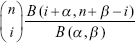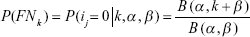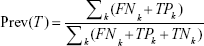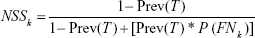Back to Journals » OncoTargets and Therapy » Volume 12
Nodal staging score and adequacy of nodal staging
Received 6 September 2018
Accepted for publication 14 November 2018
Published 8 January 2019 Volume 2019:12 Pages 449—455
DOI https://doi.org/10.2147/OTT.S186642
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Leo Jen-Liang Su
Hui-Min Chen, Ge Feng
Nanjing Jiangbei People’s Hospital, Nanjing 220000, People’s Republic of China
Aims: The number of lymph nodes (LNs) excised in patients with pathologic N0 is limited, and it is very likely that there will be recessive node disease after surgery, so they are at risk of understaging. The purpose of the present study is to develop a nodal staging score (NSS) in a mathematical way to assess the likelihood that a pathologic N0 gastric cancer (GCa) patient has, indeed, no occult nodal disease after surgery.
Patients and methods: A total of 14,033 stage I–III GCa patients were identified from Surveillance, Epidemiology and End Results database for analysis. A beta-binomial model was fitted to calculate the probability of missing a nodal disease. This probability is then used to calculate the NSS.
Results: The probability of missing a nodal disease is decreased with increasing LNs examined across all pT stages. Seven and 24 LNs removed and examined was enough for an NSS of 90% in pT1 and pT2 patients, respectively, ensuring a high confidence of correct nodal negative classification. Twenty-three and 31 LNs examined in pT3 and pT4 patients could also maintain the NSS at 80%, respectively. NSS had a significant impact on patients’ survival across all pT stages (all Ps <0.0001).
Conclusion: The probability that GCa patients are free of true nodal disease could be provided by NSS-based prediction, which is conducive to postoperative decision and survival surveillance. In addition, NSS can define a subtle standard on how many LNs examined are enough for adequate staging dependent on pT stages. However, at least 16 LNs examined is the standard recommendation to date.
Keywords: gastric cancer, nodal-negative classification, adequate staging, prognosis
Introduction
Pathologic lymph node (LN) status is critical for resectable gastric cancer (GCa) patients, because LN metastasis is a strong risk factor for disease recurrence. In recent years, platinum and fluorouracil-based adjuvant chemotherapies, which have largely improved patients’ survival, are recommended as a standard therapy for patients with resectable GCa.1–3 Considering the achievement for adjuvant chemotherapies in GCa, an accurate nodal staging system is extremely important to avoid inadequate care. However, according to the 7th Edition American Joint Committee on Cancer (AJCC) staging guideline, all pathologic nodal-negative patients are staged as pN0, even a small number of LNs are examined. Up to now, it is lack of efficient tool to assess the adequacy of nodal-negative classification for these patients staged as pN0.
There is no consensus on how many LNs should be swept for an accurate staging. Based on previous randomized trials, it is recommended that standardized extended (D2) lymphadenectomy with at least 16 LNs to be removed and examined could lead to adequate staging and better clinical outcomes.4–6 However, this cutoff-based recommendation is a one-size-fit-all approach and need to be carefully used in clinical practice. Moreover, previous studies have had implications on the understaging for pN0 GCa patients that lead to LN recurrence.7,8 Therefore, in regard to postoperative clinical decision, it is highly necessary to develop a tool to take the probability of false-negative findings of LN status into account to predict the true nodal status for pN0 patients.
The nodal staging score (NSS) is the probability that a pN0 patient is indeed free of nodal disease, in other words, the probability that a pN0 patient has no occult disease after surgery.9,10 Therefore, on the other hand, 1-NSS is an estimate of the risk of harboring occult positive LNs for a pN0 patient. In this study, with a relatively large sample size and multiple registry-derived patients from the Surveillance, Epidemiology and End Results (SEER) database, we first present a case in which NSS as a function of the number of LNs examined and patients’ pT stage can be applied to predict the true nodal status for pN0 GCa patients.
Materials and methods
Ethics statement
The data from the SEER database which was designed and maintained by the National Cancer Institute. Research was limited to secondary use of information previously collected in the course of normal care and data were anonymized before conducting the statistical analyses. This article does not contain any studies with human participants or animals performed by any of the authors.
Study population
The SEER database publishes the population-based incidence and survival data of cancer patients, covering about 30% of cancer registries from the USA. Clinical information provided by the SEER database largely facilitates clinical cancer research. According to the 7th Edition AJCC staging guideline, GCa patients with primary stage I–III resectable tumors from SEER database were included. If tumors were staged by the AJCC 6th staging system as provided by the SEER database, two dependent authors would re-stage them based on the 7th Edition AJCC staging guideline. For the current analysis, lymphoepithelial carcinoma (8,082/3), gastrointestinal stromal tumors (8,936/3), pseudosarcomatous cell carcinoma (8,033/3), and neuroendocrine tumors (8,013/3, 8,244/3, and 8,246/3) were excluded.
Statistical analysis
Step 1: Data simulation of missing a LN during surgery
To precisely assess the probability of false-negative findings – all LNs examined were negative for a factually nodal-positive patient – by a beta-binomial model, we performed a data simulation of missing an LN that should have been detected using a part of nodal-positive patients randomly selected (eg, if a patient had n LNs examined, we performed a simulation of one LN being missed, and therefore only n -1 LNs were examined). First, we generated a randomized dataset to be used for simulation for every patient, by including the top 20% and 40% of patients with pT1–2 and pT3–4, respectively. Then, assuming that n was the number of total LNs examined and i was the number of positive LNs for a patient, positive LNs for a patient in the simulation dataset were numbered 1 to i, and negative LNs were numbered i+1 to n. Finally, we generated a randomized positive integer termed rx, which was distributed uniformly, from 1 to n. Therefore, if rx ≤i, the corresponding patient was simulated by missing a positive LNs detected. Otherwise if rx >i, the corresponding patient was simulated by missing a negative LNs detected. In combination with the simulated dataset, the data for the rest of nodal-positive patients were directly fitted in a beta-binomial model without further simulation.
Step 2: Fitting a beta-binomial model to calculate the probability of false-negative findings of nodal status due to missing a nodal disease
Relevant algorithm has been reported by previous studies,9–12 in which a beta-binomial model was used as follows:
|
|
In fitting this model, n was the number of total LNs examined, and i was the number of positive LNs for a patient. Moreover, n and i were both mutually independent among patients. Then, k = n − i was the number of negative LNs for the patient. Assuming that this model was fitted by a given data including N patients, for jth patient they are respectively expressed as nj, ij, and kj and which should satisfy the condition 1 ≤ kj < nj, j =1, 2 … N.
The two parameters, a and b, in the above model were estimated by using the maximum likelihood method, and then based on the above fit, the probability of all LNs examined was negative (n = k, i = 0) for a factually nodal-positive patient due to missing a nodal disease can be obtained as follows:
|
|
In use of this model, we assumed that n = k, where, B(·) was the function of beta distribution. This model should be used under two assumptions: the first was the false-negative assumption in nodal-positive patients, the positive nodes might be missed due to a limited LNs resected during surgery. The second assumption was that there was no false-positive nodal staging, because we acquiesced that there was no pathologic technology matter in discriminating a nodal disease.
Step 3: Prevalence of nodal disease adjusted by false-negative probability
We adjusted the apparent prevalence of nodal disease, which was an underestimate, based on the false-negative probability as calculated above. Because the pT stage was an important clinical factor in the AJCC-based staging guideline, it was also taken into consideration as a stratified factor for computing the adjusted prevalence. Two steps were needed to calculate it.
|
|
where TPk and FNk were the number of true nodal-positive patients and false nodal-negative patients for a given k, which was the total number of LNs resected.
|
|
Prev(T) was the adjusted prevalence of nodal disease stratified by the pT stage, and TNk was the number of true nodal-negative patients. Similarly, the adjusted prevalence was also calculated under the assumption that there were no false-positive findings of nodal staging.
Step 4: Computing the NSS
|
|
NSS, derived from the above formula, had two meanings. The first was at population-based level: the proportion of true nodal-negative patients in the population of pN0 patients; and the second was at individual-based level: a novel estimate for the level of confidence in the judgment of true nodal-negative for a pN0 patient. On the other hand, 1 − NSS was the extent of underestimate that a nodal-positive patient was staged as pN0. The individual-based level of meaning had a more prominent clinical significance and was the focus of the present study.
All statistical analyses were performed by using SAS (version 9.4, University of North Carolina, Cary, NC, USA) and R (version 3.2.3, R Foundation, Vienna, Austria).
Results
Patients
Characteristics of the patients are presented in Table 1. A total of 14,033 stage I–III GCa patients were included in the analysis. Among these patients, 8,351 (59.51%) patients were nodal positive (pN+) and the remaining were those who have no positive LNs detected and were staged as pN0. Obviously, based on the worldwide surgery experience from SEER database, LNs removed in pT3–4 patients were significantly more than in pT1–2 patients (P<0.001).
  | Table 1 Characteristics of patients included in this study |
The probability of false-negative findings of nodal staging due to missing a nodal disease
The α and β in the fitted beta-binomial model across different pT stages are listed in Table 2. As expected, the probability of false-negative findings of LN status decreased as the increase of LNs removed across all pT stages, due to missing a nodal disease. Interestingly, this false-negative probability varied among patients with different stage. Patients with late pT stage might suffer from lower risk of false-negative findings as compared with those with early stage (Figure 1A). This result was in accordance with clinical actuality. The apparent prevalence of nodal disease in pT3–4 patients was indeed much higher than in pT1–2 patients (Table 3), so the probability of false-negative findings of LN status naturally decreased in patients with pT3–4 stage in comparison with those with pT1–2 stage.
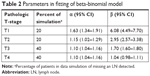  | Table 2 Parameters in fitting of beta-binomial model |
  | Table 3 Apparent and adjusted prevalence of nodal disease in GCa with different pathologic T-stage |
Prevalence of nodal disease adjusted by false-negative findings
Importantly, the prevalence of nodal disease for GCa patients, adjusted by the false-negative findings, was higher than that of apparent nodal disease. It is independent of the pT stage, indicating that a proportion of patients (adjusted minus apparent prevalence of nodal disease: 5.88%, 11.12%, 9.37%, and 7.84% for pT1–4 stages, respectively) were understaged (Table 3). According to the adjusted results, GCa patients would have a high risk of LN metastasis, especially for pT3 and pT4 patients (81.58% and 91.02% for pT3 and pT4, respectively).
Nodal staging score
For pT1 patients, NSS was 79.43%, if only a single LN was examined, which increased to 82.61% and 91.06%, if two and seven LNs were examined, respectively. For pT2 patients, however, 10 and 24 LNs needed to be examined to maintain NSS at the level of 80% and 90%, respectively. For pT3 and T4 patients, NSS could only be maintained to the levels of 80%, if 23 and 31 LNs were examined, respectively (Figure 1B). In addition, a high NSS was a strong indicator for the prolonged survival in the pN0 patients across all pT stages (P<0.0001 for all pT stages, Figure 2), indicating that the adequacy of nodal staging predicted by NSS was associated with patients’ survival and can be served as a tool to guide postoperative decision.
Discussion
To the best of our knowledge, three nodal staging systems for postoperative decision-making have been proposed to date: the pathologic AJCC-based nodal staging system,13 the log odds of positive LNs,14 and the LNs ratio,15,16 all of which have been demonstrated to be critical indicators for prognosis of GCa patients. Moreover, a series of studies have made great efforts to select a nodal staging system with the best performance in prognostic prediction.17–19 Because pN0 patients always have zero-positive LN detected by pathologists, no matter how many LNs are examined, the shared disadvantage of these three nodal staging systems is that they are definitely unresponsive to pN0 patients, and, therefore, are factually one-size-fit-all nodal staging approaches. Therefore, the major advantage of NSS, compared with the previous nodal staging systems, is that it is responsive to pN0 patients by quantifying the appropriateness of a nodal-negative diagnosis. Therefore, for pN0 patients, NSS is the optimal selection for postoperative LN status evaluation compared with previous staging systems.
Nevertheless, the threshold of LNs that should be removed for GCa patients is still controversial. According to previous studies based on the SEER database,20 and a subsequent systematic review,21 the total number of LNs resected and examined was a stage-based survival predictor. Although at least 16 LNs examined is the standard recommendation, some studies suggested that more LNs should be examined,22 especially for advanced GCa patients.20,21 The remarkable finding in the present study is that NSS increased as the number of examined LNs increased and strongly depended on pT stage. In the modeling, we observed that removing only 7 LNs was enough for an NSS of 90%, which certainly defines that a pT1 patient can be correctly staged as nodal-negative, and for pT2 patients, removing 24 LNs during surgery was enough to reach the same level of NSS. For pT3 and pT4 patients, removing 23 and 31 LNs could also hold the NSS up to 80%, ensuring a high confidence of a correct nodal-negative staging. Of note, patients with pT3–4 stage had a lower NSS as compared with those with pT1–2 stage, indicating a relatively high risk (estimated as 1-NSS) of carrying occult disease in pT3–4 patients if a limited number of LNs were examined. Therefore, our results provide a refined threshold for the number of LNs to be removed for pathologists to determine a true negative diagnosis. Moreover, because patients with late pT stage have a lower NSS as compared with those with early stage as indicated by our results, we suggest that a substantial proportion of pT3N0 and pT4N0 patients should receive effective adjuvant therapies to prevent LN recurrence based on NSS. It will be of great interest to observe the results of future clinical trials focusing on novel adjuvant therapeutic strategy for pT3N0 and pT4N0 patients based on NSS.
Because of the lack of effective methods to clarify the true LN status in pN0 patients, unlike the pN+ patients, the pT stage was the only way for us to determine the state of disease progression according to the AJCC-based cancer staging system. Importantly, our findings demonstrated that NSS could also help to predict prognosis. Taken together, we have confirmed that the pN0 subpopulation of GCa patients may benefit from NSS in addition to the traditional AJCC-based staging guideline, in terms of either postoperative clinical decision-making or recurrence prevention.
We calculated NSS under the beta-binomial model,23 a model that gives the consideration to both binomial distribution and heterogeneity distribution of the P(FN) among patients. Moreover, to avoid extrapolation to a largest extent, we make a data simulation in the condition of missing an LN, which was randomly assigned and should have been resected during surgery. Therefore, constructing a beta-binomial model using the simulated as a part of dataset could provide a more accurate fitting closer to the real situation where an LN was missed, compared to the previously reported algorism in which data were directly fitted in the model without a simulation.9–12 This model was also under an important assumption that there was no false-positive nodal staging in clinical practice. We regard this assumption as reasonable, because pathologists must have substantial expertise and follow pathological guidelines to discriminate a nodal-positive patient. Moreover, a group judgment, instead of single-minded decision, is needed to assert a nodal disease. Therefore, it is statistically and clinically reasonable to compute NSS using false-negative probability derived from beta-binomial model under such an assumption.
Besides, the extranodal extension of nodal metastasis is one of the most important prognostic indicators in GCa. Up to date, a few studies24,25 have reported on extracapsular LN involvement in relation to GCa, it demonstrates that extracapsular LN involvement is associated with higher tumor stages and is an independent negative prognostic factor. In future staging systems, not only the number of positive LNs, but also the presence of extracapsular LN involvement in GCa should be considered.
The major limitation of the current study is its retrospective design in nature. The missing therapeutic information from the SEER database is another limitation. Multicenter prospective studies with detailed clinical information are needed to validate our findings.
In summary, NSS as a function of number of LNs removed and pT stage can provide quantified information on adequacy of nodal-negative classification, which is helpful for postoperative decision and patients’ consultation on the risk of carrying occult disease. Using this quantified information, we can refine the optimal number of LNs examined for accurate staging. A relatively low level of NSS for pT3N0 and pT4N0 patients points out an unaware problem. Therefore, it is an urgent necessity for clinicians to take an improvement adjuvant therapeutic strategy in comparison with the current standard agents, to prevent the LN recurrence for pT3N0 and pT4N0 patients as illustrated by the NSS-based prediction.
Acknowledgment
This paper does not contain any studies with human participants or animals performed by any of the authors. This study was funded by National Natural Science Foundation of China (Nos. 81802347).
Disclosure
The authors report no conflicts of interest in this work.
References
Sakuramoto S, Sasako M, Yamaguchi T, et al. Adjuvant chemotherapy for gastric cancer with S-1, an oral fluoropyrimidine. N Engl J Med. 2007;357(18):1810–1820. | ||
Bang YJ, Kim YW, Yang HK, et al; CLASSIC trial investigators. Adjuvant capecitabine and oxaliplatin for gastric cancer after D2 gastrectomy (CLASSIC): a phase 3 open-label, randomised controlled trial. Lancet. 2012;379(9813):315–321. | ||
Sasako M, Sakuramoto S, Katai H, et al. Five-year outcomes of a randomized phase III trial comparing adjuvant chemotherapy with S-1 versus surgery alone in stage II or III gastric cancer. J Clin Oncol. 2011;29(33):4387–4393. | ||
Hundahl SA, Phillips JL, Menck HR. The National Cancer Data Base Report on poor survival of U.S. gastric carcinoma patients treated with gastrectomy: Fifth Edition American Joint Committee on Cancer staging, proximal disease, and the “different disease” hypothesis. Cancer. 2000;88(4):921–932. | ||
Smith DD, Schwarz RR, Schwarz RE. Impact of total lymph node count on staging and survival after gastrectomy for gastric cancer: data from a large US-population database. J Clin Oncol. 2005;23(28):7114–7124. | ||
de Steur WO, Hartgrink HH, Dikken JL, Putter H, van de Velde CJ. Quality control of lymph node dissection in the Dutch Gastric Cancer Trial. Br J Surg. 2015;102(11):1388–1393. | ||
Cao L, Selby LV, Hu X, et al. Risk factors for recurrence in T1-2N0 gastric cancer in the United States and China. J Surg Oncol. 2016;113(7):745–749. | ||
Zheng WF, Ji TT, Lin Y, Li RZ. The prognostic value of lymph nodes count on survival of patients with node-negative gastric cancer. Oncotarget. 2016;7(28):43680–43688. | ||
Robinson TJ, Thomas S, Dinan MA, Roman S, Sosa JA, Hyslop T. How many lymph nodes are enough? Assessing the adequacy of lymph node yield for papillary thyroid cancer. J Clin Oncol. 2016;34(28):3434–3439. | ||
Kluth LA, Abdollah F, Xylinas E, et al. Pathologic nodal staging scores in patients treated with radical prostatectomy: a postoperative decision tool. Eur Urol. 2014;66(3):439–446. | ||
Shariat SF, Rink M, Ehdaie B, et al. Pathologic nodal staging score for bladder cancer: a decision tool for adjuvant therapy after radical cystectomy. Eur Urol. 2013;63(2):371–378. | ||
Gönen M, Schrag D, Weiser MR. Nodal staging score: a tool to assess adequate staging of node-negative colon cancer. J Clin Oncol. 2009;27(36):6166–6171. | ||
Washington K. 7th Edition of the AJCC cancer staging manual: stomach. Ann Surg Oncol. 2010;17(12):3077–3079. | ||
Sun Z, Xu Y, Li de M, et al. Log odds of positive lymph nodes: a novel prognostic indicator superior to the number-based and the ratio-based N category for gastric cancer patients with R0 resection. Cancer. 2010;116(11):2571–2580. | ||
Kutlu OC, Watchell M, Dissanaike S. Metastatic lymph node ratio successfully predicts prognosis in western gastric cancer patients. Surg Oncol. 2015;24(2):84–88. | ||
Melis M, Masi A, Pinna A, et al. Does lymph node ratio affect prognosis in gastroesophageal cancer? Am J Surg. 2015;210(3):443–450. | ||
Spolverato G, Ejaz A, Kim Y, et al. Prognostic performance of different lymph node staging systems after curative intent resection for gastric adenocarcinoma. Ann Surg. 2015;262(6):991–998. | ||
Jian-Hui C, Shi-Rong C, Hui W, et al. Prognostic value of three different lymph node staging systems in the survival of patients with gastric cancer following D2 lymphadenectomy. Tumour Biol. 2016;37(8):11105–11113. | ||
Lee JW, Ali B, Park CH, Song KY. Different lymph node staging systems in patients with gastric cancer from Korean: what is the best prognostic assessment tool? Medicine (Baltimore). 2016;95(25):e3860. | ||
Schwarz RE, Smith DD. Clinical impact of lymphadenectomy extent in resectable gastric cancer of advanced stage. Ann Surg Oncol. 2007;14(2):317–328. | ||
Seevaratnam R, Bocicariu A, Cardoso R, et al. How many lymph nodes should be assessed in patients with gastric cancer? A systematic review. Gastric Cancer. 2012;15(Suppl 1):70–88. | ||
Huang C. How many lymph nodes should be removed to define an optimal D2 lymphadenectomy for gastric cancer in the modern era? An analysis of 2,947 patients from a two-institution database in China. J Clin Oncol. 2016;34(Suppl 15):4051. | ||
Magnussen S, Kremer A. The beta-binomial model for estimating heritabilities of binary traits. Theor Appl Genet. 1995;91(3):544–552. | ||
Veronese N, Fassan M, Wood LD, et al. Extranodal extension of nodal metastases is a poor prognostic indicator in gastric cancer: a systematic review and meta-analysis. J Gastrointest Surg. 2016;20(10):1692–1698. | ||
Alakus H, Hölscher AH, Grass G, et al. Extracapsular lymph node spread: a new prognostic factor in gastric cancer. Cancer. 2010;116(2):309–315. |
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.