Back to Journals » Journal of Pain Research » Volume 15
Nociceptors: Their Role in Body’s Defenses, Tissue Specific Variations and Anatomical Update
Authors Nikolenko VN, Shelomentseva EM , Tsvetkova MM, Abdeeva EI, Giller DB , Babayeva JV, Achkasov EE, Gavryushova LV, Sinelnikov MY
Received 19 November 2021
Accepted for publication 12 March 2022
Published 1 April 2022 Volume 2022:15 Pages 867—877
DOI https://doi.org/10.2147/JPR.S348324
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Qi Fang
Vladimir N Nikolenko,1,2 Ekaterina M Shelomentseva,2 Maria M Tsvetkova,2 Elina I Abdeeva,1 Dmitriy B Giller,1 Juliya V Babayeva,1 Evgeny E Achkasov,1 Liliya V Gavryushova,3 Mikhail Y Sinelnikov1,4
1First Moscow State Medical University Named After I.M. Sechenov (Sechenov University), Moscow, 119991, Russia; 2Lomonosov Moscow State University, Moscow, 119991, Russia; 3Saratov State Medical University, Saratov, Russian Federation; 4Research Institute of Human Morphology, Moscow, 119901, Russian Federation
Correspondence: Mikhail Y Sinelnikov, Sechenov University, Trubetskaya 8, Moscow, 119991, Russian Federation, Tel/Fax +7 89199688587, Email [email protected]
Abstract: The human body is constantly under the influence of numerous pathological factors: both external and internal. These factors can be potentially harmful and are perceived as such with a specialized nervous system subunit: the nociceptive system. The functional unit of the nociceptive system is the nociceptor. Recent studies have shown that nociceptors play a crucial role in maintaining of defensive homeostasis (responsive, immune, behavioral). Nociceptors respond to potentially harmful stimuli within viscera, bones, muscles, skin and specialized sensory organs. They function as complex predictors of harm through formation of pain stimulus. Their function and structures vary within different tissues. This variability reflects the anatomical and pathological peculiarities of varying tissues. Nociceptors play a significant role in adaptive, protective and behavioral reactions. Their functional capabilities and vast spread throughout the body make them the main units of the body’s defense system, allowing us to interact with the inner and outer environments.
Keywords: nociceptors, defensive homeostasis, tissue specific variability, pain perception
Synopsis
Current literature data shows a high variety of nociceptors and their important functions throughout different tissues. Nociceptors play an important role in defensive homeostasis, interaction with the environment and many pathological processes.
Introduction
Pain plays an important role in the body’s defense system. It provides the body with the ability to respond to potentially harmful signals and is crucial for survival and interaction with the surrounding environment.1 Nociceptive neurons are one of the key components of the somatosensory system, making it possible to perceive mechanical, chemical and thermal exteroceptive stimuli. Their function is to provide nociception: the phenomenon of nerve fiber activation in response to outer stimuli. Normally, they react to potentially harmful stimuli, however certain pathologies of the nociceptive system can create pathological pain without significant stimuli.2
Nociceptors are involved in a variety of important processes within our body. They are involved in perception of unpleasant and often pathological sensations, which are the triggers for sufficient response, both voluntary and involuntary. Pain is a complex sensation which can be associated with both high threshold stimuli or pathological stimuli. Nociceptors and specifically designated nervous tissue receptors, which subserve exteroception of noxious and pathological stimuli. In order to further understand their importance, we highlight the current understanding of anatomical, histological, clinical and pathological role and function of nociceptors.2–5
In this short essay, we revisit the nature and tissue specific characteristics of nociceptive receptors and signals in order to provide a better understanding of the pain perception mechanism and their distortion in such pathological conditions as neuropathic disorders, hypersensitivity, and chronic pain syndrome.
The Nociceptive System
Nociceptors are primary afferent pseudounipolar neurons specifically tuned to perceive stimuli arising either from actual or potential tissue damage. These neurons belong to the group of high-threshold mechanoreceptors.6 Two types of nociceptive fibers are recognized depending on their diameter and axonal conduction velocity. Type-Aδ fiber (myelinated fibers of 2–5μm in diameter with conduction velocity of up to 30m/s) nociceptors participate in the transmission of intense short-term mechanical stimuli, known as “primary pain”.7 Nociceptors with type-Aδ fibers are further subdivided based on the type of signal they perceive. Type I Aδ fibers have a high temperature threshold (>50°C) and are responsible for conducting primarily mechanical signals. Type II Aδ fibers have higher mechanical and lower temperature thresholds and are mainly responsible for conducting thermal signals.3
Type-C fiber (unmyelinated, less than 2μm in diameter with conduction velocity of under 2m/s) nociceptors transmit diffuse signals of “secondary pain” (dull pain, prolonged burning sensation) and may be subdivided into two subgroups based on neuropeptide expression.3 Receptors containing Substance P (belonging to the tachykinin neuropeptide family) and calcitonin gene-related peptide (CGRP) are known as peptidergic nociceptors. Neurons that do not express the above substances are called non-peptidergic nociceptors. Peptidergic neurons innervate the basal layer of the epidermis and internal organs and are mainly responsible for conducting thermal stimuli, while non-peptidergic neurons perceive impulses from a more superficial layer of the epidermis and perceive mechanical stimuli.3
Due to the wide dynamic signal range of myelinic nociceptors, they can also function as low-threshold mechanoreceptors (LTMR). Proper identification and differentiation of Aδ-LTMR and Type-Aδ nociceptors is critical in understanding such pain conditions as allodynia (pain occurring in response to stimuli that normally does not cause it) and hyperalgesia (abnormally high sensitivity of the body to pain stimuli). Trauma involving the peripheral nervous system can lead to a decrease in the mechanical thresholds in myelinated nociceptors and subsequent pain perception deformation.4–8
The Nociceptive Signal
Cellular bodies of nociceptors are located in the dorsal root ganglion and transmit information about potentially harmful mechanical, chemical and thermal stimuli to the second neuron located in the posterior column of the spinal cord. Fibers from second neurons cross over and ascend as part of the spinothalamic and spinoreticulothalamic pathways, ending in the ventrobasal and medial nuclei of the thalamus, from which they project onto the somatosensory cortex.9 Signal transmission in the nociceptive system is controlled at the level of the spinal cord, the thalamus and the cortex (lemniscus afferents), as well as descending influences from higher centers (a group of grey matter cells of the periaqueductal region of the midbrain).4 Such regulation affects the level at which pain is perceived.
Visceral nociceptors can transmit signals to the central nervous system via the spinal cord and also via the vagus nerve.10 Cells whose axons pass through the spinal pathways often form collaterals to the vegetative ganglion cells, thus affecting autonomic function.5 These close interconnections with elements of the autonomic nervous system explain the different sensations caused by activation of visceral and cutaneous nociceptors (Figure 1).
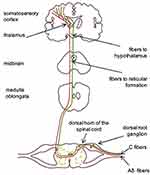 |
Figure 1 Ascending pathways of Type-C (green) and Type-Aδ (red) nociceptive fibers. |
Nociceptive Signal Reception
While Aδ fibers are surrounded by myelin sheaths synthesized by the Schwann cells, type-C fibers are organized into Remak bundles - several axons surrounded by projections of a single Schwann cell.11 Previously it was thought that when C-fibers pass through the basal membrane into the epidermis, they lose any myelin sheath and come into direct contact with keratinocytes. However, studies have shown the existence of a new type of the Schwann cells, organized into a mesh network at the subepidermal border of the skin (just below the basal membrane).8 These cells are in close contact with C fibers, forming a structure surrounded by a thick layer of collagen fibrils, oriented in the direction of the glioneuronal complex and differing in composition from the surrounding collagen. The radial projections of Schwann cells are able to penetrate, together with unmyelinated fibers, into the epidermal layer and convert mechanical stimuli into electrical signals, leading to the emergence of an action potential (Figure 2). In view of such functional and anatomical peculiarities of this complex, it may be recognized as an independent organ of nociceptive reception.
 |
Figure 2 Radial projections of a Schwann cell surround the unmyelinated fibers and penetrate with them into the epidermal layer of the skin. |
The intraepidermal relationships between C-fibers, Schwann cells and keratinocytes (which are also able to influence nociceptive signals) represent complex network of nerve microsomes (Figure 3).
 |
Figure 3 Mesh-like network of Schwann cells at the subepidermal border of the skin. |
Nociceptor Neurogenesis
Neural crest cells (arising at the junction of the neuroectoderm and ectoderm) end up within the developing dorsal root ganglia and give rise to mitotically active progenitor cells, which further differentiate into nociceptive sensory neurons, connected to specific somites.12 Before migration, neural crest cells have specific phenotypic differences, associated with the migration pattern of these cells, which occurs in two distinctive stages. During the first stage neurogenesis, large diameter neurons (proprioceptors and mechanoreceptors) are formed.13 The second stage of neurogenesis leads to the formation of small diameter neurons (thermoreceptors and nociceptors).
Most sensory neuron differentiation is regulated by neurotrophins. The expression of tropomyosin receptor kinase A (TrkA) or receptors with tyrosine kinase activity (RET) determines differentiation of nociceptors (Figure 4). It occurs due to the fact that nerve growth factors (NGFs) bind to TrkA, and the glial cell line-derived neurotrophic factor (GDNF) interacts with RET.14 These factors contribute to the development of sensory channels in nociceptors that allow them to react to stimuli.15 Further differentiation of nociceptive neurons involves axonal elongation controlled by NGFs and GDNF. Depending on the expressed receptor, nociceptors will be subdivided into peptidergic, which respond to NGF, and non-peptidergic, which respond to the GDNF.16
 |
Figure 4 Peptidergic and non-peptidergic nociceptor differentiation. |
Tissue Specific Variations of Nociceptors
Nociceptors have been shown to play a role in the complex mechanisms forming what the brain perceives as pain. This includes inflammation, nervous signaling, specific molecular changes. Nociceptors produce neurogenic inflammation, essentially acting as local immune modulators, coordinating the behavior of local immune cells.17 Interestingly, the relationship between nociceptors and the immune system can also be seen in the reactivity of nociceptors to specific immune modulators, such as interferons,18 TNF-α (tumor necrosis factor alpha), interleukin-1β19 and others. A disruption of this normal association has been linked to neuropathic pain conditions.20 As noted earlier, the localization of different types of nociceptors varies in organs and tissues. Further exploration into these variations provide valuable insight into their role and main function.
Cutaneous Nociceptors
Given the fact that the skin is the most important mechanical barrier in the body, the skin sensory system is represented primarily by Aδ nociceptors, which are responsible for primary pain reception.3 They are divided into two types: type 1 nociceptors have a low-threshold of sensitivity to mechanical stimuli, and type 2 nociceptors with low-threshold sensitivity to temperature stimuli. The skin also contains two other types of nociceptors: peptidergic nociceptors, which only respond to chemical stimuli (such as arachidonic acid, histamine, nerve growth factor (NGF), substance P (SP), calcitonin gene-related peptide (CGRP), serotonin (5-HT), acetylcholine (ACh) and others) and polymodal nociceptors with C-fibers responding to all high intensity stimuli.21
Several studies have shown an important role of cutaneous nociceptors in modification of immune response.22 According to Riol-Blanco et al, TRPV1+ neurons are often located close to dendritic cells and are required for the activation of type-17 innate immune responses.21 It’s important to note that immune response involving nociceptive system is triggered by both non-infectious factors (Imiquimod-induced dermatitis requires TRPV1+ neurons to produce IL-23 by dendritic cells)21,22 and infectious and fungal agents.23
In addition to the physiological role of nociceptors, dysregulation in one or another part of the nociceptive system is associated with pain syndromes (neuropathy, chronic pain, neurogenic inflammation24 and with conditions not accompanied by pain. For example, recently, skin irritation has been shown to be related with dysregulation of the nociceptive system. Han et al described a subpopulation of nociceptors specifically linked to the itching sensation.25 These findings may help develop new treatment targets for idiopathic pruritus and other similar diseases.
Visceral Nociceptors
The visceral nociceptive system is primarily represented by unmyelinated C-type slow fibers, including peptidergic nociceptors responding to pH changes and inflammation.19,24 In hollow organs and in the capsule of parenchymatous organs there is a special kind of mechanoreceptors responding to stretching and pressure changes in the mesenteric vessels26,27 (for example, intestinal obstruction, biliary colic or hepatic congestion).28 A major portion of all nociceptors in internal organs is represented by a specific type of nociceptors known as “sleeping” mechanoreceptors. Normally, they do not respond to mechanical stimuli, but become active when inflammation and tissue damage are present.29 Biologically active substances (BAS) released in peritonitis stimulate peptidergic nociceptors and cause pain. Similarly, nociceptors signal about other internal organ abnormalities accompanied by inflammation, swelling of the abdomen (meteorism) or increased intra-abdominal pressure. This phenomenon implies the decrease of sensitivity threshold during continuous damaging stimuli. It involves the abnormal sensibilization of nociceptors, which is associated with hyperalgesia, chronic pain syndrome, allodynia, and pain syndrome in neurogenic inflammation.30
Interrelation of Cutaneous and Visceral
Finally, it is important to note that there is a notable relationship between cutaneous and visceral nociceptors and the so-called phenomenon of “referred pain”. Fang in 2021 showed that inflammatory bowel diseases can cause hypersensitivity in a corresponding dermatome.31 According to his study, this phenomenon reflects the fact that there is common innervation of the skin and visceral organs coming from the bifurcation of the C-nociceptive neurons (Figure 5).
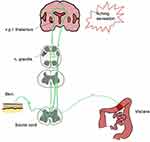 |
Figure 5 Interrelation between visceral and cutaneous nociceptive neurons. |
Research shows that irritation of C-type skin fibers leads to activation of cardiac sensory and sympathetic nerves, which results in a cardioprotective effect, known as «NIC» (nociceptor-inducing conditioning)32,33 (Figure 6). The fact that this method may use the application of capsaicin (along with direct skin incision and use of electric current) confirms the crucial role of TRPV1 channels in the regulation of nociceptive afferentation.33
 |
Figure 6 Nociceptor-inducing conditioning (NIC). |
Bronchopulmonary Nociception
The bronchopulmonary airways are innervated by nociceptive vagal C-fibers, represented by two types of receptors: non-nociceptive nociceptors and nociceptors associated with the Type 1 Vanilloid Channels (TRPV1).34 The latter are associated with bronchopulmonary hyperreactivity seen in chronic bronchitis and asthma. In these conditions, the nociceptive system is activated by subthreshold stimuli, and inflammation enhances (amplifies, exacerbates, intensifies) hyperreactivity and produces a “pathological circle”34 resulting in associated clinical manifestations. Thus, the role of TRPV1+ nociceptor hyperresponsiveness in the pathogenesis of bronchopulmonary diseases with an inflammatory component has been recognized and may be considered a potential target for therapy.
The Musculoskeletal Nociceptive System
In bones, sensory nerve fibers are primarily represented by “sleeping” nociceptors; their cell bodies are located in the dorsal root ganglia (DRG).35 The joints, ligaments, capsules, and menisci are innervated by a Aδ- and C-fibers, with polymodal C-fibers predominating in the joint nociceptive system. The skeletal muscle sensory system is represented by mechano- and heat-sensitive nociceptors, as well as peptidergic polymodal ones.
An important feature of the musculoskeletal nociceptive system is a fairly high threshold of sensitivity to mechanical stimuli (except for Aδ mechanosensors, which react to bone fracture and cause acute pain) and stretching, along with a low threshold of sensitivity to changes in pH. For example, acidosis seen in bone cancer activates “sleeping” nociceptors35 and causes pain that is difficult to treat with non-opioid analgesics.
Type I Transient Receptor Potential (TRPV1) Channels
The direct role of vanilloid channels (TRPV1) in the regulation of nociceptive endings cannot be overlooked. Vanilloid receptors are TRP family members activated by vanilloids, such as capsaicin.36,37 Their activation triggers the flux of cations, including Ca2+, which activates nociceptors and promotes pain sensitization (Figure 7). TRPV1 channels are associated with neurogenic inflammation and chronic pain syndrome specifically through TRPV1+ nociceptors, therefore, these channels may be considered as a potential target of therapy. Recent reports imply a role of TRPV1 in autoimmune and oncologic diseases,37–39 but this issue remains to be evaluated.
 |
Figure 7 TRPV1 channel. |
Nociceptive Perception Changes
Pathological and potentially pathological stimuli are transduced into electrical signals in nociceptors, which represent nerve endings with branched main axons, innervating distinct regions in the dermatomes, organosomes and angiosomes.40 The nociceptive signal is derived from afferent stimulation which leads to depolarization of peripheral terminals producing an active potential. This provides attenuation of stimuli engagement, which leads to transduction of the action potential. Since the action potential is ion dependent, a specific association is seen between Na, Ca, Cl and K ions and their role in creation of the nociceptive signal.41 Sodium, Calcium and Chloride are responsible for depolarization, suggesting that opening of ion channels permeable to Sodium, Chloride, and Calcium will cause sufficient effect for depolarization. On the other hand potassium channels when closed does not lead to depolarization, but amplifies existing fluctuations due to increased resistance of the transmembrane electrohomeostasis.42
The electrochemical and physical aspects of nociceptive signal formation are what define the ability of the receptor to play a role in both hyperalgesic and anti-hyperalgesic responses.43 Four critical functionalities of the nociceptor to provide proper signal transduction include: threshold, relaxation, allodynia and hyperalgesia.44 A normal response of a nociceptor it shows both threshold and relaxation behavior: it does not respond to weak stimulus under the threshold value and has a relaxation period, in which it repolarizes and is inactive. During relaxation, stimuli can reactivate the nociceptor at a lower threshold. Abnormal nociceptor behaviors are seen when the receptor experiences significantly increased stimuli capable of causing damage.45 Abnormal stimuli cause eithey allodynia (receptor response to signals lower than threshold) and hyperalgesia (exaggerated signal to overthreshold stimuli). These processes define that an abnormally functioning nociceptor has no specific threshold value.46 These conditions are mediated by inflammatory cytokines and cell to cell signaling, which initiate a local pathological response. Normally, an abnormally functioning nociceptors has the capability to return to threshold-dependent functioning. Understanding of these mechanisms is what can drive bioengineering of signal transduction.40
Conclusions and Outlook
Nociceptors perform various functions defining the defensive mechanisms and adaptive responses of the macroorganism. Embryological and developmental aspects of nociception explain existing understanding of relationships between nociceptors and other somite derivatives. Various subtypes of nociceptors are located in tissues depending on their physiological roles. Such differentiation results from different input signals coming from different tissues and serves as an adaptive function component. Interestingly, recent data has revealed a “non-classical” role of the nociceptive system,23,24,39 which confirms the need for further evaluation of this family of heterogeneous receptors. The opportunity to use the knowledge about the intimate relationship between nociceptive system activation and inflammatory neurotransmitters, substance P2 and TRPV1 vinyloid channels is of particular interest. The latter ones may be considered as a target for therapy in chronic pain syndromes. Understanding of the mechanisms influencing pain perception and transmission will aid in the development of new methods of targeted therapy for pathological types of pain. The nociceptor is capable of adapting abnormal responses to accommodate overall reactivity to pathological stimuli: through hyperalgesic and anti-hyperalgesic responses.
Author Contributions
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval of the version to be published; and agree to be accountable for all aspects of the work.
Funding
There was no funding/support for this study.
Disclosure
The authors declare no conflicts of interest in this work.
References
1. Hudspith MJ. Anatomy, physiology and pharmacology of pain. Anaesth Intensive Care Med. 2019;17(9):425–430.
2. Bennett DL. Neurotrophic factors: important regulators of nociceptive function. Neuroscientist. 2001;7(1):13–17. doi:10.1177/107385840100700105
3. Dubin AE, Patapoutian A. Nociceptors: the sensors of the pain pathway. J Clin Invest. 2010;120(11):3760–3772. doi:10.1172/JCI42843
4. Vardeh D, Naranjo JF. Anatomy and physiology: mechanisms of nociceptive transmission. In: Pain Medicine. Cham: Springer; 2017:3–5.
5. Ringkamp M, Dougherty PM, Raja SN. Anatomy and physiology of the pain signaling process. In: Essentials of Pain Medicine. Elsevier; 2018:3–10.
6. Meixiong J, Basso L, Dong X, Gaudenzio N. Nociceptor–mast cell sensory clusters as regulators of skin homeostasis. Trends Neurosci. 2020;43(3):130–132. doi:10.1016/j.tins.2020.01.001
7. Pace MC, Passavanti MB, De Nardis L, et al. Nociceptor plasticity: a closer look. J Cell Physiol. 2018;233(4):2824–2838. doi:10.1002/jcp.25993
8. Griffin JW, Thompson WJ. Biology and pathology of nonmyelinating Schwann cells. Glia. 2008;56(14):1518–1531. doi:10.1002/glia.20778
9. Fisher CJ, Yaksh TL, Bruno K, Eddinger KA. Basic science of pain. In: Pain Care Essentials and Innovations. Elsevier; 2021:1–14.
10. Groh A, Krieger P, Mease RA, Henderson L. Acute and chronic pain processing in the thalamocortical system of humans and animal models. Neuroscience. 2018;387:58–71. doi:10.1016/j.neuroscience.2017.09.042
11. Gondim FDAA, Barreira AA, Claudino R, et al. Definition and diagnosis of small fiber neuropathy: consensus from the peripheral neuropathy scientific department of the Brazilian academy of neurology. Arq Neuropsiquiatr. 2018;76(3):200–208. doi:10.1590/0004-282x20180015
12. Bove GM. Epi-perineurial anatomy, innervation, and axonal nociceptive mechanisms. J Bodyw Mov Ther. 2008;12(3):185–190. doi:10.1016/j.jbmt.2008.03.004
13. Meltzer S, Santiago C, Sharma N, Ginty DD. The cellular and molecular basis of somatosensory neuron development. Neuron. 2021;109(23):3736–3757. doi:10.1016/j.neuron.2021.09.004
14. Lübke JH, Idoon F, Mohasel-Roodi M, et al. Neurotrophic factors in Alzheimer’s disease: pathogenesis and therapy. Acta Neurobiol Exp. 2021;81(4):314–327.
15. Cheng X, Choi JS, Waxman SG, Dib-Hajj SD. Mini-review-sodium channels and beyond in peripheral nerve disease: modulation by cytokines and their effector protein kinases. Neurosci Lett. 2021;741:135446. doi:10.1016/j.neulet.2020.135446
16. de Nooij JC. Influencers in the somatosensory system: extrinsic control of sensory neuron phenotypes. Neuroscientist. 2022;10738584221074350. doi:10.1177/10738584221074350
17. Michoud F, Seehus C, Schönle P, et al. Epineural optogenetic activation of nociceptors initiates and amplifies inflammation. Nat Biotechnol. 2021;39(2):179–185. doi:10.1038/s41587-020-0673-2
18. Barragán-Iglesias P, Franco-Enzástiga Ú, Jeevakumar V, et al. Type I interferons act directly on nociceptors to produce pain sensitization: implications for viral infection-induced pain. J Neurosci. 2020;40(18):3517–3532. doi:10.1523/JNEUROSCI.3055-19.2020
19. Mody PH, Dos Santos NL, Barron LR, Price TJ, Burton MD. eIF4E phosphorylation modulates pain and neuroinflammation in the aged. GeroScience. 2020;42(6):1663–1674. doi:10.1007/s11357-020-00220-1
20. Shiers S, Mwirigi J, Pradhan G, et al. Reversal of peripheral nerve injury-induced neuropathic pain and cognitive dysfunction via genetic and tomivosertib targeting of MNK. Neuropsychopharmacology. 2020;45(3):524–533. doi:10.1038/s41386-019-0537-y
21. Riol-Blanco L, Ordovas-Montanes J, Perro M, et al. Nociceptive sensory neurons drive interleukin-23-mediated psoriasiform skin inflammation. Nature. 2014;510(7503):157–161; PMID: 24759321; PMCID: PMC4127885. doi: 10.1038/nature13199
22. Cohen JA, Edwards TN, Liu AW, et al. Cutaneous TRPV1+ neurons trigger protective innate type 17 anticipatory immunity. Cell. 2019. doi:10.1016/j.cell.2019.06.022
23. Kashem SW, Riedl MS, Yao C, Honda CN, Vulchanova L, Kaplan DH. Nociceptive sensory fibers drive interleukin-23 production from CD301b+ dermal dendritic cells and drive protective cutaneous immunity. Immunity. 2015;43(3):515–526. doi:10.1016/j.immuni.2015.08.016
24. Chiu IM, von Hehn CA, Woolf CJ. Neurogenic inflammation and the peripheral nervous system in host defense and immunopathology. Nat Neurosci. 2012;15(8):1063–1067. doi:10.1038/nn.3144
25. Han L, Ma C, Liu Q, et al. A subpopulation of nociceptors specifically linked to itch. Nat Neurosci. 2013;16(2):174–182; PMID: 23263443; PMCID: PMC3557753. doi: 10.1038/nn.3289
26. Brierley SM. Gut nociceptors: sentinels promoting host defense. Cell Res. 2020;30(4):279–280. doi:10.1038/s41422-020-0278-9
27. Humenick A, Brookes SJH. Activation of intestinal spinal afferent endings by changes in intra-mesenteric arterial pressure. J Physiol. 2015;593(16):3693–3709. doi:10.1113/jp270378
28. Struller F, Weinreich FJ, Horvath P, et al. Peritoneal innervation: embryology and functional anatomy. Pleura Peritoneum. 2017;2(4):153–161. doi:10.1515/pp-2017-0024
29. Schmidt R. Silent Nociceptor. In: Schmidt R, Willis W, editors. Encyclopedia of Pain. Berlin, Heidelberg: Springer; 2007. doi:10.1007/978-3-540-29805-2_4001.
30. Gold MS, Gebhart GF. Nociceptor sensitization in pain pathogenesis. Nat Med. 2010;16(11):1248–1257. doi:10.1038/nm.2235
31. Fang Y, Han S, Li X, et al. Cutaneous hypersensitivity as an indicator of visceral inflammation via c-nociceptor axon bifurcation. Neurosci Bull. 2021;37(1):45–54; PMID: 32902804; PMCID: PMC7811974. doi: 10.1007/s12264-020-00577-5
32. Sengupta JN. Visceral pain: the neurophysiological mechanism. Handb Exp Pharmacol. 2009;194:31–74. PMID: 19655104; PMCID: PMC3156094. doi:10.1007/978-3-540-79090-7_2
33. Ren X, Roessler AE, Lynch TL, et al. Cardioprotection via the skin: nociceptor-induced conditioning against cardiac MI in the NIC of time. Am J Physiol Heart Circ Physiol. 2019;316(3):H543–H553; PMID: 30575436; PMCID: PMC6415820. doi: 10.1152/ajpheart.00094.2018
34. Hadley SH, Bahia PK, Taylor-Clark TE. Sensory Nerve terminal mitochondrial dysfunction induces hyperexcitability in airway nociceptors via protein kinase C. Mol Pharmacol. 2014;85(6):839–848. doi:10.1124/mol.113.091272
35. Puntillo F, Giglio M, Paladini A, et al. Pathophysiology of musculoskeletal pain: a narrative review. Ther Adv Musculoskelet Dis. 2021;13:1759720X21995067. PMID: 33737965; PMCID: PMC7934019. doi:10.1177/1759720X21995067
36. Caterina MJ, Schumacher MA, Tominaga M, Rosen TA, Levine JD, David J. The capsaicin receptor: a heat-activated ion channel in the pain pathway. Nature. 1997;389(6653):816–824. doi:10.1038/39807
37. Bujak JK, Kosmala D, Szopa IM, Majchrzak K, Bednarczyk P. Inflammation, cancer and immunity—implication of TRPV1 channel. Front Oncol. 2019;9. doi:10.3389/fonc.2019.01087.
38. Tsuji F, Aono H. Role of transient receptor potential vanilloid 1 in inflammation and autoimmune diseases. Pharmaceuticals. 2012;5(8):837–852. doi:10.3390/ph5080837
39. Lai NY, Musser MA, Pinho-Ribeiro FA, et al. Gut-innervating nociceptor neurons regulate Peyer’s patch microfold cells and SFB levels to mediate salmonella host defense. Cell. 2020;180(1):33–49.e22; PMID: 31813624; PMCID: PMC6954329. doi: 10.1016/j.cell.2019.11.014
40. Kim Y, Kwon YJ, Kwon DE, et al. Nociceptive memristor. Adv Mater. 2018;30(8):1704320. doi:10.1002/adma.201704320
41. Carr RW, Pianova S, McKemy DD, Brock JA. Action potential initiation in the peripheral terminals of cold-sensitive neurones innervating the Guinea-pig cornea. J Physiol. 2009;587(pt 6):1249–1264. doi:10.1113/jphysiol.2008.167023
42. Hille B. Ion Channels of Excitable Membranes.
43. Anshar M, Williams MA. Simplified pain matrix method for artificial pain activation embedded into robot framework. Int J Soc Robot. 2021;13(2):187–195. doi:10.1007/s12369-020-00632-1
44. Sandkuhler J. Models and mechanisms of hyperalgesia and allodynia. Physiol Rev. 2009;89(2):707–758. doi:10.1152/physrev.00025.2008
45. Yoon JH, Song SJ, Yoo IH, et al. Highly uniform, electroforming‐free, and self‐rectifying resistive memory in the Pt/Ta2O5/HfO2‐x/TiN structure. Adv Funct Mater. 2014;24(32):5086–5095. doi:10.1002/adfm.201400064
46. Mendell LM. Computational functions of neurons and circuits signaling injury: relationship to pain behavior. Proc Natl Acad Sci. 2011;108(Supplement 3):15596–15601. doi:10.1073/pnas.1012195108
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
