cancer. 2015;121(23):4108–4123. doi:10.1002/cncr.29634.
7. Du Y, Lv G, Jing C, Liu J, Liu J. ZNF750 inhibits the proliferation and invasion of melanoma cells through modulating the Wnt/β-catenin signaling pathway. Folia Histochem Cytobiol. 2020;58(4):255–263. doi:10.5603/FHC.a2020.0026.
8. Kovacs D, Migliano E, Muscardin L, et al. The role of Wnt/β-catenin signaling pathway in melanoma epithelial-to-mesenchymal-like switching: evidences from patients-derived cell lines. Oncotarget. 2016;7(28):43295–43314. doi:10.18632/oncotarget.9232.
9. Damsky WE, Curley DP, Santhanakrishnan M, et al. β-catenin signaling controls metastasis in Braf-activated Pten-deficient melanomas. Cancer Cell. 2011;20(6):741–754. doi:10.1016/j.ccr.2011.10.030.
10. Sinnberg T, Levesque MP, Krochmann J, et al. Wnt-signaling enhances neural crest migration of melanoma cells and induces an invasive phenotype. Mol Cancer. 2018;17(1):59. doi:10.1186/s12943-018-0773-5.
11. Widlund HR, Horstmann MA, Price ER, et al. Beta-catenin-induced melanoma growth requires the downstream target Microphthalmia-associated transcription factor. J Cell Biol. 2002;158(6):1079–1087. doi:10.1083/jcb.200202049.
12. Hartman ML, Czyz M. Pro-survival role of MITF in melanoma. J Invest Dermatol. 2015;135(2):352–358. doi:10.1038/jid.2014.319.
13. Carreira S, Goodall J, Aksan I, et al. Mitf cooperates with Rb1 and activates p21Cip1 expression to regulate cell cycle progression. Nature. 2005;433(7027):764–769. doi:10.1038/nature03269.
14. Kuphal S, Lodermeyer S, Bataille F, Schuierer M, Hoang BH, Bosserhoff AK. Expression of Dickkopf genes is strongly reduced in malignant melanoma. Oncogene. 2006;25(36):5027–5036. doi:10.1038/sj.onc.1209508.
15. Xue G, Romano E, Massi D, Mandalà M. Wnt/β-catenin signaling in melanoma: preclinical rationale and novel therapeutic insights. Cancer Treat Rev. 2016;49:1–12. doi:10.1016/j.ctrv.2016.06.009.
16. Kowalik A, Jurkowska M, Mierzejewska E, et al. The prognostic role of BRAF and WNT pathways activation in kinase inhibitors-naïve clinical stage III cutaneous melanoma. Melanoma Res. 2020;30(4):348–357. doi:10.1097/cmr.0000000000000658.
17. Li P, Gao Y, Li J, et al. LncRNA MEG3 repressed malignant melanoma progression via inactivating Wnt signaling pathway. J Cell Biochem. 2018;119(9):7498–7505. doi:10.1002/jcb.27061.
18. Haass NK, Smalley KSM. Melanoma Biomarkers. Mol Diagn Ther. 2009;13(5):283–296. doi:10.1007/BF03256334.
19. Ługowska I, Kowalska M, Zdzienicki M, et al. [The prognostic role of clinical factors, VEGF, IL-8 and sTNF-R1 in cutaneous melanomas at locoregional stage]. Pol Merkur Lekarski. 2012;32(187):22–27. Croation
20. Bachmann IM, Straume O, Puntervoll HE, Kalvenes MB, Akslen LA. Importance of P-cadherin, beta-catenin, and Wnt5a/frizzled for progression of melanocytic tumors and prognosis in cutaneous melanoma. Clin Cancer Res. 2005;11(24 Pt 1):8606–8614. doi:10.1158/1078-0432.Ccr-05-0011.
21. Feldmann R, Schierl M, Fink AM, Sator PG, Maiweg J, Steiner A. Serum levels of glycoprotein Dickkopf-1 in patients with cutaneous malignant melanoma: a prospective pilot study. Dermatology. 2011;222(2):171–175. doi:10.1159/000324516.
22. Gajos-Michniewicz A, Czyz M. WNT signaling in melanoma. Int J Mol Sci. 2020;21(14):14. doi:10.3390/ijms21144852.
23. Tejera-Vaquerizo A, Pérez-Cabello G, Marínez-Leborans L, et al. Is mitotic rate still useful in the management of patients with thin melanoma? J Eur Acad Dermatol Venereol. 2017;31(12):2025–2029. doi:10.1111/jdv.14485.
24. De Carvalho N, Welzel J, Schuh S, et al. The vascular morphology of melanoma is related to Breslow index: an in vivo study with dynamic optical coherence tomography. Exp Dermatol. 2018;27(11):1280–1286. doi:10.1111/exd.13783.
25. Tas F, Erturk K. Scalp melanoma is associated with high mitotic rate and is a poor prognostic factor for recurrence and outcome. Melanoma Res. 2017;27(4):387–390. doi:10.1097/CMR.0000000000000351.
26. Balch CM, Gershenwald JE, Soong S-J, et al. Final version of 2009 AJCC melanoma staging and classification. J Clin Oncol. 2009;27(36):6199–6206. doi:10.1200/JCO.2009.23.4799.
27. Gershenwald JE, Scolyer RA. Melanoma staging: American Joint Committee on Cancer (AJCC) 8th edition and beyond. Ann Surg Oncol. 2018;25(8):2105–2110. doi:10.1245/s10434-018-6513-7.
28. Xiao L, Yang S, Hao J, et al. Endostar attenuates melanoma tumor growth via its interruption of b-FGF mediated angiogenesis. Cancer Lett. 2015;359(1):148–154. doi:10.1016/j.canlet.2015.01.012.
29. Cui C, Mao L, Chi Z, et al. A Phase II, randomized, double-blind, placebo-controlled multicenter trial of endostar in patients with metastatic melanoma. Mol Ther. 2013;21(7):1456–1463. doi:10.1038/mt.2013.79.
30. Spranger S, Bao R, Gajewski TF. Melanoma-intrinsic β-catenin signalling prevents anti-tumour immunity. Nature. 2015;523(7559):231–235. doi:10.1038/nature14404.
31. Wang M, Xu Y, Wen G-Z, Wang Q, Yuan S-M. Rapamycin suppresses angiogenesis and lymphangiogenesis in melanoma by downregulating VEGF-A/VEGFR-2 and VEGF-C/VEGFR-3 expression. Onco Targets Ther. 2019;12:4643–4654. doi:10.2147/OTT.S205160.
32. Park H, Jung HY, Choi H-J, et al. Distinct roles of DKK1 and DKK2 in tumor angiogenesis. Angiogenesis. 2014;17(1):221–234. doi:10.1007/s10456-013-9390-5.
33. Hu Y, Liu M, Xu S, et al. The clinical significance of dickkopf wnt signaling pathway inhibitor gene family in head and neck squamous cell carcinoma. Med Sci Monit. 2020;26:e927368. doi:10.12659/msm.927368.
34. Haas MS, Kagey MH, Heath H, Schuerpf F, Rottman JB, Newman W. mDKN-01, a novel anti-DKK1 mAb, enhances innate immune responses in the tumor microenvironment. Mol Cancer Res. 2020;19(4):717–725. doi:10.1158/1541-7786.MCR-20-0799.
35. Wang X, Chang Y, Gao M, Zhang F. Wogonoside attenuates cutaneous squamous cell carcinoma by reducing epithelial-mesenchymal transition/invasion and cancer stem-like cell property. Onco Targets Ther. 2020;13:10097–10109. doi:10.2147/OTT.S251806.
36. Xu J, Wei Q, He Z. Insight into the function of RIPK4 in keratinocyte differentiation and carcinogenesis. Front Oncol. 2020;10:1562. doi:10.3389/fonc.2020.01562.
37. Gerami P, Busam K, Cochran A, et al. Histomorphologic assessment and interobserver diagnostic reproducibility of atypical spitzoid melanocytic neoplasms with long-term follow-up. Am J Surg Pathol. 2014;38(7):934–940. doi:10.1097/PAS.0000000000000198.
38. Davis LE, Shalin SC, Tackett AJ. Current state of melanoma diagnosis and treatment. Cancer Biol Ther. 2019;20(11):1366–1379. doi:10.1080/15384047.2019.1640032.
39. Saldanha G, Yarrow J, Pancholi J, et al. Breslow density is a novel prognostic feature that adds value to melanoma staging. Am J Surg Pathol. 2018;42(6):715–725. doi:10.1097/PAS.0000000000001034.
40. Gillgren P, Drzewiecki KT, Niin M, et al. 2-cm versus 4-cm surgical excision margins for primary cutaneous melanoma thicker than 2 mm: a randomised, multicentre trial. Lancet. 2011;378(9803):1635–1642. doi:10.1016/S0140-6736(11)61546-8.
41. Minca EC, Billings SD, Elson P, Tetzlaff MT, Andea AA, Ko JS. Significance of epidermal mitoses in challenging melanocytic proliferations. J Cutan Pathol. 2017;44(2):135–143. doi:10.1111/cup.12855.
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.