Back to Journals » Journal of Hepatocellular Carcinoma » Volume 10
Nomogram for Estimation of Acute Liver Failure Risk in Spontaneous Ruptured Hepatocellular Carcinoma
Authors Zhao ZH, Jiang C, Wu QY, Lv GY, Wang M
Received 27 September 2023
Accepted for publication 5 December 2023
Published 12 December 2023 Volume 2023:10 Pages 2223—2237
DOI https://doi.org/10.2147/JHC.S438346
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr David Gerber
Zhi-Hao Zhao, Chao Jiang, Qing-Yuan Wu, Guo-Yue Lv, Meng Wang
Department of Hepatobiliary and Pancreatic Surgery, General Surgery Center, the First Hospital of Jilin University, Changchun, Jilin Province, People’s Republic of China
Correspondence: Meng Wang, Department of Hepatobiliary and Pancreatic Surgery, General Surgery Center, the First Hospital of Jilin University, 71 Xinmin Street, Changchun, Jilin Province, People’s Republic of China, Tel +86 13578767515, Email [email protected]
Purpose: Acute liver failure (ALF) is a severe complication of spontaneous ruptured hepatocellular carcinoma (SRHCC) that requires accurate prediction for effective treatment strategies. We aimed to develop a predictive nomogram to estimate the risk of ALF in patients with SRHCC undergoing treatment.
Patients and Methods: We performed a retrospective analysis of historical data from 284 patients diagnosed with SRHCC at the First Hospital of Jilin University over the past decade. Variables were selected through univariate and multivariate logistic regression analyses, and a predictive nomogram was constructed. We evaluated its predictive accuracy against the Child-Pugh Score, R.MELD, and ALBI by assessing discrimination, calibration, and net clinical benefit.
Results: Among the 284 patients, 65 developed ALF. The risk factors identified for model development included largest tumor size (LTS), platelet counts, prolonged prothrombin time, and elevated serum α-fetoprotein levels. The nomogram exhibited high accuracy in predicting ALF risk with a C-index of 0.91 (0.87– 0.95). The Delong test showed a significant difference between the nomogram and the other three models (p< 0.05). The calibration curve for the nomogram fit well, and the decision curve analysis revealed superior net benefit. The optimal cut-off point for the nomogram was determined to be 40, yielding sensitivity, specificity, positive predictive value, and negative predictive value of 83.10%, 87.20%, 65.90% and 94.60%, respectively.
Conclusion: The nomogram we developed provides an optimized tool for predicting ALF in SRHCC patients. Its application can help determine individual patient’s risk of ALF, enabling more rational and personalized treatment strategies.
Keywords: predict, transcatheter arterial chemoembolization, hepatectomy, prognosis, α-fetoprotein
Introduction
Liver cancer, responsible for approximately 830,000 deaths annually, is the fourth leading cause of cancer-related deaths globally. It is estimated that by 2025, there will be one million new cases of hepatocellular carcinoma (HCC) globally each year.1 Spontaneous rupture, a rare yet fatal complication of HCC, has an incidence rate of less than 3% in Western countries, but this figure dramatically rises to 12%-14% in Asia.2 The mortality rate for the acute stage of spontaneous ruptured hepatocellular carcinoma (SRHCC) varies from 25% to 75%. While recent studies have reported a decline in mortality rates, it remains a challenging issue within the realm of hepatobiliary surgery.3–7
Acute liver failure (ALF) frequently contributes to mortality in end-stage liver disease, a trend similarly observed in patients with SRHCC.5,8 During the acute phase of the disease, ALF incidence ranges from 12% to 42%.9 The prognosis for patients with SRHCC is gradually improving with advancements in diagnostic methods, surgical techniques, and perioperative management.10 This improvement is particularly attributed to the widespread recognition and utilization of therapeutic measures such as emergency or delayed partial hepatectomy and transcatheter arterial chemoembolization (TACE).3,7,11,12 However, studies indicate that ALF accounts for more than half of the in-hospital mortality post emergency hepatectomy for SRHCC.13 Furthermore, the incidence of liver failure following TACE can reach up to 12–34%.11 Therefore, physicians should constantly consider the potential occurrence of ALF during the management of patients with SRHCC.
Comprehending the predictors of ALF in SRHCC patients enables physicians to initiate diagnostic and therapeutic procedures more accurately. It also aids patients in counseling and selecting treatment options that offer the greatest likelihood of benefit. Currently, the primary models utilized in clinical practice for liver function evaluation include the Child-Pugh Score, Model for End-Stage Liver Disease (MELD), and Albumin-Bilirubin (ALBI). However, their applicability to SRHCC remains unclear.14 SRHCC patients exhibit a series of specific pathomechanisms.8,15 The large size of the tumor causes it to separate from the normal parenchyma. This, along with vascular erosion, venous occlusion, and coagulation disorders, contributes to increased pressure within the tumor, leading to subsequent hemorrhage. Moreover, opportunistic infections such as cytomegalovirus and Epstein-Barr virus may further exacerbate these processes.16,17
In light of this, we conducted a study with the objective of developing and validating a novel predictive model for the onset of ALF in SRHCC patients during treatment. This model was based on clinical data gathered over a decade from a large single center. We compared this model with other commonly used clinical liver function scoring systems, aiming to facilitate more personalized, evidence-based, and highly accurate risk assessment in SRHCC patients.
Materials and Methods
Patients
We retrospectively gathered data from patients diagnosed with SRHCC who sought consultation at the First Hospital of Jilin University between September 1, 2013, and June 30, 2022. We obtained information regarding their diagnosis, treatment, and follow-up. Patients provided informed consent for the use of their clinical data in clinical research, and no financial compensation was given. The diagnosis adhered to the guidelines published by the American Association for the Study of Liver Disease (AASLD).18 SRHCC was identified through typical diagnostic findings on computed tomography, which included peripheral high-attenuation periportal hematoma, HCC with a prominent liver contour, localized disruption of the hepatic surface, and active contrast medium extravasation. Laparotomy was not necessary for the diagnosis of SRHCC in some patients. The exclusion criteria were as follows: 1. Diagnosis of SRHCC during hospitalization; 2. ALF on admission or complications with other serious diseases; 3. Missing medical record data or follow-up information. Based on these inclusion and exclusion criteria, we included 284 patients in the study cohort. The participant selection process is illustrated in Figure 1A. The study received approval from the Ethics Committee of the First Hospital of Jilin University. Verbal consent was obtained from the patients, and due to the retrospective nature of the study, no additional written informed consent was necessary. This study was conducted in strict accordance with the principles of the Declaration of Helsinki.
Clinical Management and Follow-Up
For patients presenting with hemodynamic instability or severe complications upon admission, we employed aggressive fluid resuscitation, anti-inflammatory measures, blood transfusion, corrections of acid-base balance disorders, coagulation disorders, and other routine supportive care. Nonessential invasive procedures were minimized as much as possible. In the presence of active bleeding, TACE or emergency partial hepatectomy was prioritized. Radical resection or other additional treatments were considered once the patient’s condition has stabilized. For hemodynamically stable patients without active bleeding, partial hepatectomy was recommended, provided that the degree of cirrhosis, liver function, and stage of HCC were conducive. The implementation process was guided by a comprehensive assessment of the patient’s condition, lesions, and bleeding, as well as the family’s perspective. Important clinical procedures were undertaken with the written informed consent of the patient or their family. TACE is carried out by our seasoned interventionalists in our angiography suite. The success of TACE is determined by the stabilization of the patient’s vital signs post-procedure and the lack of a sustained drop in serum hemoglobin levels. The partial hepatectomy was conducted by experienced and skilled surgeons from the Department of Hepatobiliary and Pancreatic Surgery at our General Surgery Center.
The primary outcome in this study was the incidence of ALF during the follow-up period. Follow-up visits primarily took place in the surgical ward or intensive care unit. Patients discharged from the hospital were scheduled for a recheck in our outpatient clinic within one month. For those unable to attend the outpatient clinic, we reached out via phone or online to gather information about their health status and treatment. For patients who did not undergo invasive treatment, follow-up began on the second day of admission. For those who underwent TACE or surgical resection, follow-up commenced on the first day post-operation. For patients who underwent multiple invasive procedures during their hospital stay, follow-up began on the first day after their final operation.
Data Collection
In this study, we collected demographic information and details of the clinical management course. Basic data included sex, age, height, weight, and history of hypertension, diabetes mellitus, smoking, heavy alcohol consumption, and hepatitis. Imaging data encompassed ascites, cirrhosis, portal hypertension, tumor location and LTS, whether the tumor was single or multiple and extrahepatic metastases. Laboratory tests included blood counts, coagulation profile, liver function, blood chemistry, and α-Fetoprotein (AFP), among others. Treatment information included aspects such as supportive therapy, TACE, and partial hepatectomy. The occurrence of ALF was diagnosed based on the 2019 definition of acute-on-chronic liver failure by the Asia Pacific Association for the Study of the Liver (APASL).19 The diagnostic criteria included jaundice (serum total bilirubin >85 mmol/L) and coagulopathy (INR >1.5 or PTA <40%), complicated by clinical ascites and/or hepatic encephalopathy within 4 weeks. Simultaneously, to compare the superiority of our method with three other methods commonly used in clinical practice for liver function evaluation, we performed a quantitative analysis of their indicators:
- The Child-Pugh Score,20,21 assessed by the severity of four indicators: ascites, bilirubin, albumin, prothrombin time and encephalopathy.
- The Model for End-Stage Liver Disease (MELD) score,22 calculated using the following formula:
R=3.78 * ln(bilirubin) +11.2 * ln(INR) + 9.57 * ln(creatinine) + 6.4. Here, bilirubin is measured in μmol/L, and creatinine in mg/dl.
ALBl = log10 (bilirubin) * 0.66 + albumin * (−0.085). Here, bilirubin is measured in μmol/L, and albumin in g/L.
Statistical Analysis
Continuous variables were expressed as medians and interquartile ranges, and categorical variables were represented as counts and proportions. Based on our clinical experience and the findings of previous related studies, we selected potential variables for inclusion in a univariate logistic regression. Variables with significant impact (p<0.05) were included in a multivariate regression, ultimately generating independent influential factors, and Spearman correlation analysis was used to determine whether there is collinearity among the final variables. We then used them to construct a nomogram by the ‘rms’ package in R.
The model’s discriminatory capacity was evaluated using receiver operating characteristic (ROC) curve analysis, and quantified using the C-index, equivalent to the area under the ROC curve (AUC). An AUC value of 0.5 implies no discriminatory power, while a value of 1 signifies perfect prediction of event occurrence in the patient. The model’s calibration capability was evaluated using a calibration curve. This curve graphically represents the relationship between the frequency of observations and the predicted probability, calibrated using the bootstrap resampling method to minimize bias. In a well-calibrated model, predictions should align with a 45-degree diagonal. Decision curve analysis (DCA) was also conducted to establish the predicted net benefit threshold, which served to assess the clinical utility of the model. Superior models are indicated by curves that are further from the two null lines. The visualization analysis process is depicted in Figure 1B.
The optimal cutoff value was determined using the Youden index (sensitivity + specificity - 1). The predictive performance of this optimal cutoff value was evaluated in terms of accuracy, precision, sensitivity, specificity, positive predictive value and negative predictive value. For all analyses, a p-value less than 0.05 was considered to indicate statistical significance. All analyses were conducted using R software version 4.3.0. Data were analyzed between July 15 and July 30, 2023.
Results
Demographic and Clinical Characteristics of Patients
This study enrolled a total of 284 consecutive patients diagnosed with SRHCC, with their demographic and basic clinical characteristics detailed in Table 1. The cohort consisted of 228 males and 56 females, with a median age of 59 years (IQR, 51–67 years). The cohort’s median body mass index (BMI), calculated as weight (kg) divided by height (m) squared, was 23.3 (IQR, 21–25.4). The incidence of ALF among patients with the disease was 22.9% (65/284). A total of 57.7% (164/284) of patients had cirrhosis, and 41.5% (118/284) of patients had portal hypertension. The median LTS was 7.15cm. Slightly more patients had solitary tumor (54.6%). Moreover, ruptured tumors were more often located in the right lobe (52.5%). In terms of treatment, 70.1% (199/284) of patients underwent TACE treatment, 19.1% (54/284) of patients underwent hepatectomy, and 20.1% (57/284) patients received conservative treatment. The scoring results of patients’ admission status by the Child-Pugh Score, R.MELD, and ALBI scoring methods are also listed in Table 1. In addition, 1-, 2-, and 3-year overall survival (OS) rates for SRHCC were calculated as 50.2%, 31.5%, and 19.8%, respectively.
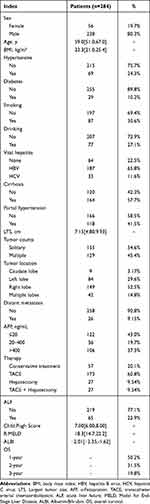 |
Table 1 Baseline Characteristics of the Enrolled Patients |
Correlation of Variables with Clinical Outcome
All examination variables in this study were obtained preoperatively. Based on clinical experience and results of previous research, potential risk factors were subjected to univariate logistic regression to determine variables with higher risk. The results are shown in Table 2, which shows that the significance of cirrhosis, portal hypertension, LTS, hemoglobin, platelet, AST, ALT, albumin, bilirubin, creatinine, pronged prothrombin time (PT), AFP, and main treatments are all less than 0.05. We included them in the multivariate regression equation. The results of the multivariate analysis are shown in Table 3, where each variable is reported as an odds ratio (95% CI). LTS (1.28 [1.12–1.47]), platelet counts (0.99 [0.98–1.00]), prothrombin time (seconds over control) (2.22 [1.57–3.32]) and high serum AFP level (for 20–400 vs ≤20 ng/mL, 4.75 [2.03–13.2]; for >400 vs 20–400 ng/mL, 0.55 [0.23–1.31)] are independent influencing factors and are independently associated with the occurrence of ALF. Correlation analysis was performed on continuous variables, the results are shown in Figure 2, to exclude the collinearity that may exist between variables.
 |
Table 2 Univariate Logistic Regression Analysis of ALF Presence Based on Variables Possible |
 |
Table 3 Multivariate Logistic Regression Analysis of ALF Presence Based on Variables Possible |
Development and Validation of Predictive Models
These independently associated risk factors were utilized to construct ALF risk estimation nomogram (Figure 3). The generated model underwent internal validation using the bootstrap validation method. The nomogram demonstrated a high accuracy in predicting ALF risk, with a C-index reaching up to 0.91 (0.87–0.95). The predictive abilities of each variable are illustrated in Figure 4. In the ROC curve, calibration curve, and clinical decision curve, the nomogram consistently achieved superior results compared to each individual variable. The optimal cut-off value for the nomogram score was determined to be 40, intended for distinguishing the occurrence of ALF. The values for Sensitivity, Specificity, Positive predictive value, and Negative predictive value used to identify the occurrence of ALF were 83.10%, 87.20%, 65.90% and 94.60% respectively.
 |
Figure 3 Nomogram for preoperative estimation of acute liver failure risk. Abbreviations: LTS, largest tumor size; PT, prothrombin time; AFP, α-Fetoprotein; ALF, acute liver failure. |
Comparison of the Predictive Performance of Different Models
The differentiation assessment of the three models - Child-Pugh Score, R.MELD, and ALBI is depicted in Figures 5 and 6. These models presented AUC values of 0.81 (0.75–0.87), 0.77 (0.70–0.84), and 0.78 (0.70–0.85) respectively, all of which are lower than our model (Figure 5A-D). Delong’s test was performed on the ROC curves between nomogram and the aforementioned three models, revealing significant differences (p<0.05) in each case. Each model was separately validated using the bootstrap method. The autonomously corrected C index was 0.87 for our model, and 0.81, 0.76, and 0.77 for the other three models, respectively. Furthermore, the calibration curves visually demonstrate better consistency between risk estimations derived from nomogram and the actual eventual occurrence of ALF compared the other three (Figure 6A-D). Following the Hosmer-Lemeshow test, the result indicated a nonsignificant difference (p=0.653). Our model yielded a Brier value of 0.087, lower than the other three models, and an R2 value of 0.591, higher than the other models. These results affirm the strong accuracy of our prediction model. Lastly, we plotted the DCA curve in Figure 7, demonstrating that the net benefit value of our model surpasses that of the other three models. The comparison of specific parameters across each model is summarized in Table 4, with nomogram outperforming the others on each criterion.
 |
Table 4 Accuracy of the Prediction Score of the Nomogram for Estimating the Risk of ALF Presence Compared with Other Models |
 |
Figure 6 The calibration curve of each model. (A) Nomogram. (B) Child.Pugh. (C) R.MELD. (D) ALBI. Abbreviations: MELD, Model for End-Stage Liver Disease; ALBI, Albumin-Bilirubin. |
 |
Figure 7 The comparison of decision curve analysis for each model. Abbreviations: MELD, Model for End-Stage Liver Disease; ALBI, Albumin-Bilirubin. |
Discussion
SRHCC is recognized as a severe and fatal complication of HCC. Past studies examining its survival rates have displayed substantial variations, though an overall trend of improvement is evident. In our study, we calculated the 1-, 2-, and 3-year OS rates for SRHCC as 50.2%, 31.5%, and 19.8%, respectively, with a median survival of 12 months. However, this outcome remains significantly worse than that observed in treated HCC cases.3,24
Liver failure significantly contributes to mortality in patients with SRHCC and is particularly linked to increased short-term mortality rates.25,26 Our findings indicate that 63.1% of patients experiencing ALF died within one month, and the one-year survival rate was a mere 8.7%. Despite considerable advancements in therapeutic measures for the disease, the incidence of ALF continues to be high. Our study found the incidence of ALF in SRHCC to be 22.9%, a figure consistent with the results of certain previous studies.8,11,27 Given the prevalence limitations, only studies with small sample sizes have been conducted in the past to explore relevant elements.11 We conducted an extended study based on a single-center 10-year sample size from a large hospital in northeastern China. We developed a model incorporating four variables to predict the occurrence of ALF in SRHCC patients. All variables included in the model were available at the time of the patient’s routine examination upon hospital admission, thereby offering the benefits of simplicity and accessibility. Our model achieved superior performance in terms of discrimination, calibration, and clinical utility compared to the predictive capabilities of existing models such as the Child-Pugh Score, R.MELD, and ALBI for liver function evaluation.
Within our model, largest tumor size, platelet, pronged prothrombin time, and AFP emerged as independent influences. These risk factors have been previously identified to collectively increase the likelihood of a poor prognosis in patients with SRHCC.4,14,28,29 Simultaneously, tumor size is also a risk factor for spontaneous rupture of the tumor.30 Larger tumors and the surrounding liver tissue exhibit more pronounced pathophysiological changes, especially in patients with SRHCC. This may also affect the occurrence of ALF.29,31 Decreased platelet count and prolonged prothrombin time both increase the tendency for bleeding, suggesting that hemorrhage and ischemia-reperfusion injury still have a significant impact on liver function. The synthesis of prothrombin indirectly reflects the liver’s reserve function.32 When liver function is impaired, the synthesis ability of coagulation factors decreases, hence, the prolongation of prothrombin time has good predictive performance for the occurrence of ALF in SRHCC. Elevated serum AFP levels usually correspond to poor cell differentiation and tumor metastasis. Qiu et al used machine learning algorithms to find that AFP value is the most important prognostic factor for predicting the short-term survival of SRHCC patients.33 Our study also found that high serum AFP levels are associated with the occurrence of ALF. Bilirubin and albumin, which still have high evaluation functions for liver function, have been confirmed by multiple studies,4,34,35 but we excluded them after multivariate regression analysis, suggesting that their role in predicting the occurrence of ALF in SRHCC is limited.
Our study also evaluated therapeutic measures. Recently, surgical procedures have gained recognition for managing SRHCC.36 It has been reported that hepatectomy for SRHCC yields a prognosis comparable to non-ruptured HCC7,10,30,37 and significantly reduces mortality rates.38 Our study affirms these results, as the incidence of ALF in patients who underwent surgical resection was significantly lower than in those who did not. However, we should note that hepatectomy can exacerbate liver injury (secondary to ischemia-reperfusion injury), leading to ALF.3 TACE is indeed effective for the disease,39 but its potential harm to liver function should not be overlooked. Particularly for SRHCC patients with severe cirrhosis, careful consideration should be given to the potential damage caused by invasive measures. In cases of active bleeding, the primary focus should be on antiresorptive and hemostatic interventions (including pharmacological and interventional treatments), with surgical procedures considered after stabilizing the patient’s condition.36 Comprehensive pre-evaluation of the patient’s degree of hepatic fibrosis, hepatic reserve function, resectable liver volume, and correction of coagulation function is essential. A previous study highlighted the effectiveness of transarterial bland embolization, which, coupled with lower hepatotoxicity, offers the benefit of reducing the incidence of adverse effects.28 This may yield better results, especially in patients with severe cirrhosis. Conversely, patients receiving only conservative treatment exhibited a very poor prognosis, which was also linked to the severity of their disease.40 However, therapeutic measures were not incorporated into our prediction model, indicating their limited impact on the occurrence of ALF.
To evaluate the clinical applicability of nomogram, we calculated its accuracy, precision, sensitivity, specificity, positive predictive value and negative predictive value in estimating the risk of ALF, using the optimal cut-off point 40 as a threshold. Furthermore, when comparing the performance of three established models—Child-Pugh Score, R.MELD, and ALBI, nomogram demonstrated superior results in most indicators. Overall, these results suggest that nomogram can provide more specific predictive information about the risk of developing ALF in patients with SRHCC. Based on these preoperative predictions, we can provide guidance for clinical management and enable patients to choose a more beneficial treatment. However, we must caution that the risk of ALF is not the sole determinant of SRHCC treatment procedures. Important factors not included in the model, such as tumor counts and location, should also be considered in practical applications.
Our study does present several limitations. Firstly, being a single-center retrospective study, it may harbor certain biases, and the results could be influenced by practices unique to our unit. Therefore, it is crucial to validate these findings with external data. Secondly, the reliability of some analyses may be limited due to the low incidence of outcome events. Additionally, as we included data from SRHCC patients over a ten-year period, the evolution of surgical techniques and treatment strategies during this period could have introduced bias in the factors influencing each patient. Lastly, the differing etiologies of HCC in Eastern and Western countries should be taken into account in future studies.
Conclusion
We developed a nomogram incorporating four straightforward and readily accessible clinical variables to predict the risk of ALF in patients with SRHCC. This model yielded favorable predictive outcomes, and demonstrated superior performance compared to existing scoring tools.
Abbreviations
HCC, Hepatocellular Carcinoma; SRHCC, Spontaneous Ruptured Hepatocellular Carcinoma; ALF, acute liver failure; TACE, transcatheter arterial chemoembolization; MELD, Model for End-Stage Liver Disease; ALBI, Albumin-Bilirubin; ROC, receiver operating characteristic; AUC, area under the ROC curve; DCA, decision curve analysis; BMI, body mass index; WBCs, white blood cells; NLR, neutrophil to lymphocyte ratio; HBV, hepatitis B virus; HCV, hepatitis C virus; LTS, largest tumor size; AST, aspartate aminotransferase; ALT, alanine aminotransferase; Na, serum sodium; OS overall survival; PT, prothrombin time; AFP, α-Fetoprotein; INR, international normalized ratio.
Data Sharing Statement
The datasets generated during the current study are available from the corresponding author on reasonable request.
Ethics Approval and Informed Consent
This retrospective observational study was approved by The First Hospital of Jilin University Review Board, and was confirmed not to require ethical approval, with no additional written informed consent needed. Patients were labeled with computer-generated numbers, with no involvement of private information. The present study was designed and conducted in accordance with the principles of the Helsinki Declaration.
Acknowledgments
We are grateful to the patients participated in the study.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
The authors received no financial support for the research, authorship, or publication of this article.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Sung H, Ferlay J, Siegel RL, et al. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 3Gut and Liver. Ca a Cancer J Clinicians. 2021;71(3):209–249. doi:10.3322/caac.21660
2. Chan ACY, Dai JWC, Chok KSH, Cheung TT, Lo CM. Prognostic influence of spontaneous tumor rupture on hepatocellular carcinoma after interval hepatectomy. Surgery. 2016;159(2):409–417. doi:10.1016/j.surg.2015.07.020
3. Wang W, Meng T, Chen Y, et al. Propensity score matching study of 325 patients with spontaneous rupture of hepatocellular carcinoma. Hepatobiliary Surgery Nutrition. 2022;11(6):808–821. doi:10.21037/hbsn-21-45
4. Wang P, Yang S, Li C, Han X, Hong D, Shao H. Nomogram-based development and evaluation for predictions of 30-day and 1-year survival in patients with spontaneously ruptured hepatocellular carcinoma. BMC Cancer. 2022;22(1):1177. doi:10.1186/s12885-022-10290-3
5. Xia F, Ndhlovu E, Zhang M, Chen X, Zhang B. Ruptured Hepatocellular Carcinoma: current Status of Research. Front Oncol. 2022;12:848903. doi:10.3389/fonc.2022.848903
6. Kudo M, Kitano M, Sakurai T, Nishida N. General Rules for the Clinical and Pathological Study of Primary Liver Cancer, Nationwide Follow-Up Survey and Clinical Practice Guidelines: the Outstanding Achievements of the Liver Cancer Study Group of Japan. Digestive Dis. 2015;33(6):765–770. doi:10.1159/000439101
7. Huang A, Guo DZ, Wang YP, Fan J, Yang XR, Zhou J. The treatment strategy and outcome for spontaneously ruptured hepatocellular carcinoma: a single-center experience in 239 patients. J Cancer Res Clin Oncol. 2022;148(11):3203–3214. doi:10.1007/s00432-022-03916-3
8. Sahu SK, Chawla YK, Dhiman RK, et al. Rupture of Hepatocellular Carcinoma: a Review of Literature. J Clin Exp Hepatol. 2019;9(2):245–256. doi:10.1016/j.jceh.2018.04.002
9. Lai ECH, Lau WY. Spontaneous rupture of hepatocellular carcinoma: a systematic review. Arch Surg. 2006;141(2):191–198. doi:10.1001/archsurg.141.2.191
10. Zhang SY, Guo DZ, Zhang X, Fan J, Zhou J, Huang A. Prognosis of spontaneously ruptured hepatocellular carcinoma: a propensity score matching study. J Cancer Res Clin Oncol. 2023;149(11):8889–8896. doi:10.1007/s00432-023-04774-3
11. Deng Z, Wang Y. Predictors of liver failure after transarterial chemoembolization in patients with spontaneously ruptured hepatocellular carcinoma: a retrospective study. J Interventional Med. 2023;6(1):35–40. doi:10.1016/j.jimed.2022.10.003
12. Sandomenico F, Arpaia V, De Rosa F, et al. Spontaneously Ruptured Hepatocellular Carcinoma: computed Tomography-Based Assessment. Diagnostics. 2023;13(6). doi:10.3390/diagnostics13061021
13. Miyamoto M, Sudo T, Kuyama T. Spontaneous Rupture of Hepatocellular Carcinoma: a Review of 172 Japanese Cases. Am J Gastroenterol. 1991;86(1):67–71. doi:10.1111/j.1572-0241.1991.tb06831.x
14. Zhang W, Zhang ZW, Zhang BX, et al. Outcomes and Prognostic Factors of Spontaneously Ruptured Hepatocellular Carcinoma. J Gastrointestinal Surgery. 2019;23(9):1788–1800. doi:10.1007/s11605-018-3930-7
15. Obeidat AE, Wong LL. Spontaneous Rupture of Hepatocellular Carcinoma: new Insights. J Clin Exp Hepatol. 2022;12(2):483–491. doi:10.1016/j.jceh.2021.05.010
16. Bouare N. Current management of liver diseases and the role of multidisciplinary approach. World J Hepatol. 2022;14(11):1920–1930. doi:10.4254/wjh.v14.i11.1920
17. Spengler U, Fischer HP, Caselmann WH. Chapter 34 - Liver Disease Associated with Viral Infections. In: Boyer TD, Manns MP, Sanyal AJ, editors. Zakim and Boyer’s Hepatology.
18. Heimbach JK, Kulik LM, Finn RS, et al. AASLD guidelines for the treatment of hepatocellular carcinoma. Hepatology. 2018;67(1):358–380. doi:10.1002/hep.29086
19. Sarin SK, Choudhury A, Sharma MK, et al. Correction to: acute-on-chronic liver failure: consensus recommendations of the Asian Pacific association for the study of the liver (APASL): an update. Hepatol Int. 2019;13(6):826–828. doi:10.1007/s12072-019-09980-1
20. Child CG, Turcotte JG. Surgery and portal hypertension. Major Probl Clin Surg. 1964;1:1–85.
21. Pugh RN, Murray-Lyon IM, Dawson JL, Pietroni MC, Williams R. Transection of the oesophagus for bleeding oesophageal varices. Br J Surg. 1973;60(8):646–649. doi:10.1002/bjs.1800600817
22. Malinchoc M, Kamath PS, Gordon FD, Peine CJ, Rank J, ter Borg PC. A model to predict poor survival in patients undergoing transjugular intrahepatic portosystemic shunts. Hepatology. 2000;31(4):864–871. doi:10.1053/he.2000.5852
23. Johnson PJ, Berhane S, Kagebayashi C, et al. Assessment of liver function in patients with hepatocellular carcinoma: a new evidence-based approach-The ALBI grade. J Clin Oncol off J Am Soc Clin Oncol. 2015;33(6):550–558. doi:10.1200/JCO.2014.57.9151
24. Alliance of Chinese Expert Consensus on Neoadjuvant Therapy for Hepatocellular Carcinoma, Committee of Digestive Surgery of Chinese Research Hospital Association, Committee of Liver Cancer, Chinese Anti‐Cancer Association. 肝癌新辅助治疗中国专家共识(2023版) [Chinese expert consensus on neoadjuvant therapy for hepatocellular carcinoma (2023 edition)]. Zhonghua Wai Ke Za Zhi. 2023;61(12):1–11. Chinese. doi:10.3760/cma.j.cn112139-20230914-00121
25. Moris D, Chakedis J, Sun SH, et al. Management, outcomes, and prognostic factors of ruptured hepatocellular carcinoma: a systematic review. J Surg Oncol. 2018;117(3):341–353. doi:10.1002/jso.24869
26. Tanaka S, Kaibori M, Ueno M, et al. Surgical Outcomes for the Ruptured Hepatocellular Carcinoma: multicenter Analysis with a Case-Controlled Study. J Gastrointest Surg. 2016;20(12):2021–2034. doi:10.1007/s11605-016-3280-2
27. Cheng YT, Teng W, Lui KW, et al. MELD score is the better predictor for 30-day mortality in patients with ruptured hepatocellular carcinoma treated by trans-arterial embolization. Am j Cancer Res. 2021;11(7):3726–3734.
28. Guo J, Wang W, Zhang Y, Xu L, Kong J. Comparison of initial tumor responses to transarterial bland embolization and drug-eluting beads-transarterial chemoembolization in the management of hepatocellular carcinoma: a propensity-score matching analysis. J gastrointestinal Oncol. 2021;12(4):1838–1850. doi:10.21037/jgo-21-370
29. Zou J, Li C, Chen Y, et al. Retrospective analysis of transcatheter arterial chemoembolization treatment for spontaneously ruptured hepatocellular carcinoma. Oncol Lett. 2019;18(6):6423–6430. doi:10.3892/ol.2019.11037
30. Chen Y, Guo D, Li X, Xu C, Zhu Q. Predictors of Spontaneous Rupture of Hepatocellular Carcinoma and Clinical Outcomes Following Hepatectomy. Front Oncol. 2022;12:820867. doi:10.3389/fonc.2022.820867
31. Ye F, Ma D, Gong XY, Yang YC, Chen YJ. Development and validation of risk score for predicting spontaneous rupture of hepatocellular carcinoma. Ann Surgical Treatment Res. 2020;99(5):268–274. doi:10.4174/astr.2020.99.5.268
32. Li S, Liu Z, Wu H. The product value of serum albumin and prothrombin time activity could be a useful biomarker for severity prediction in AP: an ordinal retrospective study. Pancreatology. 2019;19(2):230–236. doi:10.1016/j.pan.2019.02.001
33. Qiu Y, Wang T, Yang X, Shen S, Yang Y, Wang W. Development and Validation of Artificial Neural Networks for Survival Prediction Model for Patients with Spontaneous Hepatocellular Carcinoma Rupture After Transcatheter Arterial Embolization. Cancer Management Res. 2021;13:7463–7477. doi:10.2147/CMAR.S328307
34. Li WH, Cheuk ECY, Kowk PCH, Cheung MT. Survival after transarterial embolization for spontaneous ruptured hepatocellular carcinoma. J Hepatobiliary Pancreat Surg. 2009;16(4):508–512. doi:10.1007/s00534-009-0094-6
35. Zou J, Yuan J, Chen H, et al. Development of a prognostic score for recommended transarterial chemoembolization candidates with spontaneous rupture of hepatocellular carcinoma. J Gastrointest Oncol. 2022;13(3):1376–1383. doi:10.21037/jgo-22-531
36. Xie DY, Zhu K, Ren ZG, Zhou J, Fan J, Gao Q. A review of 2022 Chinese clinical guidelines on the management of hepatocellular carcinoma: updates and insights. Hepatobiliary Surgery Nutrition. 2023;12(2):216–228. doi:10.21037/hbsn-22-469
37. Huang X, Jia C, Xu L, et al. Survival of Patients Subjected to Hepatectomy After Spontaneous Rupture of Hepatocellular Carcinoma: a Meta-analysis of High-quality Propensity Score Matching Studies. Front Oncol. 2022;12:877091. doi:10.3389/fonc.2022.877091
38. Wu JJ, Zhu P, Zhang ZG, et al. Spontaneous rupture of hepatocellular carcinoma: optimal timing of partial hepatectomy. Eur j Surgical Oncol. 2019;45(10):1887–1894. doi:10.1016/j.ejso.2019.02.033
39. Xu X, Chen C, Liu Q, Huang X. A Meta-analysis of TAE/TACE Versus Emergency Surgery in the Treatment of Ruptured HCC. Cardiovascular Interventional Radiol. 2020;43(9):1263–1276. doi:10.1007/s00270-020-02514-5
40. Kerdsuknirun J, Vilaichone V, Vilaichone RK. Risk Factors and Prognosis of Spontaneously Ruptured Hepatocellular Carcinoma in Thailand. Asian Pacific j Cancer Prevention. 2018;19(12):3629–3634. doi:10.31557/APJCP.2018.19.12.3629
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.