Back to Journals » Neuropsychiatric Disease and Treatment » Volume 19
Nomogram-Based Prediction of the Futile Recanalization Risk Among Acute Ischemic Stroke Patients Before and After Endovascular Therapy: A Retrospective Study
Authors Guan J, Wang Q, Hu J, Hu Y, Lan Q, Xiao G, Zhou B , Guan H
Received 6 December 2022
Accepted for publication 27 March 2023
Published 13 April 2023 Volume 2023:19 Pages 879—894
DOI https://doi.org/10.2147/NDT.S400463
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Yuping Ning
Jincheng Guan,1 Qiong Wang,1 Jiajia Hu,2 Yepeng Hu,1 Qiaoyu Lan,1 Guoqiang Xiao,1 Borong Zhou,2 Haitao Guan1
1Department of Neurology, The Third Affiliated Hospital of Guangzhou Medical University, Guangzhou, Guangdong, People’s Republic of China; 2Department of Psychiatry, The Third Affiliated Hospital of Guangzhou Medical University, Guangzhou, Guangdong, People’s Republic of China
Correspondence: Borong Zhou, Department of Psychiatry, the Third Affiliated Hospital of Guangzhou Medical University, No. 63, Duobao Road, Liwan District, Guangzhou, Guangdong, 510150, People’s Republic of China, Email [email protected] Haitao Guan, Department of Neurology, the Third Affiliated Hospital of Guangzhou Medical University, No. 63, Duobao Road, Liwan District, Guangzhou, Guangdong, 510150, People’s Republic of China, Email [email protected]
Background and Purpose: Futile recanalization (FRC) is common among large artery occlusion (LAO) patients after endovascular therapy (EVT). We developed nomogram models to identify LAO patients at a high risk of FRC pre- and post-EVT to help neurologists select the optimal candidates for EVT.
Methods: From April 2020 to July 2022, EVT and mTICI score ≥ 2b LAO patients were recruited. Nomogram models was developed by two-step approach for predicting the outcomes of LAO patients. First, the least absolute shrinkage and selection operator (LASSO) regression analysis was to optimize variable selection. Then, a multivariable analysis was to construct an estimation model with significant indicators from the LASSO. The accuracy of the model was verified using receiver operating characteristic (ROC), calibration curve, and decision curve analyses (DCA), along with validation cohort (VC).
Results: Using LASSO, age, sex, hypertension history, baseline NIHSS, ASPECTS and baseline SBP upon admission were identified from the pre-EVT variables. Model 1 (pre-EVT) showed good predictive performance, with an area under the ROC curve (AUC) of 0.815 in the training cohort (TrC) and 0.904 in VC. Under the DCA, the generated nomogram was clinically applicable where risk cut-off was between 15%– 85% in the TrC and 5%– 100% in the VC. Moreover, age, ASPECTS upon admission, onset duration, puncture-to-recanalization (PTR) duration, and lymphocyte-to-monocyte ratio (LMR) were screened by LASSO. Model 2 (post-EVT) also demonstrated good predictive performance with AUCs of 0.888 and 0.814 for TrC and VC, respectively. Under the DCA, the generated nomogram was clinically applicable if the risk cut-off was between 13– 100% in the TrC and 22– 85% of VC.
Conclusion: In this study, two nomogram models were generated that showed good discriminative performance, improved calibration, and clinical benefits. These nomograms can potentially accurately predict the risk of FRC in LAO patients pre- and post-EVT and help to select appropriate candidates for EVT.
Keywords: acute ischemic stroke, AIS, futile recanalization, endovascular therapy, nomogram model, predictive model
Introduction
Large artery occlusion (LAO)-induced acute ischemic stroke (AIS) has a massive disease burden and carries an elevated rate of disability and mortality.1 The current international guidelines2 indicate that endovascular therapy (EVT) is the primary intervention for LAO stroke involving the anterior circulation. In fact, multiple randomized clinical trials (RCTs) demonstrated the efficacy and safety of this intervention.3 The EVT goal is recanalization, which can enhance patient functional prognosis. Unfortunately, futile recanalization (FRC) is relatively common after EVT in AIS patients.3,4 Several studies reported FRC in approximately 40.5–54.5% of patients after EVT.5–8 FRC is generally described as worse clinical prognosis (mRS score >2 at 3 months), or therapy failure after meaningful recanalization (MRC, TIMI grade 2b or 3) among AIS patients after EVT.4 FRC patients who receive EVT may also experience reperfusion injury, which consumes additional time and resources. Given these challenges, it is critical to identify suitable LAO candidates who can undergo EVT without risk of FRC.
Till date, multiple FRC-related indicators have been identified in LAO patients following EVT. These include advanced age, female gender, and progressive neurological deficits.5,7 In addition, neuroimaging profiles involving baseline Alberta Stroke Program Early Computed Tomography Score (ASPECTS), poor collateral circulation, and final infarction volume are also strongly linked to FEC incidences.5,9,10
Several investigations studying noncontrast CT risk factors (RFs) among mechanical thrombectomy-treated AIS patients reported that leukoaraiosis and brain atrophy are strongly correlated with worse prognosis among recanalized patients.11,12 Another report employing diffusion-weighted magnetic resonance imaging (DWI) for FRC prediction revealed that patients with large preintervention DWI lesions in the deep white matter have poor outcomes following EVT.6 Furthermore, laboratory assessments of interleukins (IL), tumor necrosis factors (TNF), and several inflammatory markers, namely, high-sensitivity C-reactive protein, homocysteine, neutrophil-to-lymphocyte ratio (NLR), lymphocyte-to-monocyte ratio (LMR), platelet-to-lymphocyte ratio (PLR), and platelet volume are also linked to AIS patient outcome.13–17 Likewise, the systemic inflammation response (SIRI) and systemic immune-inflammation indexes (SII) are also related to FRC in LAO patients following EVT.18 Lastly, certain peri-interventional features like general anesthesia and delayed PTR are also correlated with FRC occurrence.19
There are limited investigations on FRC predictive models in LAO patients who receive EVT. As such, currently, there is no consensus on a predictive model for FRC prediction within this patient population. A nomogram is a robust, accurate, and visually presented tool of outcome prediction based on data incorporation and scoring methodology. Using the nomogram model, one can evaluate the risk probability of a single clinical event and stratify patients accordingly. Hence, it is essential to develop a nomogram model to estimate FRC probability among LAO patients following EVT. Herein, we assessed FRC-related prognostic indicators using nomogram, and established a pre-EVT (Early) and post-EVT model (Late) to accurately estimate FRC risk, and empower patients and their relatives with reliable prognostic information.
Materials and Methods
Patient Selection
For this retrospective investigation, we retrieved information on all acute ischemic stroke patients who underwent EVT within 24 hours after onset at the National Advanced Stroke Center of the Third Affiliated Hospital of Guangzhou Medical University (China) between April 2020 and July 2022. EVT reperfusion was assessed according to the modified Thrombolysis in Cerebral Infarction (mTICI) scales. Eligible patients received EVT and had an mTICI score ≥2b. An mTICI score of 2b/3 represented recanalization following EVT. Patients who were eliminated from analysis if there was missing 3-month mRS, age <18 years, pre-stroke mRS score >2, and onset to arrival (OTA) duration >24 hours. The reasons for our exclusion of patients with a pre-stroke mRS score >2 from the analysis need to be specified. On the one hand, the patients with pre-stroke mRS score >2 implied a previous disability, who may have additional risk factors for cerebrovascular diseases.20 On the other hand, in most clinical studies, patients with a pre-stroke mRS score >2 are excluded from trials of EVT of acute ischemic stroke in the anterior circulation.21–24 This is because patients with a pre-stroke mRS score >2 do not have a good outcome with EVT, but rather increase mortality.25,26 Therefore, we chose to exclude this group of patients to reduce bias in the results. This study was reviewed and approved by the Ethics Committee of the Third Affiliated Hospital of Guangzhou Medical University, and all patients involved signed the informed consent form.
Patient Clinical and Radiological Variables
We analyzed the following clinical and radiological profiles across patients: age, sex, RFs, baseline National Institutes of Health Stroke Scale (NIHSS) score (ranging between 0–42; rising score indicates worse neurological deficit), baseline ASPECTS, ischemic stroke pathogenesis, arterial occlusion location, intravenous thrombolysis, and mTICI grade. We also assessed patient medical and lifestyle histories, namely, hypertension, diabetes, dyslipidemia, dyslipidemia, coronary artery disease, as well as smoking and drinking histories. Using ASPECTS via CT, we also calculated the extent of early infarct, which were based out of a score between 0–10, whereby elevated scores represented smaller early ischemic alterations. Ischemic stroke pathogenesis was stratified using the ORG 10172 Trial of in Acute Stroke Treatment (TOAST) criteria.27 In short, the following variable durations were assessed: onset, onset to recanalization (OTR), OTA, onset to puncture (OTP), arrival to recanalization (AOR), and puncture to recanalization (PTR) durations. Moreover, we evaluated complications, such as symptomatic intracerebral hemorrhage (sICH), infarct hemorrhagic transformation (HT), and heating conditions. SICH was described as any intracranial hemorrhage that increases the total NIHSS score by four points.
Laboratory Measurements
Among the retrieved laboratory information were baseline systolic (SBP), and diastolic blood pressure (DBP), as well as glucose upon admission, white blood cell differential counts, platelets, mean platelet volume (MPV), hemoglobin (HGB), albumin (ALB), globulin (GLB), total cholesterol (TC), triglyceride (TG), high-density lipoprotein cholesterol (HDL-C), low-density lipoprotein cholesterol (LDL-C), creatinine (Cr), homocysteine (HCY), HbA1c, BNP, fibrinogen, and D-dimer. NLR was computed via division of the neutrophil count (NC) by the lymphocyte count (LC). LMR was computed via division of the LC by the monocyte count (MC). PLR was computed via division of the platelet count (PC) by the LC.16 The SIRI and SII were described as follows: SIRI=NC ×MC/LC and SII=PC ×NC/LC, respectively.28,29
Patient Outcome
The study participants with mTICI ≥2b were next stratified using the 90-day mRS score, which encompassed the FRC (90-day mRS of 3–6) and MRC groups (90-day mRS of 0–2). The 90-day mRS was gathered via telephonic conversation or outpatient appointment at the 3 months follow-up after onset.
Statistical Analysis
Data analyses were conducted via the SPSS 26.0 (IBM Corporation, Armonk, New York, USA) and R software (version number 4.2.1). Clinical comparison was performed between the FRC and MRC patient cohorts, and continuous variable normality was assessed via the Shapiro–Wilk test. Data with normal distribution were assessed via the t-test and are displayed as mean and SD. Data with non-normal distributions were assessed via the Mann–Whitney U-test and are displayed as median and interquartile range (IQR). The Pearson’s chi-squared or Fisher’s exact test was employed for categorical information. Missing data was imputed with the miss Forest algorithm. Overall, 101 participants were arbitrarily separated into a training (TrC, n = 76) and validation cohorts (VC, n = 25), conforming to a ratio of 3:1. All investigations were two-sided and a P-value <0.05 was set as the significance threshold.
Construction and Evaluation of Models
The least absolute shrinkage and selection operator (LASSO) regression analysis facilitates the shrinkage and variable selection of linear regression models. To identify indicators, LASSO analysis reduces estimation inaccuracy for a given quantitative response by using constraints on model parameters such that the regression coefficients (RCs) for certain variables reduce to zero. Using this mechanism, variables with RC = 0 are eliminated from the model, whereas variables with RC > 0 are considered strongly linked to the response variable. Then, using a −2log-likelihood and binomial family, LASSO computes 10-folds K cross-verification to centralize and normalize relevant variables, prior to selecting the ones with the optimal lambda values. “Lambda.1se” is reported to generate a model with good performance but with minimal quantity of independent variables.30 Given these advantages, LASSO was chosen to assess TC data for the selection of significant RF indicators. The multicollinearities of variable combinations were evaluated using variance inflation factors and condition index. Variables identified as significant in LASSO analysis were used to generate the multivariable logistic regression analysis,31 and the variables are presented as odds ratio and P value with 95% confidence interval (CI). Lastly, the resulting significant indicators were employed for nomogram construction. All variables within the nomogram received a weighted score. The total of all variable scores was used to assess the individual FRC risk using a function between the sum score and outcome probability. Finally, a conventional nomogram was generated using the “rms” package in R.
We also employed several verification techniques to assess the precision of risk estimation of TrC and VC. The area under the receiver operating characteristic curves (AUCs) was employed to evaluate the model’s predictability, and to identify the cut-off values that distinguished between the MRC and FRC cohorts. A calibration curve (CC) was plotted using 1000 bootstrap resampling to examine the relationship between actual events and estimated outcome. The decision curve analysis (DCA) was employed to quantify the total benefit across all cut-off probabilities to further assess the benefit of the aforementioned nomograms.32,33 Lastly, the online “DynNom” package in R was employed to generate dynamic nomogram models to predict undesirable outcomes in AIS patient at 3 months post intervention.
Result
Between April 2020 and July 2022, we recruited 134 patients. Following exclusion of patients with failed recanalization (n=16), a pre-stroke mRS score >2 (n=6), lost following up (n=5), OTA duration > 24 hours (n=2), and age <18 years (n=1), 101 patients were remaining, among which 76 were stratified into TrC and 25 into VC (Figure 1). No marked difference was evidenced between these two patient populations, except for the fibrinogen counts (Table 1).
 |
Table 1 Differences in Characteristics Between the Training Cohort and the Validation Cohort |
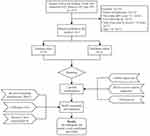 |
Figure 1 Flow chart presenting the process of patient inclusion and exclusion in this study. |
Evaluation of Patient Clinical Profiles
TrC was further separated into two sub-cohorts, namely, MRC (mRS ≤ 2, good prognosis) and FRC (mRS > 2, poor prognosis) (Table 2). In all, 40 patients (52.6%) were placed in the FRC cohort, among which 24 were males (60%, P > 0.05 relative to the MRC cohort), with a median age of 78 (69, 86) years (P<0.001 relative to the MRC cohort), 29 patients (72.5%, P=0.044) had hypertension, NIHSS was assessed upon admission in 17 patients (13, 21) (P=0.002), and only 14 patients (35%, P=0.012) had ASPECTS upon admission ≤7. The passage quantity between the two cohorts was also markedly different (P=0.009). Lastly, the FRC patients exhibited markedly elevated baseline SBP, relative to the MRC patients (P=0.011).
 |
Table 2 Comparison of Characteristics Between the Futile Recanalization Group and the Favorable Recanalization Group |
Based on our laboratory assessment, the NC was considerably high among FRC patients (P=0015), whereas the MC was markedly elevated among MRC patients (P=0.003). Moreover, the FRC patients exhibited enhanced NLR with reduced LMR, relative to the MRC patients (P=0.010, P<0.001). Lastly, the SIRI and SII values were markedly diminished in the MRC patients, relative to the FRC patients (P<0.001, P=0.009).
Prediction Model Development
We analyzed 19 FRC-related variables recorded before EVT using LASSO, using lambda. min = 0.041 [log (lambda. min) = −1.386] and lambda.1se = 0.095 [log(lambda.1se) = −1.022]. Owing to our small patient population, we selected lambda.1se for analysis. Using lambda.1se = 0.095, we filtered the following variables, namely, age, gender, hypertension history, baseline NIHSS, ASPECTS upon admission ≤7 and baseline SBP (Figure 2). Next, we conducted LASSO analysis to identify the FRC-associated variables from 52 candidates, providing us with lambda. min = 0.072 [log (lambda. min) = −1.142] and lambda.1se = 0.115 [log(lambda.1se) = −1.143]. We chose lambda.1se again. Using lambda.1se = 0.115, the following variables were screened, namely, age, ASPECTS upon admission ≤7, onset time, PTR, LMR, SBP and sICH (Figure 3).
To establish two predictive nomogram models for the pre- and post-operative FRC risk evaluations, we entered the aforementioned significant variables into multivariate analysis. No obvious multicollinearity existed for the stand-alone indicators that were entered into multivariate analysis. (Table 3). Model 1 revealed that patient age (odds ratio (OR), 1.08; 95% confidence interval [CI], 1.01 to 1.12), baseline NIHSS (OR, 1.12; 95% CI, 1.01 to 1.25), and ASPECTS upon admission ≤7 (OR, 7.13; 95% CI, 1.46 to 34.76) were stand-alone FRC indicators (Table 4). Additionally, model 2 revealed that patient age (OR, 1.07; 95% CI, 1.112 to 17.587), ASPECTS upon admission ≤7 (OR, 11.82; 95% CI, 1.47 to 94.77), onset duration (OR, 0.12; 95% CI, 0.02 to 0.76), PTR (OR, 1.04; 95% CI, 1.01 to 1.07), and LMR (OR, 0.52; 95% CI, 0.28–0.97) were stand-alone FRC indicators (Table 4).
 |
Table 3 Multicollinearity Assessment |
 |
Table 4 The Multivariable Logistic Regression Analysis |
Next, two predictive nomogram models were established using the aforementioned stand-alone FRC indicators (Figure 4 and 5). In addition, we established a dynamic nomograph based on the network so that clinicians and researchers can access it more widely and easily https://model1pre-evt.shinyapps.io/DynNomapp-pre-EVT, https://model2post-evt.shinyapps.io/DynNomapp-pre-EVT Get it online. Clinicians and researchers can input individual variables of patients on the website to easily obtain the risk of invalid recanalization.
Prediction Model Assessment and Validation
For the predictive Models 1 and 2, the pooled nomogram AUCs were 0.815 (95% CI, 0.720 to 0.911) and 0.888 (95% CI, 0.813 to 0.964) in the TrC, respectively, and 0.904 (95% CI, 0.790 to 1.000) and 0.814 (95% CI, 0.623 to 1.000) in the VC, respectively (Figures 6A and FB), thereby demonstrating good performance. Both nomogram CCs utilized 1000 resampling for bootstrapping verification, which also displayed good agreements between the estimated and actual values (Figures 7A, B, 8A and B). The mean squared error of models 1 and 2 were 0.039 and 0.025, respectively, again revealing good predictive performance. Subsequently, using decision-curve analysis (DCA), we assessed whether the generated nomograms can delineate FRC from MRC patients. In case of model 1, based on the DCA, the nomogram predictability was enhanced when the risk cut-off was between 0–80% in the TrC and between 0–100% in the VC (Figure 9A and B). In case of model 2, the nomogram predictability was enhanced when the risk cut-off was between 15–85% in the TrC, and >5% in the VC (Figure 10A and B).
 |
Figure 6 Receiver operating characteristic curve (ROC) validation of the Model 1 (A) and Model 2 (B) nomogram prediction. |
Discussion
Herein, we generated and verified two estimation nomograms that integrated AIS patient demographic, clinical, and laboratory profiles for the individualized prediction of FRC prognosis before and after EVT. Even though a worse AIS prognosis is primarily due to disease severity, a precise prognosis is crucial for the clinical decision-making process involving AIS patients. To minimize overfitting or skewed indicator distributions that are common with classical logistic regression analyses, we employed LASSO regression to evaluate the 19 potential FRC-related variables prior to EVT, and 52 indicator candidates after EVT. We further assessed the estimated outcomes by narrowing down the RCs using LASSO. Lastly, we employed multivariate analysis to identify stand-alone FRC indicators, and a nomogram was subsequently generated.
Nomograms are highly reliable and practical estimation tools. They generate distinct possibilities of clinical events by combining different prognostic and determinant data, and by incorporating several strong event indicators to assess individual risk.34 Nomograms are a great resource for integrating biological and clinical findings, and they are critical for the development of personalized therapy. Lastly, it has good user-friendly digital interfaces, which facilitates prognosis prediction and enhances clinical decision-making.31
Model 1 nomogram included three variables, namely age, baseline NIHSS, and baseline ASPECTS. Meanwhile, model 2 nomogram included five variables, namely, age, baseline ASPECTS, onset duration, PRT, and LMR. Both models demonstrated good discriminatory power, calibration, and clinical utility.
Relative to younger participants, older participants who receives EVT, experiences a reduced rate of good clinical outcomes. Similar to earlier publications,9,10,19,35,36 we revealed that advanced age was strongly correlated with functional dependence, even with MRC. This is potentially due to an elevated incidence of underlying diseases and complications, along with a reduced potential for rehabilitation among older versus younger patients. Owing to the increased presence of underlying diseases among older patients, enhanced neuronal plasticity is not easy to achieve, even with post-reperfusion therapy. As a result, leukoaraiosis and the weak blood–brain barrier may contribute to intracranial hemorrhage (ICH) among older patients. In addition, collateral blood supply may be worse in older versus younger patients. Of note, in terms of the optimal AIC therapy, older patients, particularly, those with good pre-stroke functional status may still benefit from EVT.37 Given these evidence, older patients should not be exempt from thrombectomy; however, the newly developed prognostic indicator can potentially inform patients and their families about post-stroke patient outcome.
Baseline NIHSS is generally considered a robust FRC indicator following thrombectomy. Herein, we also demonstrated that an elevated NIHSS score upon admission was intricately linked to FRC incidence, which was consistent with prior publications.7,9,19,35,36,38,39 Interestingly, Lee et al reported that the reperfusion benefits following EVT enhanced with stroke severity, in spite of augmented FRC rate.39 Likewise, a meta-analysis revealed that patients with severe mechanical stroke (NIHSS score >20) achieved higher benefits from thrombectomy, compared to pharmaceutical therapy. This is likely because patients with severe stroke typically becomes functionally dependent, if left untreated, and only few patients with sufficient salvageable brain tissue can potentially recover following reperfusion. Based on these evidence, when deemed as a non-modifiable RF, an elevated NIHSS score should not exclude a patient from receiving EVT.
Emerging reports suggest that the proper selection of EVT recipients using imaging criteria enhances patient outcome.40–43 Herein, we demonstrated that ASPECTS upon admission can aid in the prediction of functional outcomes following LAO MRC. Moreover, we stratified at ≤7 vs >7 to estimate patient prognosis at 90 days. Patients with reduced scores represented massive brain ischemic infarction lesions, indicating that despite MRC, good functional outcomes were difficult to achieve.
Early vascular recanalization following AIS can protect patients from more ischemic penumbra, reduce core infarct volume, enhance clinical prognosis, and reduce FRC incidence. One prospective IMS III; (interventional management of stroke III) investigation examining the outcome of mechanical thrombectomy of AIS venous thrombolysis bridge within 3 hours of onset revealed that the OTP duration is longer among FRC patients, and multivariate analysis further revealed that the OTP duration is a stand-alone RF for FRC.7 Similarly, in a HERMES meta-analysis, the mean OTP duration was 238min in patients with MRC, and the time delay in reperfusion was found to be strongly associated with FRC.44 A Japanese study prospectively examined 2420 patients with anterior circulation large vessel occlusive AIS who received EVT within 12 hours of onset in 46 centers. Based on its post-mortem analysis, a femoral artery PTR duration <80 min made mTICI2b patients more susceptible than mTICI3 patients to FRC. However, when the PTR duration was ≥80 min, no marked difference was evident in the FRC prevalence between the two groups.45
In this investigation, the onset and PTR durations were identified as stand-alone RFs for FRC prediction among EVT-treated LAO patients. This is a novel find in the field of FRC research. One possible rationale for our findings is that patients experiencing prolonged operation durations may have occlusions that are challenging to recanalize. Hence, a longer duration likely represents multiple attempts at achieving a good TICI score. This can, unfortunately, enhance the risk of intra- and post-procedural intracranial vasculature surgery-related complications.
Our study established that the LMR in peripheral blood samples accurately predict FRC following EVT. Inflammation accelerates AIS progress, and leukocytes, namely, neutrophils, lymphocytes, and monocytes, possess distinct roles in an inflammatory reaction.46–48 Neutrophils typically stimulate the inflammatory process, thereby inducing brain damage via release of inflammatory mediators. In contrast, monocytes interact with platelets and endothelial cells to enhance inflammation via inflammatory and prothrombotic networks. Lymphocytes, on the other hand, downregulate inflammation.49,50 Prior investigations suggested that inflammatory markers like NLR and LMR are closely correlated with AIS patient prognosis.51,52 Reduced NLR and enhanced LMR indicate desirable AIS patient outcome.51–53 Hence, elevated LMR levels are correlated with enhanced prognosis following EVT, which is brought about by inhibition of the inflammatory response via downregulation of monocytes and upregulation of the anti-inflammatory property of lymphocytes.
SIRI is newly discovered inflammatory indicator, and it performs better than NLR or LMR in predicting inflammation. This is likely due to SIRI (neutrophil× monocyte/lymphocyte), which concurrently represents the status of three variables, while NLR (neutrophil/lymphocyte) and LMR (lymphocyte/monocyte) represent only two variables. Based on a prior publication, both SIRI and SII are stand-alone indicators of FRC in EVT-treated patients.54 Nevertheless, herein, our analyses did not identify SIRI and SII as stand-alone indicators of FRC in EVT-treated LAP patients.
However, the present study still has some limitations. First, the study is a single-center retrospective study with a relatively small sample size, and some bias are inevitable. Therefore, prospective and external validation studies with large samples are needed. Second, we excluded the population with pre-stroke mRS score >2, as in most previous clinical studies. Therefore, the predictive power of our study for this group of patients needs to be further analyzed and studied.
Conclusion
This study showed that the establishment of our dynamic and visualized nomogram model could be applied preoperatively and postoperatively to accurately predict the risk of futile recanalization in patients with LAO and two predictive models demonstrated good discriminative performances, along with enhanced calibration, and clinical benefits, which assist in the selection of clinical treatment modalities. In the future, the emergence of more multi-center studies will be more conducive to the application of our model in clinical settings.
Ethics Approval and Informed Consent
This study protocol was reviewed and approved by the Institutional Research Ethic Committee of The Third Affiliated Hospital of Guangzhou Medical, approval number [IIT20220240B-R1]. All participants wrote informed consent. We followed the guidelines outlined in the Declaration of Helsinki.
Acknowledgments
We thank our research group that helped and supported us for this study at the Department of psychiatry and Neurology, The Third Affiliated Hospital of Guangzhou Medical, China.
Disclosure
The authors have no conflicts of interest to declare in this work.
References
1. Emberson J, Lees KR, Lyden P, et al. Effect of treatment delay, age, and stroke severity on the effects of intravenous thrombolysis with alteplase for acute ischaemic stroke: a meta-analysis of individual patient data from randomised trials. Lancet. 2014;384(9958):1929–1935. doi:10.1016/S0140-6736(14)60584-5
2. Powers WJ, Rabinstein AA, Ackerson T, et al. Guidelines for the early management of patients with acute ischemic stroke: 2019 Update to the 2018 Guidelines for the early management of acute ischemic stroke: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2019;50(12):e344–e418. doi:10.1161/STR.0000000000000211
3. Goyal M, Menon BK, van Zwam WH, et al. Endovascular thrombectomy after large-vessel ischaemic stroke: a meta-analysis of individual patient data from five randomised trials. Lancet. 2016;387(10029):1723–1731. doi:10.1016/S0140-6736(16)00163-X
4. Hussein HM, Georgiadis AL, Vazquez G, et al. Occurrence and predictors of futile recanalization following endovascular treatment among patients with acute ischemic stroke: a multicenter study. AJNR Am J Neuroradiol. 2010;31(3):454–458. doi:10.3174/ajnr.A2006
5. van Horn N, Kniep H, Leischner H, et al. Predictors of poor clinical outcome despite complete reperfusion in acute ischemic stroke patients. J Neurointerv Surg. 2021;13(1):14–18. doi:10.1136/neurintsurg-2020-015889
6. Tateishi Y, Wisco D, Aoki J, et al. Large deep white matter lesions may predict futile recanalization in endovascular therapy for acute ischemic stroke. Interv Neurol. 2015;3(1):48–55. doi:10.1159/000369835
7. Hussein HM, Saleem MA, Qureshi AI. Rates and predictors of futile recanalization in patients undergoing endovascular treatment in a multicenter clinical trial. Neuroradiology. 2018;60(5):557–563. doi:10.1007/s00234-018-2016-2
8. Hassan AE, Shariff U, Saver JL, et al. Impact of procedural time on clinical and angiographic outcomes in patients with acute ischemic stroke receiving endovascular treatment. J Neurointerv Surg. 2019;11(10):984–988. doi:10.1136/neurintsurg-2018-014576
9. Pan H, Lin C, Chen L, et al. Multiple-factor analyses of futile recanalization in acute ischemic stroke patients treated with mechanical thrombectomy. Front Neurol. 2021;12:704088. doi:10.3389/fneur.2021.704088
10. Zhou T, Yi T, Li T, et al. Predictors of futile recanalization in patients undergoing endovascular treatment in the DIRECT-MT trial. J Neurointerv Surg. 2022;14(8):752–755. doi:10.1136/neurintsurg-2021-017765
11. Gilberti N, Gamba M, Premi E, et al. Leukoaraiosis is a predictor of futile recanalization in acute ischemic stroke. J Neurol. 2017;264(3):448–452. doi:10.1007/s00415-016-8366-y
12. Kaginele P, Beer-Furlan A, Joshi KC, et al. Brain atrophy and leukoaraiosis correlate with futile stroke thrombectomy. J Stroke Cerebrovasc Dis. 2021;30(8):105871. doi:10.1016/j.jstrokecerebrovasdis.2021.105871
13. Lambertsen KL, Biber K, Finsen B. Inflammatory cytokines in experimental and human stroke. J Cereb Blood Flow Metab. 2012;32(9):1677–1698. doi:10.1038/jcbfm.2012.88
14. Yu H, Huang Y, Chen X, et al. High-sensitivity C-reactive protein in stroke patients - The importance in consideration of influence of multiple factors in the predictability for disease severity and death. J Clin Neurosci. 2017;36:12–19. doi:10.1016/j.jocn.2016.10.020
15. Altintas O, Altintas MO, Tasal A, et al. The relationship of platelet-to-lymphocyte ratio with clinical outcome and final infarct core in acute ischemic stroke patients who have undergone endovascular therapy. Neurol Res. 2016;38(9):759–765. doi:10.1080/01616412.2016.1215030
16. Oh SW, Yi HJ, Lee DH, et al. Prognostic significance of various inflammation-based scores in patients with mechanical thrombectomy for acute ischemic stroke. World Neurosurg. 2020;141:e710–e717. doi:10.1016/j.wneu.2020.05.272
17. Kumral E, Saruhan G, Aktert D, et al. Association of hyperhomocysteinemia with stroke recurrence after initial stroke. J Stroke Cerebrovasc Dis. 2016;25(8):2047–2054. doi:10.1016/j.jstrokecerebrovasdis.2016.05.008
18. Lattanzi S, Norata D, Divani AA, et al. Systemic inflammatory response index and futile recanalization in patients with ischemic stroke undergoing endovascular treatment. Brain Sci. 2021;11(9):1164. doi:10.3390/brainsci11091164
19. Xu H, Jia B, Huo X, et al. Predictors of futile recanalization after endovascular treatment in patients with acute ischemic stroke in a multicenter registry study. J Stroke Cerebrovasc Dis. 2020;29(10):105067. doi:10.1016/j.jstrokecerebrovasdis.2020.105067
20. Goyal N, Tsivgoulis G, Pandhi A, et al. Impact of pretreatment with intravenous thrombolysis on reperfusion status in acute strokes treated with mechanical thrombectomy. J Neurointerv Surg. 2019;11(11):1073–1079. doi:10.1136/neurintsurg-2019-014746
21. Zi W, Qiu Z, Li F, et al. Effect of endovascular treatment alone vs intravenous alteplase plus endovascular treatment on functional independence in patients with acute ischemic stroke: the devt randomized clinical trial. JAMA. 2021;325(3):234–243. doi:10.1001/jama.2020.23523
22. Goyal N, Tsivgoulis G, Frei D, et al. Comparative safety and efficacy of combined IVT and MT with direct MT in large vessel Occlusion. Neurology. 2018;90(15):e1274–e1282. doi:10.1212/WNL.0000000000005299
23. LeCouffe NE, Kappelhof M, Treurniet KM, et al. A randomized trial of intravenous alteplase before endovascular treatment for stroke. N Engl J Med. 2021;385(20):1833–1844. doi:10.1056/NEJMoa2107727
24. Yang P, Zhang Y, Zhang L, et al. Endovascular Thrombectomy with or without intravenous alteplase in acute stroke. N Engl J Med. 2020;382(21):1981–1993. doi:10.1056/NEJMoa2001123
25. Bala F, Beland B, Mistry E, et al. Endovascular treatment of acute ischemic stroke in patients with pre-morbid disability: a meta-analysis. J Neurointerv Surg. 2022;38(9):Neurintsurg-2021–018573. doi:10.1136/jnis-2022-019064
26. Zhao H, Bai X, Li W, et al. Influence of pre-stroke dependency on safety and efficacy of endovascular therapy: a systematic review and meta-analysis. Front Neurol. 2022;13:956958. doi:10.3389/fneur.2022.956958
27. Adams HP, Bendixen BH, Kappelle LJ, et al. Classification of subtype of acute ischemic stroke. Definitions for use in a multicenter clinical trial. TOAST. Trial of Org 10172 in Acute Stroke Treatment. Stroke. 1993;24(1):35–41. doi:10.1161/01.STR.24.1.35
28. Hong X, Cui B, Wang M, et al. Systemic immune-inflammation index, based on platelet counts and neutrophil-lymphocyte ratio, is useful for predicting prognosis in small cell lung cancer. Tohoku J Exp Med. 2015;236(4):297–304. doi:10.1620/tjem.236.297
29. Wei L, Xie H, Yan P. Prognostic value of the systemic inflammation response index in human malignancy: a meta-analysis. Medicine. 2020;99(50):e23486. doi:10.1097/MD.0000000000023486
30. Mo R, Shi R, Hu Y, et al. Nomogram-based prediction of the risk of diabetic retinopathy: a retrospective study. J Diabetes Res. 2020;2020:7261047. doi:10.1155/2020/7261047
31. Wang H, Zhang L, Liu Z, et al. Predicting medication nonadherence risk in a Chinese inflammatory rheumatic disease population: development and assessment of a new predictive nomogram. Patient Prefer Adherence. 2018;12:1757–1765.
32. van Calster B, Wynants L, Verbeek JFM, et al. Reporting and interpreting decision curve analysis: a guide for investigators. Eur Urol. 2018;74(6):796–804. doi:10.1016/j.eururo.2018.08.038
33. Vickers AJ, Elkin EB. Decision curve analysis: a novel method for evaluating prediction models. Med Decis Making. 2006;26(6):565–574. doi:10.1177/0272989X06295361
34. Liang G, Chen X, Zha X, et al. A nomogram to improve predictability of small-incision lenticule extraction surgery. Med Sci Monit. 2017;23:5168–5175. doi:10.12659/MSM.904598
35. Shi ZS, Liebeskind DS, Xiang B, et al. Predictors of functional dependence despite successful revascularization in large-vessel occlusion strokes. Stroke. 2014;45(7):1977–1984. doi:10.1161/STROKEAHA.114.005603
36. Olivot JM, Heit JJ, Mazighi M, et al. What predicts poor outcome after successful thrombectomy in early time window? J Neurointerv Surg. 2022;14(11):1051–1055. doi:10.1136/neurintsurg-2021-017946
37. Khan MA, Baird GL, Miller D, et al. Endovascular treatment of acute ischemic stroke in nonagenarians compared with younger patients in a multicenter cohort. J Neurointerv Surg. 2017;9(8):727–731. doi:10.1136/neurintsurg-2016-012427
38. van de Graaf RA, Samuels N, Chalos V, et al. Predictors of poor outcome despite successful endovascular treatment for ischemic stroke: results from the MR CLEAN Registry. J Neurointerv Surg. 2022;14(7):660–665. doi:10.1136/neurintsurg-2021-017726
39. Lee SH, Kim BJ, Han MK, et al. Futile reperfusion and predicted therapeutic benefits after successful endovascular treatment according to initial stroke severity. BMC Neurol. 2019;19(1):11. doi:10.1186/s12883-019-1237-2
40. Lu WZ, Lin HA, Bai CH, et al. Posterior circulation acute stroke prognosis early CT scores in predicting functional outcomes: a meta-analysis. PLoS One. 2021;16(2):e0246906. doi:10.1371/journal.pone.0246906
41. Pereira D, Fragata I, Amorim J, et al. ADC quantification in basilar artery occlusion as an indicator of clinical outcome after endovascular treatment. Neuroradiol J. 2017;30(6):586–592. doi:10.1177/1971400917706197
42. Coutts SB, Lev MH, Eliasziw M, et al. ASPECTS on CTA source images versus unenhanced CT: added value in predicting final infarct extent and clinical outcome. Stroke. 2004;35(11):2472–2476. doi:10.1161/01.STR.0000145330.14928.2a
43. Karameshev A, Arnold M, Schroth G, et al. Diffusion-weighted MRI helps predict outcome in basilar artery occlusion patients treated with intra-arterial thrombolysis. Cerebrovasc Dis. 2011;32(4):393–400. doi:10.1159/000330644
44. Saver JL, Goyal M, van der Lugt A, et al. Time to treatment with endovascular thrombectomy and outcomes from ischemic stroke: a meta-analysis. JAMA. 2016;316(12):1279–1288. doi:10.1001/jama.2016.13647
45. Kitano T, Todo K, Yoshimura S, et al. Futile complete recanalization: patients characteristics and its time course. Sci Rep. 2020;10(1):4973. doi:10.1038/s41598-020-61748-y
46. Ren H, Liu X, Wang L, et al. Lymphocyte-to-monocyte ratio: a novel predictor of the prognosis of acute ischemic stroke. J Stroke Cerebrovasc Dis. 2017;26(11):2595–2602. doi:10.1016/j.jstrokecerebrovasdis.2017.06.019
47. Song Q, Pan R, Jin Y, et al. Lymphocyte-to-monocyte ratio and risk of hemorrhagic transformation in patients with acute ischemic stroke. Neurol Sci. 2020;41(9):2511–2520. doi:10.1007/s10072-020-04355-z
48. Herz J, Sabellek P, Lane TE, et al. Role of neutrophils in exacerbation of brain injury after focal cerebral ischemia in hyperlipidemic mice. Stroke. 2015;46(10):2916–2925. doi:10.1161/STROKEAHA.115.010620
49. Lux D, Alakbarzade V, Bridge L, et al. The association of neutrophil-lymphocyte ratio and lymphocyte-monocyte ratio with 3-month clinical outcome after mechanical thrombectomy following stroke. J Neuroinflammation. 2020;17(1):60. doi:10.1186/s12974-020-01739-y
50. Yang Y, Sun G, Diao S, et al. Diagnostic performances of neutrophil to lymphocyte ratio and lymphocyte to monocyte ratio in acute ischemic stroke caused by cervicocranial arterial dissection. J Clin Lab Anal. 2020;34(12):e23515. doi:10.1002/jcla.23515
51. Switonska M, Slomka A, Korbal P, et al. Association of neutrophil-to-lymphocyte ratio and lymphocyte-to-monocyte ratio with treatment modalities of acute ischaemic stroke: a pilot study. Medicina. 2019;55(7):342. doi:10.3390/medicina55070342
52. Park MG, Kim MK, Chae SH, et al. Lymphocyte-to-monocyte ratio on day 7 is associated with outcomes in acute ischemic stroke. Neurol Sci. 2018;39(2):243–249. doi:10.1007/s10072-017-3163-7
53. Wang L, Song Q, Wang C, et al. Neutrophil to lymphocyte ratio predicts poor outcomes after acute ischemic stroke: a cohort study and systematic review. J Neurol Sci. 2019;406:116445. doi:10.1016/j.jns.2019.116445
54. Yi HJ, Sung JH, Lee DH. Systemic inflammation response index and systemic immune-inflammation index are associated with clinical outcomes in patients treated with mechanical thrombectomy for large artery occlusion. World Neurosurg. 2021;153:e282–e9. doi:10.1016/j.wneu.2021.06.113
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.








