Back to Journals » Journal of Inflammation Research » Volume 16
Nomogram Based on Inflammatory Factor to Predict Therapeutic Response of Thrombocytopenia in Patients with Primary Sjögren’s Syndrome
Authors Gan M, Peng Y, Zhu M, Ying Y
Received 27 March 2023
Accepted for publication 2 June 2023
Published 12 June 2023 Volume 2023:16 Pages 2449—2459
DOI https://doi.org/10.2147/JIR.S414320
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Ning Quan
Minzhi Gan, Yong Peng, Mengya Zhu, Ying Ying
Department of Rheumatology, Ningbo NO.2 Hospital, Ningbo, Zhejiang, 315010, People’s Republic of China
Correspondence: Minzhi Gan, Ningbo NO.2 Hospital, No. 41, Xibei Street, Ningbo, Zhejiang, 315010, People’s Republic of China, Tel + 86-0574-83870999, Email [email protected]
Objective: Thrombocytopenia is a common manifestation of blood system involvement in primary Sjögren’s syndrome (pSS) patients, and the treatment approach involves glucocorticoids and immune agents. However, a proportion of patients do not respond well to this therapy and failed to achieve remission. Accurate prediction of therapeutic response in pSS patients with thrombocytopenia is of great significance for improving the prognosis. This study aims to analyze the influencing factors of no remission to treatment in pSS patients with thrombocytopenia and establish an individualized nomogram to predict the treatment response of patients.
Materials and Methods: The demographic data, clinical manifestations and laboratory examinations of 119 patients with thrombocytopenia pSS in our hospital were retrospectively analyzed. According to the 30-day treatment response, patients were divided into remission group and non-remission group. Logistic regression was used to analyze the influencing factors related to the treatment response of patients, and then a nomogram was further established. The discriminative ability and clinical benefit of the nomogram were evaluated by receiver operating characteristic (ROC) curve, calibration chart and decision curve analysis (DCA).
Results: After treatment, there were 80 patients in the remission group and 39 in the non-remission group. Comparative analysis and multivariate logistic regression analysis identified hemoglobin (P=0.023), C3 level (P=0.027), IgG level (P=0.040), and bone marrow megakaryocyte counts (P=0.001) as independent predictors of treatment response. The nomogram was constructed based on the above four factors, and the C-index of the model was 0.882 ( 95% CI 0.810– 0.934). The calibration curve and DCA proved that the model has better performance.
Conclusion: The nomogram incorporating hemoglobin, C3 level, IgG level, and bone marrow megakaryocyte counts could be used as an auxiliary tool to predict the risk of treatment non-remission in pSS patients with thrombocytopenia.
Keywords: primary Sjögren’s syndrome, thrombocytopenia, nomogram, bone marrow megakaryocyte
Introduction
Primary Sjögren’s syndrome (pSS) is an autoimmune disease characterized by lymphocytic infiltrates in exocrine glands, resulting in classic symptoms of oral and ocular dryness. Although primary manifestations of pSS target salivary and lachrymal glands, its impact extends to other organ systems.1,2 Hematologic abnormalities are frequently encountered in the setting of pSS, with approximately 30% experiencing cytopenia, mild anemia, and leukopenia. Thrombocytopenia is a serious manifestation of hematological system involvement, with an incidence rate of 5–13%, and can develop at any stage of the disease.3 Studies have shown that pSS patients with thrombocytopenia are more likely to have renal involvement and positive anti-SSB antibodies, which seriously affect the quality of life, shorten life expectancy, and bring challenges to clinical management.4
The pathogenesis of pSS with thrombocytopenia is complex and has not yet been completely elucidated. It has been suggested that the increase in peripheral blood destruction is the primary cause of thrombocytopenia in pSS patients, which may be mediated by autoantibody.5,6 A few studies have shown that T cell-mediated immune responses seem to play an important role in pSS patients with thrombocytopenia. Activated T cells release various cytokines that facilitate the activation and proliferation of B cells, in which the antibodies generated bind to the platelet surface, leading to platelet destruction and ultimately thrombocytopenia.7,8 The current treatment options for such diseases include glucocorticoids, immunosuppressants, and intravenous immunoglobulin.9 However, a proportion of patients may develop resistance to this therapy or exhibit dependency on corticosteroids and require the application of second-line treatments. Even after receiving treatment with glucocorticoids and more than one immunosuppressant, some patients remain refractory or encounter unacceptable toxicity.10 Therefore, the prediction of treatment response in pSS patients with thrombocytopenia is helpful in choosing appropriate treatment strategies and further improving survival outcomes.
The nomogram, a prediction tool that estimates an individualized risk based on regression models, has recently become the preferred choice. Several nomograms have been established to predict the probability of kidney and nervous system involvement in patients with pSS.11–13 To the best of our knowledge, no studies have constructed a nomogram for pSS patients with thrombocytopenia. In this study, we aimed to develop specialized nomograms that can predict possible treatment outcomes of pSS patients with thrombocytopenia.
Materials and Methods
Study Design and Patient Selection
This study was designed as a single-center retrospective analysis. All adult patients with pSS complicated with thrombocytopenia who were admitted to the Ningbo NO.2 Hospital during December 2015 and January 2022 were assessed for eligibility. The clinical information of the eligible patients was retrospectively obtained from medical records. The disease activity in pSS patients was quantified by the European League Against Rheumatism Sjögren’s syndrome disease activity index (ESSDAI), which consists of 12 organ-specific domains. This study was approved by the Ethics Committee of Ningbo NO.2 Hospital, and informed consent was obtained from all patients. It was planned in accordance with the Declaration of Helsinki.
Diagnostic Criteria
All participants fulfilled the criteria for pSS by the American College of Rheumatology (ACR)-European League Against Rheumatism (EULAR) classification criteria in 201614 and were simultaneously diagnosed with thrombocytopenia. The clinical presentation of pSS includes: general symptoms, symptoms of exocrine gland involvement (dry mouth, dry eyes, foreign body sensation, parotid gland enlargement, rampant caries, etc.), and extraglandular system involvement symptoms (arthritis, Raynaud’s phenomenon, skin manifestations, pulmonary complications, nephropathy, liver function, hemorrhagic manifestations, central and peripheral neuropathy, etc.). Further confirmation of the diagnosis was made through Schirmer’s test, tear break-up time measurement, salivary gland biopsy, imaging examination, and laboratory examination. Thrombocytopenia was defined as a platelet count <100 × 109/L. All patients underwent bone marrow puncture of the posterior superior iliac spine after informed consent to exclude other causes of thrombocytopenia. Before proceeding with the puncture, a highly experienced physician meticulously assessed the patient’s physical condition. Bone marrow aspiration is deemed unsuitable for individuals presenting with severe coagulation disorders, serious hemorrhage, psychiatric illnesses, and systemic or puncture site infection.
Exclusion Criteria
The exclusion criteria included: (1) patients who had other causes of thrombocytopenia, such as primary hematological disease, liver cirrhosis, drug-induced or infection; (2) patients with other autoimmune diseases, malignant tumors, or recent history of blood transfusion; (3) patients received medications, chemotherapy or radiotherapy which might affect bone marrow during the 6 months prior to admission; (4) patients with insufficient data in medical records. Finally, a total of 119 patients were enrolled and the flow diagram of the study was depicted in Figure 1.
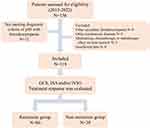 |
Figure 1 Flowchart of the inclusion and study design. Abbreviations: pSS, primary Sjögren’s syndrome; GCS, glucocorticoids; ISAs, immunosuppressive agents; IVIG, intravenous immunoglobulin. |
Data Collection
Demographic data consisting of age, sex, body mass index (BMI), disease duration and comorbidities were collected. Laboratory tests including routine blood tests, immunoglobulin, serum autoantibodies and bone marrow examination were conducted in our hospital laboratory. Complete blood counts and erythrocyte sediment rate (ESR) were obtained with the Beckman Coulter LH 750 analyzer (Beckman Coulter Inc., California, USA). The levels of rheumatoid factor (RF), serum C-reactive protein (CRP), immunoglobulin G (IgG), immunoglobulin M (IgM), immunoglobulin A (IgA), complement 3 (C3), and complement 4 (C4) were detected by routine turbidimetric. Immunoblotting was used for the detection of anti-Sjögrens syndrome-A/Ro (anti-SSA/Ro) antibody and anti-Sjögren syndrome-B/La (anti-SSB/La) antibody. The anti-double-stranded DNA (anti-dsDNA) and anti-Smith antigen (anti-Sm) antibodies were analyzed using commercial enzyme-linked immunosorbent assay kits. The antinuclear antibodies (ANA) and anti-centromere antibody (ACA) were tested by an indirect immunofluorescence assay. Additionally, bone marrow megakaryocyte was also quantified.
Response Criteria
According to disease conditions, patients were treated with glucocorticoids (prednisone, methylprednisolone) and immunosuppressants (cyclophosphamide, tacrolimus, cyclosporin). For patients with severe thrombocytopenia, biologic agents (Rituximab) or intravenous immunoglobulin were often used. All patients underwent routine laboratory assessments 1 month after the end of treatment. The response criteria were as follows:15 (1) complete remission: platelet count ≥100×109/L and absence of bleeding; (2) partial remission: platelet count ≥ 30×109/L or increased above baseline by at least 2 times and no bleeding episodes; (3) no remission: platelet count <30×109/L or bleeding, or increase lower than 2 times of baseline. Patients with complete remission and partial remission were considered in the remission group and no remission was the non-remission group.
Statistical Analysis
The measurement data for normal distribution were assessed using Kolmogorov–Smirnov test. Data with normal distribution were expressed as mean ± standard deviation (Mean±SD), and the two groups were compared by independent sample t-test. Non-normally distributed data were presented as median and quartiles 25 and 75% [M (Q1, Q3)], and the Mann–Whitney U-test was used for two group comparisons. Categorical data were expressed as a number with percentage (%), and compared using the χ2 test or Fisher exact test. Variables with a P-value of <0.05 were considered statistically significant for the univariate analysis. Multiple logistic regression analysis was further performed to evaluate the factors associated with efficacy and then a nomogram was established based on the results of the multivariate logistic model. The receiver operating characteristic (ROC) curve and C-index were used to assess the discriminant power of the model in the training set. In addition, the calibration curve was introduced to verify the prediction performance, and the clinical practicability of the new model was determined with decision curve analysis (DCA). Statistical analyses in the present study were performed using SPSS (version 25.0, USA) and R software (version 3.5, USA).
Results
Demographic Data and Clinical Manifestations of Two Groups
At day 30 post-treatment, 80 patients were classified as the remission group, with 52 cases of complete remission and 28 cases of partial remission. The remaining 39 cases were considered as the non-remission group. Demographic data showed that the median ages of patients in the two groups were 51 and 53 years, respectively, and the majority of them were female. The median duration of the disease was 62 months in the remission group and 67 months in the non-remission group. Evidently, Xerophthalmia and xerostomia occurred most frequently among all the symptoms in both groups, followed by rampant caries. Based on the data in Table 1, no significant differences were found between the remission and non-remission groups in terms of gender, age, BMI, duration of disease, various clinical manifestations, and ESSDAI (all P>0.05; see Table 1).
 |
Table 1 Clinical Characteristics of Patients in Remission and Non-Remission Groups |
Clinical and Biochemical Parameters of the Two Groups
Laboratory test results are shown in Table 2. The ANA titer (P=0.001), lgG level (P=0.010), and lgA level (P=0.013) in the non-remission group of patients exhibited statistically significant elevation in comparison to the remission group. Conversely, the hemoglobin content (P=0.004), C3 level (P=0.005), and bone marrow megakaryocyte counts (P<0.001) in the non-remission group were significantly lower than those of the remission group. However, there was no significant difference in platelet, leukocyte, creatinine and other indicators between the two groups (P>0.05).
 |
Table 2 Laboratory Indicators of Patients in Remission and Non-Remission Groups |
Multivariate Analysis of Therapeutic Response in Patients
Multivariate logistic regression analysis was performed using the efficacy outcome (remission /non-remission) as the dependent variable and the ANA titer, lgG level, lgA level, hemoglobin content, C3 level, and bone marrow megakaryocyte counts as the independent variables. The results showed that hemoglobin (OR=0.935, P=0.023), C3 level (OR=0.055, P=0.027), lgG level (OR=1.197, P=0.040), and bone marrow megakaryocyte counts (OR=0.723, P=0.001) were the independent influencing factors for no remission after treatments, as shown in Table 3.
 |
Table 3 Logistic Regression Analysis of Therapeutic Response in Patients with pSS and Thrombocytopenia |
ROC Curve of the Prediction Model
ROC curves were constructed for four variables in the prediction model (Figure 2). The results showed that the area under the curve (AUC) of bone marrow megakaryocyte counts was the highest at 0.759 (95% CI 0.672–0.833), followed by C3 with an AUC of 0.717 (95% CI 0.627–0.796). However, the AUCs for hemoglobin and IgG were relatively low at 0.703 (95% CI 0.612–0.783) and 0.646 (95% CI 0.553–0.731), respectively.
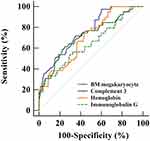 |
Figure 2 ROC curves of four variables for the prediction of the therapeutic response. |
Establishment of Nomogram Prediction Model
Based on the results of Logistic regression and ROC curve analysis, an individualized nomogram prediction model incorporating 4 independent factors was established for pSS patients with thrombocytopenia (Figure 3). In the nomogram, each factor corresponds to a score, and the sum of these factors was calculated as the total score. The predicted risk corresponding to the total score was the probability of no-remission after treatment in pSS patients with thrombocytopenia.
 |
Figure 3 Nomogram predicting no remission to treatment in pSS patients with secondary thrombocytopenia. |
Validation of Nomogram Prediction Model
Internal validation was performed using bootstrap methods with 1000 bootstrap repetitions. The ROC curve revealed that the nomogram model exhibited excellent predictive ability, with a C-index of 0.882 (95% CI 0.810–0.934), which was higher than that of the four indicators separately (Figure 4A). The calibration plot showed that the calibration curve was well-fitted with the standard curve, indicating the high accuracy of the model (Figure 4B). Moreover, the DCA curve also demonstrated that the nomogram model had favorable clinical utilization. For predicted risk thresholds less than 88.26%, the model offered a net benefit over the “admit all” or “admit none” strategy (Figure 4C).
Discussion
Thrombocytopenia, a prevalent hematological disorder, is often present in individuals afflicted with rheumatic autoimmune maladies, with its prevalence in pSS being second only to systemic lupus erythematosus. Corticosteroids or immunosuppressants are currently the main therapeutic options for pSS patients with secondary thrombocytopenia.16,17 Nevertheless, a subset of patients exhibits poor responses and resistance to diverse immunotherapies, thereby resulting in a worse prognosis.18 Early prediction of curative effects and adjusting treatment intensity through analyzing relevant indicators contribute significantly to improving the prognosis of patients. In this study, a nomogram model was constructed by integrating diverse clinical indicators for the prediction of non-remission risk in pSS patients with secondary thrombocytopenia. The established individualized nomogram showed excellent discrimination and calibration, providing the potential to identify high-risk patients and facilitate early intervention.
Most studies performed to date have focused on the clinical characteristics of pSS patients. Liu et al19 analyzed the clinical manifestations of autoimmune disease-associated thrombocytopenia in 2016 and concluded that the response to treatment should be closely monitored because non-remission of thrombocytopenia has a close association with mortality. Recently, Wu et al20 conducted a detailed examination of the clinical and laboratory features of pSS complicated with thrombocytopenia, emphasizing the correlation between mild to severe thrombocytopenia and serious clinical manifestations in pSS patients. They suggested that thrombocytopenia may be present at the onset of pSS development without any involvement of exocrine glands. Another retrospective study involving 639 pSS patients found that those with secondary immune thrombocytopenic purpura exhibited a poor prognosis, with disease activity linked directly to the severity of thrombocytopenia.19 Currently, only a few studies have analyzed the prognosis of hematological abnormalities in pSS patients.21,22 Furthermore, there are no studies that systematically evaluated the role of inflammatory factors, autoantibodies, and biopsy results in the therapeutic response of pSS patients with thrombocytopenia.
For patients with pSS complicated by mild to moderate thrombocytopenia, oral corticosteroids and immunosuppressants are effective. Those with severe thrombocytopenia have a high risk of bleeding and often require adjunctive intravenous gamma globulin pulse therapy.23 Nevertheless, reduction of hormone dosage frequently leads to a relapse of thrombocytopenia. Moreover, the use of common immunosuppressants such as cyclophosphamide is accompanied by pronounced bone marrow suppression effects, which pose significant challenges for clinical management.24,25 Recurrent application of such aggressive therapeutic interventions may increase the risk of multiple infections and cause significant economic losses.26 Consequently, the identification of predictive biomarkers for individual responses to immunotherapy is of great clinical importance. In the present investigation, the curative effect of pSS patients with was evaluated after 30 days of treatment. The observed overall response rate of 67.23% and non-remission rate of 32.77% were generally in agreement with the extant literature.27,28 Comparative analysis between non-remission and remission groups showed that ANA titers, IgG and IgA levels were significantly increased in non-remission patients, whereas hemoglobin concentration, C3 level, and bone marrow megakaryocyte counts were significantly decreased. These findings indicated a potential involvement of the above parameters in the immunotherapeutic management of thrombocytopenia. Antibody components traditionally used in the diagnosis and monitoring of pSS and other autoimmune diseases, such as RF, ANA, anti-La/SSB, and anti-Ro/SSA, had comparable positive rates to earlier reported data.29,30
The underlying mechanisms of thrombocytopenia in patients with pSS may be multifactorial, including increased immune-mediated platelet destruction as well as impaired production of megakaryocyte and platelet production. The main pathogenic feature of pSS is the exaggerated activation of B cells triggered by T cell-mediated immune responses.31 This B cell dysfunction causes the production of a series of autoantibodies, ultimately leading to platelet destruction.32 During this process, pSS patients exhibit excessive complement activation and depletion, resulting in a decline in the levels of C3 and C4.33 Approximately 10–15% of pSS patients were reported to have reduced C3 levels, and about 5–20% of patients had decreased C4 levels. In some cases, this may reflect a link between disease activity and complement consumption in immune complex formation.34 Several investigators have demonstrated that the prognostic value of C3 is superior to that of C4.35–37 A previous study from Solans-Laqué et al38 indicated that a reduced level of C3 in patients with pSS was a risk factor for the development of secondary hematologic malignancies. Similarly, Cheloff et al39 reported that a reduction in C3 levels was an independent predictor of unfavorable outcomes in individuals with immune-related thrombocytopenia. Moreover, due to the disruption of regular immune tolerance mechanisms, pSS is known to trigger the production of high-titer IgG antibodies directed at self-antigens. Elevated levels of IgG have been proven to be closely associated with lung lesions and skin purpura and could thus serve as a prognostic indicator for the disease.40,41 In our study, a decline in C3 levels and an increase in IgG levels were more prevalent in the non-remission group, indicating a heightened disease activity and significant inflammatory response in these patients.
As mentioned previously, a decrease in megakaryocytes is closely linked to increased platelet apoptosis, shortened lifespan, and reduced production. This can be attributed to the disturbance of the hematopoietic microenvironmental balance by autoantibodies, resulting in decreased production of bone marrow blood cells.42,43 A recent study reported that the importance of bone marrow aspiration in predicting treatment of thrombocytopenia associated with autoimmune disease.44 Khodadi et al45 illustrated that patients with immune thrombocytopenia have megakaryocytes that show signs of impaired maturation and degradation. There is a significant relationship between megakaryocyte maturation and platelet production. A study in 2016 by Zhao et al46 suggested that megakaryocyte count could be a predictor of response to immunotherapy for severe thrombocytopenia in SLE patients. Multivariate logistic regression analysis of this study revealed that bone marrow megakaryocyte counts was an independent factor affecting the non-remission of treatment. Consistent with previous reports, a decrement in megakaryocyte count in bone marrow was associated with poor response to treatment, further validating the underlying mechanism of pSS.44,45 It was also observed in a previous cohort that the levels of the inflammatory marker C3 correlate with hematological involvement in pSS.35 Importantly, Anemia is a common symptom of blood system involvement in pSS, and low hemoglobin was also associated with poor response to treatment in some studies.47 On the basis of the above studies, we found for the first time that hemoglobin, C3 level, IgG level and bone marrow megakaryocyte counts have the potential to predict the therapeutic response of thrombocytopenia in pSS patients. However, the ROC curves of this study showed limited independent predictive capability for hemoglobin, C3 levels, IgG levels, and bone marrow megakaryocyte counts. Therefore, we developed a multi-factorial nomogram model predicated on these indicators, and the results revealed that the combination of these indicators improved the overall discrimination of the model with a C-index of 0.882. Moreover, the calibration curve validated the high predictive accuracy of the nomogram model in predicting treatment responses, which underscored the utility of this model in directing therapeutic strategies for pSS patients with thrombocytopenia.
There were several limitations to this study. First, due to its retrospective design and relatively small sample size, there was a potential for misclassification or missing data, thus limiting the strength of our findings. Second, selection bias could not be rule out because only patients who underwent bone marrow punctures were included, which may affect the generalizability of our findings. Third, as the available studies were limited, the subgroup analysis according to the treatment regimens was not performed. Fourth, we did not conduct external verification due to the small amount of data. In the follow-up study, a prospective study with a large sample size will be performed to confirm the conclusion. Notably, the nomogram developed in this study is helpful to predict the treatment effect of pSS patients with thrombocytopenia, but further investigations are needed on whether this conclusion in other types of thrombocytopenia.
In conclusion, our study suggested that hemoglobin, C3, IgG levels, and bone marrow megakaryocyte counts were influencing factors of therapeutic response in pSS patients with thrombocytopenia. The nomogram established based on the above indicators exhibited a better predictive ability for the risk of non-remission in the current study, which provided a reference for the formulation and adjustment of individualized treatment paradigms, but larger sample studies are still needed for external validation.
Data Sharing Statement
The data during the current study are available from the corresponding author on reasonable request.
Ethical Approval
This study was approved by the Ethics Committee of Ningbo NO.2 Hospital.
Informed Consent
Informed consents were obtained from all patients.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
This research is supported by HwaMei Research Foundation of Ningbo NO.2 Hospital, Grant No.2020HMKY14 and Medical Scientific Research Foundation of Zhejiang Province, Grant No.2021KY1008.
Disclosure
The authors declare that they have no conflicts of interest in this work.
References
1. Bowman SJ, Fox R, Dörner T, et al. Safety and efficacy of subcutaneous ianalumab (VAY736) in patients with primary Sjögren’s syndrome: a randomised, double-blind, placebo-controlled, phase 2b dose-finding trial. Lancet. 2022;399(10320):161–171. doi:10.1016/s0140-6736(21)02251-0
2. Theander E, Mandl T. Primary Sjögren’s syndrome: diagnostic and prognostic value of salivary gland ultrasonography using a simplified scoring system. Arthritis Care Res. 2014;66(7):1102–1107. doi:10.1002/acr.22264
3. Manganelli P, Fietta P, Quaini F. Hematologic manifestations of primary Sjögren’s syndrome. Clin Exp Rheumatol. 2006;24(4):438–448.
4. Zhang S, Qu J, Wang L, et al. Activation of Toll-Like Receptor 7 Signaling Pathway in Primary Sjögren’s Syndrome-Associated Thrombocytopenia. Front Immunol. 2021;12:637659. doi:10.3389/fimmu.2021.637659
5. Dai F, Yang G, Rao P, et al. Clinical Characteristics of Secondary Immune Thrombocytopenia Associated With Primary Sjögren’s Syndrome. Front Med. 2020;7:138. doi:10.3389/fmed.2020.00138
6. Zhang W, Wang F, Wang H, Hua B, Feng X. Severe thrombocytopenia in connective tissue diseases: a single-center review of 131 cases. Clinical Rheumatology. 2018;37(12):3337–3344. doi:10.1007/s10067-018-4312-y
7. Luo J, Song WJ, Chen JQ, et al. Factors associated with secondary immune thrombocytopenia in patients with primary Sjögren’s syndrome: a retrospective study of 639 cases. Clin Exp Rheumatol. 2022;40(12):2245–2252. doi:10.55563/clinexprheumatol/8hgmjm
8. Li X, Xu B, Ma Y, et al. Clinical and laboratory profiles of primary Sjogren’s syndrome in a Chinese population: a retrospective analysis of 315 patients. Int J Rheum Dis. 2015;18(4):439–446. doi:10.1111/1756-185x.12583
9. Xu L, Zhang Y, Lin N. Eltrombopag improves refractory thrombocytopenia in patients with Sjögren’s syndrome. Science Progress. 2022;105(2):368504221102786. doi:10.1177/00368504221102786
10. Nakamura N, Tsunemine H, Sakai T, Arima N. Biomarkers for predicting response to corticosteroid therapy for immune thrombocytopenic purpura. Br J Haematol. 2023;201(4):774–782. doi:10.1111/bjh.18670
11. Huang X, Wang X, Yu D. Development and validation of a nomogram for renal involvement in primary Sjögren syndrome patients: a retrospective analysis. Modern Rheumatology. 2023;33(1):169–174. doi:10.1093/mr/roab123
12. Ye W, Chen S, Huang X, et al. Clinical features and risk factors of neurological involvement in Sjögren’s syndrome. BMC Neurosci. 2018;19(1):26. doi:10.1186/s12868-018-0427-y
13. Xu X, Shi Y, Yang X, Zhang Y, Qin L, Cai L. Risk factors for hypertension in primary Sjögren’s syndrome patients: a nomogram was constructed. Journal of Human Hypertension. 2022;36(11):996–1002. doi:10.1038/s41371-021-00603-7
14. Shiboski CH, Shiboski SC, Seror R, et al. 2016 American College of Rheumatology/European League Against Rheumatism classification criteria for primary Sjögren’s syndrome: a consensus and data-driven methodology involving three international patient cohorts. Ann Rheum Dis. 2017;76(1):9–16. doi:10.1136/annrheumdis-2016-210571
15. Jiang B, Li T, Guo L, Shen H, Ye S, Chen S. Efficacy and Safety of Rituximab in Systemic Lupus Erythematosus and Sjögren Syndrome Patients With Refractory Thrombocytopenia: a Retrospective Study of 21 Cases. J Clin Rheumatol. 2015;21(5):244–250. doi:10.1097/rhu.0000000000000273
16. Baimpa E, Dahabreh IJ, Voulgarelis M, Moutsopoulos HM. Hematologic manifestations and predictors of lymphoma development in primary Sjögren syndrome: clinical and pathophysiologic aspects. Medicine. 2009;88(5):284–293. doi:10.1097/MD.0b013e3181b76ab5
17. Sellam J, Proulle V, Jüngel A, et al. Increased levels of circulating microparticles in primary Sjögren’s syndrome, systemic lupus erythematosus and rheumatoid arthritis and relation with disease activity. Arthritis Res Ther. 2009;11(5):R156. doi:10.1186/ar2833
18. Koh JH, Lee J, Chung SH, Kwok SK, Park SH. Relation of Autoimmune Cytopenia to Glandular and Systemic Manifestations in Primary Sjögren Syndrome: analysis of 113 Korean Patients. J Rheumatol. 2015;42(10):1817–1824. doi:10.3899/jrheum.150058
19. Liu Y, Chen S, Sun Y, et al. Clinical characteristics of immune thrombocytopenia associated with autoimmune disease: a retrospective study. Medicine. 2016;95(50):e5565. doi:10.1097/md.0000000000005565
20. Wu J, Chang X, Zhang J, Liu C, Liu M, Chen W. Clinical and laboratory features of primary Sjögren’s syndrome complicated with mild to severe thrombocytopenia. Ann Transl Med. 2022;10(6):300. doi:10.21037/atm-22-162
21. Yıldız F, Gökmen O. Haematologic indices and disease activity index in primary Sjogren’s syndrome. International Journal of Clinical Practice. 2021;75(3):e13992. doi:10.1111/ijcp.13992
22. Zhao X, Gan Y, Jin Y, et al. Interleukin 17E associates with haematologic involvement and autoantibody production in primary Sjögren’s syndrome. Clin Exp Rheumatol. 2021;39(2):378–384. doi:10.55563/clinexprheumatol/gbjatf
23. Choung BS, Yoo WH. Successful treatment with intravenous immunoglobulin of severe thrombocytopenia complicated in primary Sjögren’s syndrome. Rheumatol Int. 2012;32(5):1353–1355. doi:10.1007/s00296-010-1395-4
24. Klepfish A, Friedman J, Schechter Y, Schattner A. Autoimmune neutropenia, thrombocytopenia and Coombs positivity in a patient with primary Sjögren’s syndrome. Rheumatology. 2001;40(8):948–949. doi:10.1093/rheumatology/40.8.948
25. Liu Y, Chen S, Yang G, et al. ANA-positive primary immune thrombocytopaenia: a different clinical entity with increased risk of connective tissue diseases. J Med. 2021;8(1). doi:10.1136/lupus-2021-000523
26. El Hasbani G, Chahine R, Uthman I, Taher AT. Intravenous immunoglobulin, neonatal alloimmune thrombocytopenia, and Sjogren’s syndrome: a case report. Eur J Obstet Gynecol Reprod Biol. 2021;258:476–477. doi:10.1016/j.ejogrb.2021.01.053
27. Harris EN, Asherson RA, Gharavi AE, Morgan SH, Derue G, Hughes GR. Thrombocytopenia in SLE and related autoimmune disorders: association with anticardiolipin antibody. Br J Haematol. 1985;59(2):227–230. doi:10.1111/j.1365-2141.1985.tb02988.x
28. Kamimura T, Sato H, Iwamoto M, et al. Sjögren’s syndrome associated with chronic hepatitis C, severe thrombocytopenia, hypertrophic cardiomyopathy, and diabetes mellitus. Intern Med. 2005;44(6):657–661. doi:10.2169/internalmedicine.44.657
29. Stefanski AL, Tomiak C, Pleyer U, Dietrich T, Burmester GR, Dörner T. The Diagnosis and Treatment of Sjögren’s Syndrome. Dtsch Arztebl Int. 2017;114(20):354–361. doi:10.3238/arztebl.2017.0354
30. Negrini S, Emmi G, Greco M, et al. Sjögren’s syndrome: a systemic autoimmune disease. Clin Exp Med. 2022;22(1):9–25. doi:10.1007/s10238-021-00728-6
31. Lu C, Pi X, Xu W, et al. Clinical significance of T cell receptor repertoire in primary Sjogren’s syndrome. EBioMedicine. 2022;84:104252. doi:10.1016/j.ebiom.2022.104252
32. Levesque MC. Translational Mini-Review Series on B Cell-Directed Therapies: recent advances in B cell-directed biological therapies for autoimmune disorders. Clin Exp Immunol. 2009;157(2):198–208. doi:10.1111/j.1365-2249.2009.03979.x
33. Zhou M, Yuan F. Hypocomplementemia in Primary Sjogren’s Syndrome: a Retrospective Study of 120 Treatment-Naive Chinese Patients. Int J Gen Med. 2022;15:359–366. doi:10.2147/ijgm.s346188
34. Lin W, Xin Z, Wang J, et al. Hypocomplementemia in primary Sjogren’s syndrome: association with serological, clinical features, and outcome. Clinical Rheumatology. 2022;41(7):2091–2102. doi:10.1007/s10067-022-06135-w
35. Jordán-González P, Gago-Piñero R. Characterization of a subset of patients with primary Sjögren’s syndrome initially presenting with C3 or C4 hypocomplementemia. Clin Med J. 2020;7(3):112–117. doi:10.5152/eurjrheum.2020.19132
36. Campar A, Isenberg DA. Primary Sjögren’s syndrome activity and damage indices comparison. Eur J Clin Invest. 2010;40(7):636–644. doi:10.1111/j.1365-2362.2010.02303.x
37. Lundtoft C, Sjöwall C, Rantapää-Dahlqvist S, et al. Strong Association of Combined Genetic Deficiencies in the Classical Complement Pathway With Risk of Systemic Lupus Erythematosus and Primary Sjögren’s Syndrome. Arthritis Rheumatol. 2022;74(11):1842–1850. doi:10.1002/art.42270
38. Solans-Laqué R, López-Hernandez A, Bosch-Gil JA, Palacios A, Campillo M, Vilardell-Tarres M. Risk, predictors, and clinical characteristics of lymphoma development in primary Sjögren’s syndrome. Semin Arthritis Rheum. 2011;41(3):415–423. doi:10.1016/j.semarthrit.2011.04.006
39. Cheloff AZ, Kuter DJ, Al-Samkari H. Serum complement levels in immune thrombocytopenia: characterization and relation to clinical features. Research and Practice in Thrombosis and Haemostasis. 2020;4(5):807–812. doi:10.1002/rth2.12388
40. Maślińska M, Wojciechowska B, Mańczak M. Serum immunoglobulin G4 in Sjögren’s syndrome: a pilot study. Rheumatology International. 2020;40(4):555–561. doi:10.1007/s00296-020-04529-0
41. Pu J, Wang X, Riaz F, et al. Effectiveness and Safety of Iguratimod in Treating Primary Sjögren’s Syndrome: a Systematic Review and Meta-Analysis. Front Pharmacol. 2021;12:621208. doi:10.3389/fphar.2021.621208
42. Wang Y, Xie X, Zhang C, et al. Rheumatoid arthritis, systemic lupus erythematosus and primary Sjögren’s syndrome shared megakaryocyte expansion in peripheral blood. Annals of the Rheumatic Diseases. 2022;81(3):379–385. doi:10.1136/annrheumdis-2021-220066
43. Szyszko EA, Aqrawi LA, Jonsson R, Brokstad KA, Skarstein K. Non-proliferating plasma cells detected in the salivary glands and bone marrow of autoimmune NOD.B10.H2b mice, a model for primary Sjögren’s syndrome. Autoimmunity. 2016;49(1):41–49. doi:10.3109/08916934.2015.1079820
44. Tripathi AK, Mishra S, Kumar A, Yadav D, Shukla A, Yadav Y. Megakaryocyte morphology and its impact in predicting response to steroid in immune thrombocytopenia. Platelets. 2014;25(7):526–531. doi:10.3109/09537104.2013.845875
45. Khodadi E, Asnafi AA, Shahrabi S, Shahjahani M, Saki N. Bone marrow niche in immune thrombocytopenia: a focus on megakaryopoiesis. Ann Hematol. 2016;95(11):1765–1776. doi:10.1007/s00277-016-2703-1
46. Zhao L, Xu D, Qiao L, Zhang X. Bone Marrow Megakaryocytes May Predict Therapeutic Response of Severe Thrombocytopenia in Patients with Systemic Lupus Erythematosus. J Rheumatol. 2016;43(6):1038–1044. doi:10.3899/jrheum.150829
47. Szanto A, Szodoray P, Kiss E, Kapitany A, Szegedi G, Zeher M. Clinical, serologic, and genetic profiles of patients with associated Sjögren’s syndrome and systemic lupus erythematosus. Hum Immunol. 2006;67(11):924–930. doi:10.1016/j.humimm.2006.06.006
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

