Back to Journals » Neuropsychiatric Disease and Treatment » Volume 12
Nine traditional Chinese herbal formulas for the treatment of depression: an ethnopharmacology, phytochemistry, and pharmacology review
Authors Feng D, Tang T, Lin X, Yang Z, Yang S, Xia Z, Wang Y, Zheng P, Wang Y, Zhang C
Received 8 June 2016
Accepted for publication 23 August 2016
Published 20 September 2016 Volume 2016:12 Pages 2387—2402
DOI https://doi.org/10.2147/NDT.S114560
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Wai Kwong Tang
Dan-dan Feng, Tao Tang, Xiang-ping Lin, Zhao-yu Yang, Shu Yang, Zi-an Xia, Yun Wang, Piao Zheng, Yang Wang, Chun-hu Zhang
Laboratory of Ethnopharmacology, Institute of Integrated Traditional Chinese and Western Medicine, Xiangya Hospital, Central South University, Changsha, People’s Republic of China
Abstract: Depression is a major mental disorder, and is currently recognized as the second-leading cause of disability worldwide. However, the therapeutic effect of antidepressants remains unsatisfactory. For centuries, Chinese herbal formulas (CHFs) have been widely used in the treatment of depression, achieving better therapeutic effects than placebo and having fewer side effects than conventional antidepressants. Here, we review the ethnopharmacology, phytochemistry, and pharmacology studies of nine common CHFs: “banxia houpo” decoction, “chaihu shugansan”, “ganmaidazao” decoction, “kaixinsan”, “shuganjieyu” capsules, “sinisan”, “wuling” capsules, “xiaoyaosan”, and “yueju”. Eight clinical trials and seven meta-analyses have supported the theory that CHFs are effective treatments for depression, decreasing Hamilton Depression Scale scores and showing few adverse effects. Evidence from 75 preclinical studies has also elucidated the multitarget and multipathway mechanisms underlying the antidepressant effect of the nine CHFs. Decoctions, capsules, and pills all showed antidepressant effects, ranked in descending order of efficacy. According to traditional Chinese medicine theory, these CHFs have flexible compatibility and mainly act by soothing the liver and relieving depression. This review highlights the effective treatment choices and candidate compounds for patients, practitioners, and researchers in the field of traditional Chinese medicine. In summary, the current evidence supports the efficacy of CHFs in the treatment of depression, but additional large-scale randomized controlled clinical trials and sophisticated pharmacology studies should be performed.
Keywords: traditional Chinese medicine, Chinese herbal formula, antidepressant, phytochemistry, pharmacological bioactivity
Introduction
Depression is a common chronic mental health problem,1–3 with a global prevalence of 4.4%–5%.4 It is characterized by a loss of interest in daily activities and feelings of low self-worth, disappointment, sadness, and hopelessness. According to the Global Burden of Disease Study 2013, major depressive disorder (MDD) is the second-leading global cause of years lived with disability.5 The population-attributable risk for depression and all-cause death is 12.7%, and for depression and suicide it is 11.2%.6 The economic burden of individuals with MDD in the US increased by 21.5% between 2005 and 2010 (from $173.2 billion to $210.5 billion, inflation-adjusted).7 A recent meta-analysis of the prevalence of depressive symptoms in older Chinese adults from 1987 to 2012 reported an overall prevalence of 23.6%.8–10
Conventional antidepressants, such as selective reuptake inhibitors of serotonin (5-HT) or norepinephrine (NE), monoamine oxidase inhibitors, tricyclic antidepressants, or N-methyl-d-aspartate receptor (NMDAR) antagonists, fail to help at least 40% of depressed patients.11 Existing treatments for MDD usually take several weeks or months to achieve their therapeutic effects.12,13 During this lag period, patients continue to have depressive symptoms and risk self-harm.12 Despite this, there has been little notable progress in the development of drugs to treat depression over the last few decades.14 Scientists are seeking new, improved therapeutic approaches to deal with depression, and the drive to develop next-generation drugs with improved safety profiles has intensified.15,16
Traditional Chinese medicine (TCM) is attracting increasing attention as a method for meeting the demands for higher remission rate, faster onset, persistent antidepressant action, and fewer adverse effects.17,18 TCM is one of the oldest medical systems in the world, and includes Chinese herbal medicine, acupuncture, and massage. Herbal medicine has been used for the treatment of depression in People’s Republic of China for centuries, and is becoming more frequently used in Western countries. “Yuzheng”, meaning “depression syndrome”, first appeared in the Ming Dynasty Tuan Yu’s Yi Xue Zheng Zhuan, although discussions about depression can be traced back to Huangdi Neijing (The Inner Canon of Huangdi).19 Then, in the Eastern Han Dynasty, Jingui Yaolue (Synopsis of Prescriptions of the Golden Chamber) first described the symptoms of lily disease, globus hystericus, and “zangzao” syndrome, which are thought to belong to depression syndrome; this document also recorded “banxia houpo” decoction (BHD) and “ganmaidazao” decoction (GMDZD), both used very frequently for depression.20
Interestingly, modern TCM for the treatment of depression has started to be integrated into evidence-based medicine. A PubMed search revealed that the first randomized controlled trial (RCT) of Chinese herbal formulas (CHFs) in depression investigated “gengnianle”, a defined formula of Chinese medicinal herbs for the relief of perimenopausal depression.21 The earliest herbal pharmacology study investigated BHD.22 A systematic review of the treatment of menopausal symptoms started with the study of “erxian” decoction,23 and the first meta-analysis was conducted on the clinical effects of “chaihu shugansan” (CSS).24 Further studies showed that liver depression and chi stagnation were the main syndromes,25 and that the main treatment principle is the regulation of chi, relief of depression, and empathy.26
Following Synopsis of Prescriptions of the Golden Chamber, numerous antidepressant CHFs were recorded in ancient books. Although some systematic reviews summarized the most frequent antidepressant formulas and patterns of TCM use,25,27 there have been no detailed descriptions of effective and classical CHFs available to date, which is a major impediment to the use of CHFs in modern medical practice. Here, we introduce nine common and important antidepressant CHFs with regard to their source, composition, chemical constituents, indications, TCM and antidepressant effects, mechanisms, and side effects from a review of ethnopharmacology, phytochemistry, and pharmacology studies.
Materials and methods
Available references that recorded the use of the nine CHFs in depression were analyzed via PubMed and Web of Science (from inception to June 2, 2016). Further comprehensive resources were searched for each CHF in PubMed, Web of Science, Science Direct, China National Knowledge Infrastructure, Wanfang Data, Chinese Scientific Journals Full-Text Database, and Google Scholar. Relevant TCM information was from http://www.tcmwiki.com/w/TCM_Wiki and the Pharmacopoeia of the People’s Republic of China (2015).
Database searches included the following keywords: (“depression” or “depressive disorder” or “antidepressant”) and (“Chinese prescription” or “Chinese formula” or “Chinese decoction” or “Chinese herbal formula” or each CHF by name). The keywords were modified for use with different databases. Full-text articles in English or Chinese were included.
Results
The source, TCM effects, and syndromes of the nine most commonly prescribed CHFs for treating depression are provided in Table 1. Photographs of each formula are presented in Figure 1. All correspond to the typical principles of TCM treatment of yuzheng according to their effects and Chinese-syndrome patterns, including soothing the liver, relieving depression, promoting the circulation of chi, and resolving phlegm. Importantly, we found a considerable amount of evidence-based research for most of the CHFs we examined, meaning the results can be used to guide clinical practice and research in related fields.
  | Table 1 The source, TCM effects, and syndromes of the nine Chinese herbal formulas |
Banxia houpo decoction
Composition
Banxia (Pinellia rhizome) 12 g, “fuling” (Poria) 12 g, houpo (Magnolia officinalis cortex) 9 g, “shengjiang” (Zingiber officinale rhizome) 9 g, “suye” (Perilla folium) 6 g.
Chemical constituents
Zingiberol, guanosine, rosmarinic acid,28 magnolol, honokiol,29 volatile oils (including linalool, citral, nerolidol, caryophyllene, bisabolene, caryophyllene oxide, apiole, α- and β-eudesmol, and α-farnesene),30 polysaccharides.31
Pharmacology and bioactivity
A clinical report of 46 patients with globus hystericus showed that the modified BHD granule (BHD + “foshou” [Citrus medica var. sarcodactylis fructus] 15 g; 46 cases), taken at a dose of one pack three times a day, was more effective than Manyanshuning (sore-throat treatment; 49 cases) in reducing symptoms of depression and anxiety on Symptom Checklist 90 and improving patients’ psychological state.32
A study using an orthogonal array showed that the interactions of the BHD constituents houpo × suye and fuling × suye were the core herb pairs to alleviate depression-like serotonergic and dopaminergic changes in mice.33 Furthermore, aqueous and lipophilic extracts of BHD showed the greatest antidepressant effects, whereas the polyphenol fraction showed a moderate effect.22 BHD reduced immobility time in the forced-swim test (FST) and tail-suspension test (TST), increased 5-HT and 5-hydroxyindoleacetic acid levels in the hippocampus and striatum, and decreased serum and liver malondialdehyde (MDA) levels in mice with a depression-like phenotype.34 Ethanol and water extracts of BHD reduced c-Fos expression in cerebral regions of rats subjected to chronic mild stress (CMS) to a level comparable to that of fluoxetine.35 BHD significantly increased high-density lipoprotein levels, decreased serum triglyceride levels, improved the activity of splenic natural and lymphokine-activated killer cells, reduced the activity of liver SOD and nitric oxide synthase, and decreased the level of serum MDA by inhibiting lipid peroxidation in rats after CMS.36 Furthermore, BHD normalized changes in the metabolites of rats after CMS, and exerted antidepressant effects by regulating amino acid and energy metabolism.37
Indications and usage
BHD can be used to promote chi, calm adverse chi, eliminate stagnation, and dissolve phlegm. It is also used to treat the syndrome of phlegm-chi stagnation and binding, especially for globus hystericus32 (which manifests as an obstruction in the throat that is hard to cough up or swallow), fullness and oppression in the chest and diaphragm, white fur on the tongue, and “wiry” pulse (which means a small, tense pulse).
Dosage
Decocted in water for oral use; taken twice a day.
Chaihu shugansan
Composition
Chaihu (bupleuri radix) 6 g, “chenpi” (Citrus reticulata pericarp) 6 g, “chuanxiong” (Ligusticum chuanxiong rhizome) 4.5 g, “xiangfu” (Cyperus rhizome) 4.5 g, “zhiqiao” (Citrus aurantium fructus) 4.5 g, “shaoyao” (Paeonia radix) 4.5 g, “gancao” (Glycyrrhiza radix) 1.5 g.
Chemical constituents
Synephrine, paeoniflorin, naringin, hesperidin, neohesperidin, saikosaponin A, glycyrrhizic acid, nobiletin, tangeretin, ferulic acid,38 gallic acid, oxypaeoniflorin, albiflorin, liquiritin, benzoic acid, narirutin, meranzin hydrate, liquiritigenin, quercetin, benzoylpaeoniflorin, isoliquiritigenin, formononetin.39
Pharmacology and bioactivity
An in vitro study suggested that the antioxidant activity of CSS contributes to its antidepressant effect. CSS showed a ferric-reducing antioxidant power equivalent to 0.24 mM FeSO4/g CSS aqueous extract and scavenging free radical activity.40 A meta-analysis of ten RCTs with a total of 835 subjects showed CSS acts at multiple targets, without side effects. CSS, with or without combined antidepressant treatment, significantly improved symptoms of depression, with better efficacy and recovery rate than antidepressants alone.24
CSS reverses the stress-induced disruption of ERK5 and p-ERK1/2 activation in the rat hippocampus41,42 and increases the messenger RNA (mRNA)-expression ratio of ERα to ERβ in the rat hippocampus after CMS, but shows no significant change in estradiol levels or mRNA expression of ERα or GPR30.43 CSS also inhibits the expression of the c-Jun N-terminal kinase via signal transduction involving stress-activated protein kinase in the hippocampus of rats after CMS.44 Nevertheless, CSS upregulates the expression of 5-HT1A-receptor mRNA and cellular proliferation in the hippocampal dentate gyrus in epileptic rats with depression.45 In addition, CSS inhibits lipid peroxidation, relieves oxidative injury, and regulates antioxidant capacity. Restrained mice treated with CSS have higher SOD and catalase activities in liver, lower MDA values, and higher glutathione levels in the blood compared with untreated restrained mice.40 A urinary metabonomic study on rats after CMS showed that the therapeutic effect of CSS may involve reversal of energy and tryptophan-metabolism dysfunction, increase in bone density, and liver detoxification.46 Furthermore, in depressed patients, CSS improves both clinical symptoms and impairments in cerebral blood flow in multiple regions, revealed by single-photon emission computed tomography.47
Indications and usage
CSS is indicated to soothe the liver, regulate chi, and promote blood circulation to relieve pain. It can be used to treat the syndrome of liver-chi stagnation (manifesting as pain in the lateral thorax, pressure in the chest, and tendency to sigh), as well as depression or irritability, belching, abdominal fullness and distention, white fur on the tongue, or a “wiry” pulse.
Precautions and side effects
In TCM theory, CSS should be used with caution in patients with yin deficiency. Like antidepressants (such as fluoxetine or melitracen–flupentixol), modified CSS can cause nausea, stomach upset, dry mouth, drowsiness, or insomnia. However, CSS ranks lower on the treatment-emergent side-effect scale than antidepressants, and does not affect patients’ routine blood-examination results or hepatic or renal function.24
Dosage
Decocted in water for oral use, or taken in its powder form; taken twice a day.
Ganmaidazao decoction
Composition
Gancao (Glycyrrhiza radix) 9 g, “xiaomai” (Triticum aestivum fructus) 9–15 g, 5–7 DZ (jujube [type of date] fructus).
Chemical constituents
Known constituents are glycyrrhizic acid, isoliquiritin, and isoliquiritigenin, plus unknown extracts of three herbs: tritici fructus, Glycyrrhiza, and jujube.48
Pharmacology and bioactivity
A meta-analysis of GMDZD for depression, including ten RCTs with a total of 968 subjects, suggested that GMDZD showed antidepressant action with few side effects. GMDZD in combination with antidepressants reduces side effects and enhances antidepressant efficacy.48 Another study of modified GMDZD (43 patients) showed equivalent efficacy to melitracen–flupentixol (43 patients) in climacteric depression, and was more effective than melitracen–flupentixol in improving sleep quality, which may have been a result of adjusted levels of 5-HT, NE, estradiol, follicle-stimulating hormone, and luteotrophic hormone in climacteric depression.49 Subchronic administration of GMDZD reduces CMS-induced depression-like behavior in rats, and this is associated with a reduction in glutamate levels and increased expression of NMDAR subunits (NR2A and NR2B) in the frontal cortex and hippocampus.50,51
Indications and usage
GMDZD is used to nourish the heart, tranquilize the mind, regulate the function of the middle jiao, relieve spasms, and alleviate pain. It is also used to treat deficiency of heart yin, malnutrition of heart/mind, and disharmony of liver chi (which manifests as hysteria, climacteric syndrome, neurasthenia, or nocturnal fretfulness in infants), especially for zangzao syndrome (visceral irritation, in which female patients present with low mood, tendency to cry, possessive behavior, and frequent yawning).48
Precautions and side effects
GMDZD is contraindicated in cases with excessive “phlegm fire” inside manic-depressive psychosis. Adverse events associated with modified GMDZD include dry mouth, constipation, melancholia, insomnia, irritability,48 abdominal distension, and acid regurgitation.52
Dosage
Decocted in water for oral use; taken three times a day.
Kaixinsan
Composition
“Rensheng” (ginseng radix and rhizome [GR]), “yuanzhi” (Polygala radix [PR]), “shichangpu” (Acorus tatarinowii rhizome [ATR]), fuling (Poria [PO]).
Source
Kaixinsan (KXS) is known by different names, and has different dose-ratio compositions. For example, the ratio of GR:PR:ATR:PO is 1:1:25:50 in the formula KXS-652, 3:2:2:3 in “dingzhi xiaowan” (DZW-652) (both from Essential Prescriptions Worth a Thousand in Gold for Every Emergency), and 1:1:1:2 in KXS-984 (from Prescriptions from the Heart of Medicine).53
Chemical constituents
Ginsenosides (27 in total, including Ro, Ra1, Rb1, Rb2, Rc, Rd, Re, Rf, Rg1, Rg3, Rg5, Rg6, Rh1, Rh2, Roa, zingibroside R1, notoginsenoside R2 and R4, and pseudoginsenoside Rc1), Polygala saponins (arillatanoside A, tenuifolin), terpenoids (tumulosic acid, pachymic acid, dehydropachymic acid), oligosaccharide esters (18, including sibiricoses A1 and A6 and tenuifolisides A, B, C, E, G, H, I, K, L, M, and O), and other compounds, including 3,6′-disinapoyl sucrose, polygalaxanthone III, α- and β-asarone, and pachymic acid.53–55
Pharmacology and bioactivity
A study in cultured astrocytes suggested that the antidepressant action of KXS is mediated by an increase in mRNA and protein expression and secretion of neurotrophic factors, including NGF, BDNF, and GDNF. Moreover, individual aqueous extracts of the four herbs that constitute KXS did not significantly influence the expression of neurotrophic factors, which suggests that these herbs act synergistically to produce their effects.56 Furthermore, KXS attenuates cell death induced by BDNF knockdown (mediated by a lentiviral vector – LV-shBDNF-3) in primary hippocampal neurons; it also increased sucrose intake in the sucrose preference test, exploration in the open-field test, and learning ability in the Morris water maze in rats with an LV-shBDNF-3-induced depression-like phenotype. Western blot analysis has confirmed that KXS can reverse the LV-shBDNF-3-induced reduction in BDNF both in vitro and in vivo.57 An optimized KXS with a GR:PR:ATR:PO ratio of 1:1:5:10 has shown a greater capability to promote neurofilament expression in pheochromocytoma (PC12) cells. Furthermore, the PKA inhibitor H89 significantly inhibited induced neurofilament-promoter activity, which indicated that the underlying mechanism was mediated by the PKA-signaling pathway.58
In patients after 2 and 4 weeks’ treatment, Hamilton Depression Scale (HAM-D) scores were decreased significantly more after KXS (DZW-652, 40 cases, 60 g/day) than after fluoxetine (40 cases, 20 mg/day) in patients with mild or moderate depression. Also, after 8 weeks’ treatment, the clinical:control ratio was greater in the KXS group than in the fluoxetine group.59
KXS likely exerts its antidepressant and nootropic effects in CMS rat models of depression by modulating the hypothalamic–pituitary–adrenal (HPA) axis and monoaminergic and cholinergic systems. DZW-652 reduces depression-like behaviors in CMS rat models, increasing the sucrose preference index, increasing active avoidance and active movement distances in the two-way active avoidance test, and decreasing the prolonged latency of novelty-suppressed feeding. In addition, KXS significantly reduces CMS-induced elevation in serum levels of adrenocorticotropic hormone, decreases hippocampal acetylcholinesterase expression, and upregulates monoaminergic neurotransmitters (dopamine, NE, and 5-HT) in the brain.60,61 Another study showed that KXS increased 5-HT synthesis and reduced 5-HT reuptake. It significantly increased protein and mRNA expression of tryptophan hydroxylase during the 5-HT synthetic process in the hippocampus and prefrontal cortex of rat models of depression, and downregulated 5-HT transporter protein and mRNA levels.62 It also regulates the activity of aralkylamine N-acetyltransferase (the penultimate and key enzyme in the hormone biosynthetic pathway) in the pineal body, melatonin biosynthesis, and the expression of the melatonin receptor Mel1A in rat models of depression.63,64
Indications and usage
KXS invigorates vital energy to nourish the heart and tranquilize and sedate the mind. It can be used to treat the syndrome of heart-chi deficiency and disharmony of the heart and kidney, manifesting as sadness, insomnia, and amnesia.
Precautions and side effects
It is relatively safe to take KXS orally. The observed adverse-effect level is 19.67 g/kg/day for mice, 73-fold higher than the currently recommended dose for a 60 kg human. Adverse effects in mice include slow movement, a decrease in aggression, anorexia, and weight loss.65 In humans, adverse effects include dry mouth and abdominal distension, which alleviate spontaneously without any treatment.59
Dosage
Decocted in water for oral use, taken twice a day.
Shuganjieyu capsules
Composition
Extracts of “guanye jinsitao” (Hypericum perforatum) and “ciwujia” (Acanthopanax).
Chemical constituents
Twenty-two compounds were identified by ultraperformance liquid chromatography tandem mass spectrometry: neochlorogenic acid, syringin, chlorogenic acid, 1′-O-caffeoylquinic acid, 1′,5′-, 3′,5′-, and 4′,5′-O-dicaffeoylquinic acid, eleutheroside E, rutin, hyperoside, isoquercitrin, isofraxidin, quercetin-3-β-d-arabinose, quercitrin, acetyl-hyperoside, quercetin, II3,II8-biapigenin, protopseudohypericin, hyperforin, adhyperforin, hypericin, and pseudohypericin.66
Pharmacology and bioactivity
A systematic review of shuganjieyu capsules for mild-to-moderate depression included 15 RCTs with 1,240 patients, and concluded that the total effective rate and HAM-D scores of the shuganjieyu group were higher than those of the control group (paroxetine, citalopram, sertraline, fluoxetine, or melitracen–flupentixol), and the incidence of adverse drug reactions in the shuganjieyu group was also better than in the control group.67 Another meta-analysis investigating MDD in adults comprised seven RCTs with 595 participants. The capsule was superior to placebo in terms of response and remission rates, and mean changes from baseline in the HAM-D and TCM-syndrome scales. Moreover, shuganjieyu plus venlafaxine had significantly higher response and remission rates than venlafaxine alone, and was superior to venlafaxine in terms of average change from baseline on the treatment-emergent symptom scale.68 The capsule was also effective and safe in the treatment of geriatric depression.69
In rat models of depression, shuganjieyu capsules increased spontaneous activity, sucrose consumption, and sucrose preference, reduced immobility time in the FST, decreased the number of apoptotic cells, and decreased protein levels of caspase 3.70 They also increased levels of p-CREB and BDNF expression in the medial prefrontal cortex and hippocampal CA3 region.71
Indications and usage
Shuganjieyu capsules soothe the liver, relieve depression, strengthen the spleen, and calm the nerves. They are used for mild and moderate single-phase depression with the syndrome of liver-chi stagnation and spleen deficiency, manifesting as low spirits, falling interest, slow movement, sleep disorder, anxiety, irritability, indigestion, loss of appetite, fatigue, sweating, white or greasy tongue, and wiry or small pulse.
Precautions and side effects
Nausea and vomiting, dry mouth, headache, dizziness, nasal congestion, insomnia, poor appetite or anorexia, diarrhea, and constipation. All of these adverse effects disappear spontaneously upon cessation of treatment.68,69
Dosage
Two capsules (380 mg each) orally, twice a day for 6 weeks continuously, is usually recommended. However, a multicenter, randomized, controlled, open-label trial found that doubling the dose for the first week of treatment can significantly enhance symptom relief. Therefore, in some cases, recommended dosage is four capsules twice daily during week 1, three capsules twice daily during week 2, and two capsules twice daily during weeks 3–6.72
Sinisan
Composition
“Zhishi” (Citrus aurantium fructus) 6 g, chaihu (bupleuri radix) 6 g, shaoyao (Paeonia radix) 9 g, “zhigancao” (Glycyrrhiza radix preparation) 6 g.
Chemical constituents
Sinisan (SNS) contains hundreds of constituents. These include gallic acid, oxypaeoniflorin, albiflorin, paeoniflorin, liquiritin, benzoic acid, narirutin, naringin, hesperidin, neohesperidin, meranzin hydrate, liquiritigenin, quercetin, benzoylpaeoniflorin, isoliquiritigenin, formononetin, quercetin-3-O-rhamnoside, isoliquiritin,39 quercetin-3-O-glucoside, naringenin, licorice saponin A3 and G2, glycyrrhizic acid, saikosaponin A and D, glycyrrhetinic acid,73 paeoniflorin sulfonate, liquiritin apioside, uralsaponin A and B, ononin, hesperetin, nobiletin, licochalcone, 5-methoxyflavone,74 synephrine, catechin, tangeretin, and β-sitosterol.75
Pharmacology and bioactivity
In rats subjected to chronic restraint stress, SNS reverses impairments in spatial learning and memory, reduces aggressive behavior, and increases growth rate.76 However, SNS has a relatively long lag. After 4 weeks’ treatment, SNS alone was less effective than fluoxetine alone or in combination with SNS in reducing depression-like behavior in rats after CMS, although the combination was more effective than fluoxetine alone. However, after 8 weeks’ treatment, SNS alone was more effective in reducing depression-like behavior than fluoxetine alone, and markedly increased central 5-HT and reduced peripheral 5-HT in model rats.77
The main active ingredients of SNS are saikosaponins, paeoniflorin, naringin, and glycyrrhizic acid. SNS exerts its antidepressant effects via modulation of cerebral monoamine-neurotransmitter expression, hippocampal neuroprotection, inhibition of HPA-axis hyperfunction, and immune and second-messenger regulation.78 A single dose of SNS (1.3 g/kg) significantly decreased immobility time in the mouse TST, but not FST, comparably to a fluoxetine group. In addition, pretreatment with p-chlorophenylalanine methyl ester (an inhibitor of 5-HT synthesis) or α-methyl-p-tyrosine (an inhibitor of tyrosine hydroxylase) prevented the anti-immobility effect of SNS in mice exposed to the TST. Moreover, acute SNS decreased serum corticosterone levels and elevated 5-HT, NE, and dopamine levels without affecting BDNF levels in the whole brain after the TST.79 A study using the auxiliary mechanism-elucidation system for Chinese medicine and network pharmacology methods found that 263 chemical constituents in SNS directly or indirectly affected 19 targets for the treatment of depression, such as the β2-adrenergic receptor, 5-HT1A, 5-HT2A, 5-HT2C, and the dopamine D2 receptor. It also revealed that SNS exerted its antidepressant effects via a multitarget and multipathway mechanism, involving G-protein coupled-receptor signaling, the cAMP pathway, neurological systems, neurotransmitter secretion, and the inflammatory response, among others.80
Indications and usage
SNS expels pathogens and resolves depression, soothes the liver, and regulates the spleen. It treats the syndrome of yang stagnation (manifesting as cold limbs, abdominal pain, diarrhea, or tenesmus), or the syndrome of liver–spleen disharmony (manifesting as distending pain in the hypochondrium and a wiry pulse).
Dosage
Decocted in water for oral use, or taken in its powder form, twice a day.
Wuling capsules
Composition
Made from the mycelia of Xylaria nigripes (Kl.) Sacc. in People’s Republic of China.
Chemical constituents
Nucleosides (adenosine, uridine, and guanosine),81 5-methylmellein, 5-hydroxymellein, 5-carboxylmellein, and genistein,82,83 and 15 amino acids (aspartic acid, glutamic acid, serine, histidine, glycine, arginine, proline, alanine, γ-aminobutyric acid, threonine, methionine, valine, phenylalanine, isoleucine, leucine).84
Pharmacology and bioactivity
A total of 16 RCTs for poststroke depression involving 1,378 patients were identified for the meta-analysis. Wuling capsules showed some antidepressant effects compared to the no-treatment control. Combined with conventional antidepressants (melitracen–flupentixol, fluoxetine, sertraline, paroxetine, or citalopram), the capsules improved treatment effects and reduced the incidence of adverse effects as an alternative choice in the treatment of poststroke depression.85 They also relieved depression and anxiety in female climacteric patients.86 A multicenter randomized double-blind placebo-controlled study started with 104 patients and finished with 81. The study showed that wuling capsules (three tablets, three times daily for 12 weeks) alleviated depressive symptoms in patients with epilepsy, and enhanced some aspects of quality of life without increasing the risk of exacerbating epileptic seizures.87
Wuling capsules improved hippocampal neurogenesis in rats exposed to CMS via a mechanism involving an increase in the expression of connexin 43 mRNA and protein, but not involving BDNF.88 Another study also found that the mechanism by which wuling capsules improve impairments in learning and memory in rat models of poststroke depression is not mediated by BDNF.89 The antidepressant effect of wuling capsules might involve facilitation of the 18 kDa TSPO-mediated mitophagy pathway. In a learned helplessness to inescapable electric shock model of depression in mice, wuling capsules showed antidepressant effects across multiple behavioral tests, including the shuttle-box test, novelty-suppressed feeding, and the FST. It also altered the expression of multiple proteins related to the TSPO-mediated mitophagy pathway in the mouse mesencephalon: it significantly upregulated the expression of TSPO, voltage-dependent anion channel 1, and PTEN-induced putative kinase 1, and downregulated parkin expression. Wuling capsules also exerted neuroprotective effects by increasing the expression of Beclin 1 and kinesin family member C2 in the mesencephalon, and increased the relative integrated optical density of synaptogyrin 3-positive nerve fibers in the ventral striatum.90 Furthermore, wuling capsules exerted an antidepressant effect in rats exposed to CMS, via the l-arginine–nitric oxide–cyclic guanosine monophosphate pathway. After 4 weeks of administration, wuling capsules (at 1 and 2 g/kg) increased sucrose preference, open-field activity, and food consumption in rats exposed to CMS. Furthermore, these antidepressant effects were suppressed by pretreatment with l-arginine and sildenafil, and enhanced by pretreatment with 7-nitroindazole and methylene blue.91
Indications and usage
Wuling capsules tonify the kidney and brain, nourish the heart, and tranquilize the mind. According to the syndrome-differentiation theory of TCM, they treat heart–kidney disharmony or heart–spleen deficiency, which present with various symptoms, such as insomnia, dreaminess, amnesia, palpitations, listlessness, soreness and weakness of waist and knees, dizziness, tinnitus, thin and white tongue fur, and deep, weak, and wiry pulse.92 According to modern diagnostic criteria, wuling is suitable for neurasthenia and fatigue syndrome, and it can enhance immunity function and ameliorate the fatigue caused by chemotherapy or blood loss. It is used to treat such conditions as hemorrhagic anemia, irregular menstruation, climacteric syndrome, and benign prostatic hyperplasia.93
Precautions and side effects
Cigarettes, alcohol, and spicy or greasy food should be avoided while taking this formula. Wuling capsules are a safe drug with mild side effects, including diarrhea, dry mouth, dizziness, fatigue, abdominal discomfort, elevated liver enzymes, constipation, increased saliva, anterior thoracic discomfort, skin rash, and sneezing.87,94
Dosage
Three capsules (0.33 g each) taken orally, three times daily for 20 continuous days. The dosage may be doubled if necessary.
Xiaoyaosan
Composition
Chaihu (bupleuri radix) 9 g, “danggui” (Angelica sinensis radix) 9 g, “baishao” (Paeonia lactiflora radix) 9 g, fuling (Poria) 9 g, baizhu (Atractylodes macrocephala rhizome) 9 g, bohe (Mentha haplocalyx) 3 g, shengjiang (Zingiber officinale rhizome) 6 g, zhigancao (Glycyrrhiza radix preparation) 4.5 g.
Chemical constituents
Gallic acid, catechin, albiflorin, paeoniflorin, liquiritin apioside, liquiritin, ferulic acid,95 volatile components (Z-ligustilide, palmitic acid, atractylenolide I, atractylenolide II),96 isorhamnetin.97
Pharmacology and bioactivity
Anxiolytic and antidepressant components of “xiaoyaosan” (XYS) can be isolated using a room-temperature superextraction system.98 XYS treated in this way (300 μg/mL) reduces the reserpine-induced death rate of glial cells more than either untreated XYS (300 μg/mL) or fluoxetine (30 μM) at 24 hours after application.98
A systematic review of 26 randomized trials (1,837 patients) showed that XYS combined with antidepressants was more effective than antidepressants alone in decreasing HAM-D and self-rating depression-scale scores, with no adverse effects.99 Petroleum ether extracts of XYS were the most effective when compared to 30%, 60%, and 95% ethanol extracts and anhydrous ethanol residue extracts, suggesting that the lipophilic constituents of the formula contribute most to its antidepressant effects.100 XYS exerts an antidepressant effect via α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) receptor-related synaptic plasticity of the hippocampus. XYS ameliorates behavioral changes and ultrastructural damage to the hippocampal CA1 region, and also reverses chronic decreases in GluR2 mRNA and increases in GluR1 mRNA in the CA1 after immobilization stress.101 It may also act via cerebral 5-HT1A-receptor activation98 and the inhibition of locus coeruleus NE neuron activity. XYS decreases serum NE concentrations and protein levels of tyrosine hydroxylase, dopamine-β-hydroxylase, and corticotrophin-releasing factor in the locus coeruleus of rat models of depression.102 Furthermore, XYS ameliorates HPA hyperactivation by downregulating corticosterone and urocortin 2. It reduces the level of serine/threonine-protein phosphatase 2A regulatory subunit B and increases β-arrestin 2, BDNF, mTOR, and tyrosine receptor kinase B.97 Another study found that the CNP–NPR2 pathway is upregulated in the rectum of rat models of depression; XYS partially antagonizes this pathway by inhibiting rectal tissue expression of CNP and NPR2.103
Changes in endogenous metabolites in depressed patients have been measured before and after treatment with XYS using dynamic urinalysis and plasma metabolomics based on 1H nuclear magnetic resonance. After XYS treatment, urinary and plasma measures revealed metabolic changes in energy, tryptophan, taurine, and gut microbes.104,105 Quantitative urinary metabolomics using gas chromatography–mass spectrometry provided further support that the antidepressant action of XYS involves the regulation of neurotransmitter and amino acid metabolism and promotion of energy production in depressed patients.106
Indications and usage
XYS soothes the liver and strengthens the spleen, nourishes the blood, and regulates menstruation. It is used to treat the syndrome of liver stagnation with blood deficiency, as well as spleen weakness (manifesting as pain, headache, dizziness, dry mouth and throat, spiritlessness, anorexia, irregular menstruation, breast-distending pain, and wiry and weak pulse).92
Side effects
Modified XYS can cause headache, dizziness, fatigue, and mild diarrhea.99
Dosage
Decocted in water for oral use, or taken in its powder form, twice daily. The decoction shows better effects than the capsule at the same dosage, whereas the capsule is more effective than the pill at the same dosage.107
Yueju
Composition
“Cangzhu” (Atractylodes rhizome) 6 g, xiangfu (Cyperus rhizome) 6 g, chuanxiong (Ligusticum chuanxiong rhizome) 6 g, “shenqu” (medicated leaven) 6 g, “zhizi” (Gardenia fructus) 6 g.
Chemical constituents
Gardenoside,108 ligustilide, α-cyperone, atractylodin.109
Pharmacology and bioactivity
A randomized double-blind, fluoxetine-adjunct, placebo-controlled, pilot clinical study in patients with MDD indicated that the “yueju” pill contributes to fast-onset antidepressant effects. Patients taking yueju (23 g/day) + fluoxetine (20 mg/day; ten patients) showed a significant decrease in HAM-D score compared with baseline or patients taking placebo + fluoxetine (20 mg/day; eight patients) on days 3–7 of treatment, while placebo + fluoxetine had no significant changes in HAM-D score over the first 7 days of treatment.18
Yueju ethanol extract (YJ-E) decreases the duration of immobility in the TST and FST in mouse models of depression, whereas the water extract has no notable effects.110 The petroleum ether fraction and n-butanol fraction also appear to be active fractions of YJ-E.111 YJ-XCC1Z3 (a type of YJ-E) shows potent antidepressant effects by improving depression-like behavior in mice and decreasing 5-HT metabolism.112 In imprinting control region (ICR) mice, acute administration of YJ-E rapidly alleviates depression-like behavior in the TST, and this effect is maintained for at least 24 hours. Additionally, YJ-E rapidly increases the expression of BDNF in the hippocampus, comparably to ketamine, although mRNA expression remains unchanged. YJ-E quickly increases BDNF synthesis by decreasing phosphorylation levels of EEF2. Yueju sustains both BDNF expression and eEF2 phosphorylation for 24 hours after administration, which was longer than ketamine.113 The NMDAR subunit NR1 and the Akt–mTOR pathway are important therapeutic targets of yueju for depression. An improvement in the sucrose-preference test was observed as early as 2 hours after administration of yueju and ketamine, but lasted for 6 days only with yueju and 2 days with ketamine. In Kunming mice exposed to CMS, both yueju and ketamine increased phosphorylation of the mTOR effectors 4E-BP1 and p70S6K, their upstream regulators ERK and Akt, and downstream targets, including synaptic protein GluR1, 2 days after drug administration, but only p-Akt remained upregulated with yueju 6 days after drug administration. Both yueju and ketamine decreased NR1 expression 2 days after administration, but by day 6 this effect was maintained only by yueju.114
Another study in Kunming mice showed that both yueju and ketamine decreased immobility time in the FST and hippocampal NR1 expression from 30 minutes to 5 days after a single administration (2.7 g/kg). Both also reduced hippocampal NR2B protein expression at 30 minutes, which returned to baseline levels within 24 hours, whereas NR2A expression was decreased only by ketamine at 30 minutes. Yueju relieved depression-like symptoms in a learned-helplessness model in mice for at least 3 days after administration, and decreased NR1 expression in mouse models of depression at 1 or 3 days after administration. Antidepressant effects were not influenced by blockade of the AMPA receptor, indicating that the mechanism of action of yueju is different from that of ketamine.115 Furthermore, prolonged increases in hippocampal BDNF may account for the enduring and strain-dependent antidepressant responses to yueju and ketamine, but only yueju was mediated via the PKA–CREB pathway. Yueju and ketamine upregulated hippocampal expression of total and phosphorylated CREB, as well as the CREB-signaling activator PKA 1 day after administration in Kunming but not ICR mice. Yueju and ketamine increased BDNF gene expression at 3 hours in both strains, but this was sustained for only 1 day in Kunming mice only. However, blockade of the PKA–CREB pathway reversed the increase in BDNF gene expression and the antidepressant effects of yueju but not ketamine. Conversely, the mTOR inhibitor rapamycin reversed the antidepressant effects of ketamine but not yueju.116
Indications and usage
Yueju regulates chi, relieves depression, regulates the function of the middle jiao, and resolves chi stagnation. It is used to treat the syndrome of six stagnations (chi, dampness, fire, phlegm, blood, and food), manifesting as fullness and stuffiness in the chest and abdomen, dietetic stagnation, belching, and acid regurgitation.92
Side effects
Gastrointestinal symptoms, such as diarrhea and constipation.18
Dosage
All herbs can be crushed into a fine powder, sieved, mixed, then made into pills with water; 6–9 g of the pills should be taken twice daily. Alternatively, the herbs can be decocted in water for oral administration.92
Discussion
A literature search identified nine CHFs with evidence-based antidepressant effects: BHD, CSS, GMDZD, KXS, shuganjieyu capsules, SNS, wuling capsules, XYS, and yueju. The present study is the first to describe these formulas in terms of source, TCM syndromes and effects, composition, chemical constituents, clinical trials, antidepressant mechanisms, indications, and side effects. This information will help to guide treatment of depression, and will be of value to neurologists, neuroscientists, and those interested in CHFs.
Depression is a serious affective disorder characterized by persistent low mood with frequent residual depressive symptoms and relapse.117 Furthermore, there is a significant association between depression and excess mortality.118 Despite considerable research efforts, antidepressant treatments remain unsatisfactory. Existing Western medicines for MDD have a delayed onset of therapeutic action, severe side effects, withdrawal symptoms, and even treatment-related suicidal behavior.24 The NMDAR antagonist ketamine, recently indicated as an antidepressant, has consistently shown antidepressant effects within a few hours after administration (intranasal or intramuscular).119 However, many obstacles remain before it can gain widespread clinical approval.120 Ketamine is an anesthetic with neurotoxic and hallucinogenic side effects, such as visual or auditory hallucinations, out-of-body experiences, or abnormal sensations.121 Its long-term use is associated with cardiotoxicity122 and impairments in neurocognitive function and psychological well-being.123 Abuse potential and addictive properties are significant barriers to its widespread use.120 There is an increasing need to develop a practical and effective antidepressant, due to the growing prevalence of depression worldwide. A search on the Web of Science shows that the number of reports on antidepressant CHFs is growing every year, revealing that the subject is drawing increasing attention, likely due to treatment efficacy and minimal side effects.
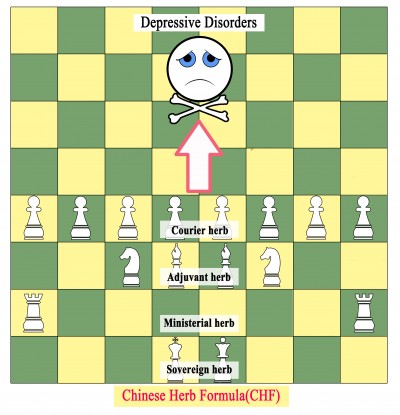
A characteristic of Chinese herbal medicines is their use as formulas rather than single herbs. Thousands of years ago, TCM practitioners generally used a single herb for disease treatment, but this was increased to two or more herbs, constituting a formula, to achieve additive or synergistic actions and reduce adverse effects.124,125 As clinical experience in Chinese herbal medicine accumulates, practitioners modify their CHFs based on the holistic philosophy and syndrome differentiation of TCM, while carefully following the rules of herb synergism and compatibility.126 Each single herb has its own flavor and channel tropism, whereas typical CHFs are mostly built on the “sovereign, minister, assistant, courier” rule.127 According to the seven methods (“emotions”) in prescription compatibility, mutual promotion and mutual enhancement can synergistically enhance curative effects, and mutual restraint and mutual detoxification can alleviate toxicity and adverse effects.128,129 An increasing number of studies have demonstrated the advantages of formula completeness and herb compatibility, such as comprehensive disease intervention,130 favorable pharmacokinetics,131,132 alleviation of drastic effects, and fewer adverse effects than single herbs.133 Owing to the “sovereign, minister, assistant, courier” rule and the seven methods in prescription compatibility, CHFs are more commonly used than single herbs for disease treatment, and have become the predominant application of TCM.131,134 As illustrated in Figure 2, the relationship between CHFs and MDDs can be likened to playing chess. A single herb represents a chess piece, which cannot win the game single-handedly; however, when different herbs are combined to create a formula, their effectiveness in the treatment of depression becomes apparent.
Most CHFs have liver-soothing and depression-relieving effects. A systematic review revealed that the most common TCM-syndrome pattern of depression was liver-chi depression, followed by liver depression, spleen deficiency, and dual deficiency of the heart and spleen. In addition, XYS was the most commonly used prescription for liver-chi stagnation and liver stagnation with spleen deficiency, whereas CSS was usually used for liver-chi stagnation.25 An analysis based on the TCM inheritance support platform software V2.0 compiled 358 TCM prescriptions for depression from modern literature databases. The main effects of these prescriptions were soothing the liver and relieving depression, strengthening the spleen and resolving phlegm, activating and enriching blood, clearing and purging heat, and tranquilizing and sedating the mind; these follow the principle of “regulating chi, relieving depression, and empathy”.26
Multicomponent CHFs exert their effects via multitarget and multipathway mechanisms.80,135,136 Antidepressant CHFs comprise a series of active compounds, including terpenoids, flavonoids, xanthones, phenylpropanoids, and phenols, providing a shortcut to finding novel antidepressant agents.137 In vitro and in vivo pharmacology and bioactivity studies of CHFs in combination with conventional antidepressants have indicated that CHFs not only enhance the antidepressant response and speed of onset18,48 but also reduce adverse effects of traditional psychotherapeutic drugs in patients with severe depression.27,138 CHFs have a multitude of biological actions, including effects on reuptake and receptor binding of various monoamines (eg, NE, 5-HT) and endocrine and neuropsychoimmunological modulation.139 CHFs regulate dysfunctions of the hypothalamic–pituitary–adrenal, –thyroid, and –gonadal axes and locus coeruleus NE system, as well as improving impairments in endogenous metabolic processes. Constructing a disease-related compound–target pathway network can help elucidate the antidepressant mechanism of CHFs.140 Future studies should no longer focus on single pathways, but include multiple pathways and networks.
There is an urgent need for more and higher-quality phytochemical and pharmacological studies of these nine CHFs. Many chemical constituents have been detected and reported for most of the CHFs, but reports relating to shuganjieyu and wuling capsules have been published in Chinese only, which cannot be understood worldwide. Furthermore, to date, few chemical constituents have been identified in BHD, GMDZD, or yueju. Various bioactive chemical constituents are expected to be identified by chromatography, mass spectrometry, and nuclear magnetic resonance. Studies of the pharmacological mechanisms of these constituents are also needed. Today’s high-throughput screening technology enables us to explore bioactivity and mechanisms rapidly, resulting in a high discovery rate of candidate drugs.
There is evidence to support the effective and safe use of these nine CHFs in the treatment of depression, with demonstrations of better efficacy than placebo and synergistically increased effects of conventional therapy. However, some evidence should be interpreted with caution. First, whereas BHD, KXS, and yueju have been investigated in RCTs, and CSS, GMDZD, shuganjieyu and wuling capsules, and XYS have been the subject of meta-analyses or systematic reviews, SNS has not been included in any such systematic studies, because of its variations in formula. Second, most clinical trials of these formulas have been published in Chinese, and the randomization and blinding methods were not clearly reported. Third, only four trials have registered online at https://ClinicalTrials.gov (for wuling capsules, XYS, and two for shuganjieyu capsules), and only two multicenter RCTs were identified (for shuganjieyu and wuling capsules).72,87 No large-scale RCTs have been performed. Moreover, there was considerable variation between the formulas used in the trials: only a few used the traditional formulas, whereas most used a modification of the original formula, adding or subtracting several herbs based on patients’ symptoms. Although this does not change the “sovereign, minister, assistant, courier” structure of the traditional formulas, experimental evidence is needed to support the use of added or subtracted herbs. In addition, a systematic review and meta-analysis of 13 RCTs concluded that a previous meta-analysis of GMDZD for depression48 did not use a comprehensive search strategy. This latter review, with a high risk of bias, found no evidence that GMDZD showed a significant antidepressant effect in MDD, postsurgical depression, or geriatric depression; it was only deemed to have an effect in poststroke depression.141 Therefore, more large-scale and rigorous RCTs comparing the efficacy of each formula with placebo and established pharmacotherapy need to be registered and conducted to obtain robust evidence on the antidepressant effects of these formulas.
Conclusion
In the present study, we reviewed important data regarding the use of CHFs in the treatment of depression. In summary, diverse CHFs have been used for various types of depression for many years, with experimentally and clinically demonstrable effects. This review highlights the possibility of CHFs as evidence-based treatment options for depression, and could help modern TCM doctors prescribe appropriate CHFs for depressed patients. We have provided a comprehensive list of the constituents, clinical effects, putative mechanisms, TCM patterns, and use for each CHF. This research will benefit patients, practitioners, and researchers in the field of TCM. Further well-designed, well-reported, placebo-controlled randomized trials and psychopharmacological studies should be carried out to substantiate clinical claims of the antidepressant actions of CHFs and contribute to the building of a compound–target pathway network.
Acknowledgments
The authors would like to thank Zhi-qing Li for data collection and manuscript preparation. This review was supported by the National Natural Science Foundation of China (81403259, 81303074, 81173175, and 81673951).
Disclosure
The authors report no conflicts of interest in this work.
References
Patten SB, Williams JV, Lavorato DH, Wang JL, McDonald K, Bulloch AG. Descriptive epidemiology of major depressive disorder in Canada in 2012. Can J Psychiatry. 2015;60(1):23–30. | ||
Al-Hamzawi AO, Bruffaerts R, Bromet EJ, AlKhafaji AM, Kessler RC. The epidemiology of major depressive episode in the Iraqi general population. PLoS One. 2015;10(7):e0131937. | ||
Hankin BL. Depression from childhood through adolescence: risk mechanisms across multiple systems and levels of analysis. Curr Opin Psychol. 2015;4:13–20. | ||
Ferrari AJ, Somerville AJ, Baxter AJ, et al. Global variation in the prevalence and incidence of major depressive disorder: a systematic review of the epidemiological literature. Psychol Med. 2013;43(3):471–481. | ||
Global Burden of Disease Study 2013 collaborators. Global, regional, and national incidence, prevalence, and years lived with disability for 301 acute and chronic diseases and injuries in 188 countries, 1990–2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet. 2015;386(9995):743–800. | ||
Walker ER, McGee RE, Druss BG. Mortality in mental disorders and global disease burden implications: a systematic review and meta-analysis. JAMA Psychiatry. 2015;72(4):334–341. | ||
Greenberg PE, Fournier A, Sisitsky T, Pike CT, Kessler RC. The economic burden of adults with major depressive disorder in the United States (2005 and 2010). J Clin Psychiatry. 2015;76(2):115–155. | ||
Kessler RC, Bromet EJ. The epidemiology of depression across cultures. Annu Rev Public Health. 2013;34:119–138. | ||
Zhong BL, Liu TB, Chan SS, et al. Prevalence and correlates of major depressive disorder among rural-to-urban migrant workers in Shenzhen, China. J Affect Disord. 2015;183:1–9. | ||
Li D, Zhang DJ, Shao JJ, Qi XD, Tian L. A meta-analysis of the prevalence of depressive symptoms in Chinese older adults. Arch Gerontol Geriatr. 2014;58(1):1–9. | ||
Simon GE, Savarino J, Operskalski B, Wang PS. Suicide risk during antidepressant treatment. Am J Psychiatry. 2006;163(1):41–47. | ||
Zarate CA, Singh JB, Carlson PJ, et al. A randomized trial of an N-methyl-D-aspartate antagonist in treatment-resistant major depression. Arch Gen Psychiatry. 2006;63(8):856–864. | ||
Machado-Vieira R, Salvadore G, Diazgranados N, Zarate CA. Ketamine and the next generation of antidepressants with a rapid onset of action. Pharmacol Ther. 2009;123(2):143–150. | ||
Monteggia LM, Malenka RC, Deisseroth K. Depression: the best way forward. Nature. 2014;515(7526):200–201. | ||
Plesničar BK. Efficacy and tolerability of agomelatine in the treatment of depression. Patient Prefer Adherence. 2014;8:603–612. | ||
Hickie IB, Rogers NL. Novel melatonin-based therapies: potential advances in the treatment of major depression. Lancet. 2011;378(9791):621–631. | ||
Liu J, Fang Y, Yang L, Qin X, Du G, Gao X. A qualitative, and quantitative determination and pharmacokinetic study of four polyacetylenes from radix bupleuri by UPLC-PDA-MS. J Pharm Biomed Anal. 2015;111:257–265. | ||
Wu R, Zhu D, Xia Y, et al. A role of yueju in fast-onset antidepressant action on major depressive disorder and serum BDNF expression: a randomly double-blind, fluoxetine-adjunct, placebo-controlled, pilot clinical study. Neuropsychiatr Dis Treat. 2015;11:2013–2021. | ||
Qu M, Tang QS. [Investigation on the relationship between depression and TCM depression syndrome]. Beijing Zhong Yi Yao Da Xue Xue Bao. 2004;27(1):11–13. Chinese. | ||
Chang HS, Duan XH, Liang JC, Wang QG. [Origin of TCM doctrine of depression syndrome]. Beijing Zhong Yi Yao Da Xue Xue Bao. 2011;34(10):653–658, 661. Chinese. | ||
Qu F, Cai X, Gu Y, et al. Chinese medicinal herbs in relieving perimenopausal depression: a randomized, controlled trial. J Altern Complement Med. 2009;15(1):93–100. | ||
Luo L, Wang JN, Kong LD, Jiang QG, Tan RX. Antidepressant effects of banxia houpu decoction, a traditional Chinese medicinal empirical formula. J Ethnopharmacol. 2000;73(1–2):277–281. | ||
Chen HY, Cho WC, Sze SC, Tong Y. Treatment of menopausal symptoms with Er-xian decoction: a systematic review. Am J Chin Med. 2008;36(2):233–244. | ||
Wang Y, Fan R, Huang X. Meta-analysis of the clinical effectiveness of traditional Chinese medicine formula chaihu-shugan-san in depression. J Ethnopharmacol. 2012;141(2):571–577. | ||
Yeung WF, Chung KF, Ng KY, et al. Prescription of Chinese herbal medicine in pattern-based traditional Chinese medicine treatment for depression: a systematic review. Evid Based Complement Alternat Med. 2015;2015:160189. | ||
Zhao YQ, Teng J, Yang HJ. [Analysis on medication regularity of modern traditional Chinese medicines in treating melancholia based on data mining technology]. Zhongguo Zhong Yao Za Zhi. 2015;40(10):2042–2046. Chinese. | ||
Yeung WF, Chung KF, Ng KY, Yu YM, Ziea ET, Ng BF. A systematic review on the efficacy, safety and types of Chinese herbal medicine for depression. J Psychiatr Res. 2014;57:165–175. | ||
Wang DL. [Target component analysis of banxia houpo decoction]. Chin Tradit Pat Med. 1991;13(10):47. Chinese. | ||
Xu Q, Ou Yang Z, Chang Y, Fu DS, Kong LD. [Principal, assistant, compleaent [sic], guide compatibility of banxiahoupo decoction on contents of honokioi and magnolol]. Zhongguo Shi Yan Fang Ji Xue Za Zhi. 2008;14(10):1–3. Chinese. | ||
Xu Q, Wu LL, Wang CP, Wang SJ, Kong LD. [Effects of different compatibility of banxiahoupu decoction on changes of volatile oils by GC-MS]. Zhongguo Shi Yan Fang Ji Xue Za Zhi. 2009;15(2):5–10. Chinese. | ||
Guo Y, Kong L, Wang Y, Huang Z. Antidepressant evaluation of polysaccharides from a Chinese herbal medicine banxia-houpu decoction. Phytother Res. 2004;18(3):204–207. | ||
Bo P, Chen QM, Zhu HH, et al. Clinical observations on 46 cases of globus hystericus treated with modified banxia houpu decoction. J Tradit Chin Med. 2010;30(2):103–107. | ||
Yi LT, Zhang L, Ding AW, Xu Q, Zhu Q, Kong LD. Orthogonal array design for antidepressant compatibility of polysaccharides from banxia-houpu decoction, a traditional Chinese herb prescription in the mouse models of depression. Arch Pharm Res. 2009;32(10):1417–1423. | ||
Wang Y, Kong L, Chen Y. Behavioral and biochemical effects of fractions prepared from banxia houpu decoction in depression models in mice. Phytother Res. 2005;19(6):526–529. | ||
Zhang W, Li J, Zhu J, Shi Z, Wang Y, Kong L. Chinese medicine banxia-houpu decoction regulates c-Fos expression in the brain regions in chronic mild stress model in rats. Phytother Res. 2004;18(3):200–203. | ||
Li JM, Kong LD, Wang YM, Cheng CH, Zhang WY, Tan WZ. Behavioral and biochemical studies on chronic mild stress models in rats treated with a Chinese traditional prescription banxia-houpu decoction. Life Sci. 2003;74(1):55–73. | ||
Ma Z, Ji W, Qu R, et al. Metabonomic study on the antidepressant-like effects of banxia houpu decoction and its action mechanism. Evid Based Complement Alternat Med. 2013;2013:213739. | ||
Su ZH, Zou GA, Preiss A, Zhang HW, Zou ZM. Online identification of the antioxidant constituents of traditional Chinese medicine formula chaihu-shu-gan-san by LC-LTQ-Orbitrap mass spectrometry and microplate spectrophotometer. J Pharm Biomed Anal. 2010;53(3):454–461. | ||
Qiu XJ, Huang X, Chena ZQ, Ren P. Simultaneous determination of 16 chemical constituents in the traditional Chinese medicinal prescription si-ni-san and chaihu-shugan-san by ultra performance liquid chromatography coupled with photodiode array detection. Asian J Chem. 2011;23(12):5301–5307. | ||
Li SQ, Su ZH, Peng JB, Zou ZM, Yu CY. In vitro and in vivo antioxidant effects and the possible relationship between the antidepression efficacy of traditional Chinese medicine formulation chaihu shugan san. Chin J Nat Med. 2010;8(5):353–361. | ||
Qiu J, Hu SY, Shi GQ, Wang SE. Changes in regional cerebral blood flow with chaihu-shugan-san in the treatment of major depression. Pharmacogn Mag. 2014;10(40):503–508. | ||
Wang S, Hu S, Zhang C, Qiu J, Li Y. Antidepressant-like activity of chaihu-shugan-san aqueous extract in rats and its possible mechanism. Pharmacogn Mag. 2014;10 Suppl 1:S50–S56. | ||
Chen S, Asakawa T, Ding S, et al. Chaihu-shugan-san administration ameliorates perimenopausal anxiety and depression in rats. PLoS One. 2013;8(8):e72428. | ||
Li YH, Zhang CH, Qiu J, et al. Antidepressant-like effects of chaihu-shugan-san via SAPK/JNK signal transduction in rat models of depression. Pharmacogn Mag. 2014;10(39):271–277. | ||
Yang P, Li L, Liu XJ, et al. Effect of chaihu-shugan-san on the mRNA expression of the 5-HT1A receptor and cellular proliferation in the hippocampus of epileptic rats with depression. Exp Ther Med. 2016;11(1):124–130. | ||
Su ZH, Li SQ, Zou GA, et al. Urinary metabonomics study of anti-depressive effect of chaihu-shu-gan-san on an experimental model of depression induced by chronic variable stress in rats. J Pharm Biomed Anal. 2011;55(3):533–539. | ||
Qiu J, Hu SY, Zhang CH, et al. The effect of chaihu-shugan-san and its components on the expression of ERK5 in the hippocampus of depressed rats. J Ethnopharmacol. 2014;152(2):320–326. | ||
Yeung WF, Chung KF, Ng KY, Shi GQ, Wang SE, Xiang T. A meta-analysis of the efficacy and safety of traditional Chinese medicine formula ganmai dazao decoction for depression. J Ethnopharmacol. 2014;153(2):309–317. | ||
Ma XJ, Zhao J, Feng ZY, et al. [Effects of modified ganmai dazao decoction on neuroendocrine system in patients with climacteric depression]. Zhongguo Zhong Yao Za Zhi. 2014;39(23):4680–4684. Chinese. | ||
Lou JS, Li CY, Yang XC, Fang J, Yang YX, Guo JY. Protective effect of gan mai da zao decoction in unpredictable chronic mild stress-induced behavioral and biochemical alterations. Pharm Biol. 2010;48(12):1328–1336. | ||
El-Alfy AT, Abourashed EA, Matsumoto RR. Nature against depression. Curr Med Chem. 2012;19(14):2229–2241. | ||
Zhou XY, Qin XD, Geng LF. [Ganmai dazao decoction in clinical observation on treatment of 30 cases of post stroke depression]. J Sichuan Tradit Chin Med. 2009;27(10):75–76. Chinese. | ||
Zhu KY, Fu Q, Xie HQ, et al. Quality assessment of a formulated Chinese herbal decoction, kaixinsan, by using rapid resolution liquid chromatography coupled with mass spectrometry: a chemical evaluation of different historical formulae. J Sep Sci. 2010;33(23–24):3666–3674. | ||
Zhang X, Li Q, Lv C, et al. Characterization of multiple constituents in kai-xin-san prescription and rat plasma after oral administration by liquid chromatography with quadrupole time-of-flight tandem mass spectrometry. J Sep Sci. 2015;38(12):2068–2075. | ||
Liu JY, Xiao SY, Shang WF, Xu LZ, Yang SL. A new minor triterpene saponin from kaixin-san prescription. J Asian Nat Prod Res. 2005;7(4):643–648. | ||
Zhu KY, Xu SL, Choi RC, et al. Kai-xin-san, a Chinese herbal decoction containing ginseng radix et rhizoma, polygalae radix, acori tatarinowii rhizoma, and poria, stimulates the expression and secretion of neurotrophic factors in cultured astrocytes. Evid Based Complement Alternat Med. 2013;2013:731385. | ||
Hu Y, Zhou XJ, Liu P, et al. Antidepressant and neuroprotective effect of the Chinese herb kaixinsan against lentiviral shRNA knockdown brain-derived neurotrophic factor-induced injury in vitro and in vivo. Neuropsychobiology. 2014;69(3):129–139. | ||
Yan L, Xu SL, Zhu KY, et al. Optimizing the compatibility of paired-herb in an ancient Chinese herbal decoction kai-xin-san in activating neurofilament expression in cultured PC12 cells. J Ethnopharmacol. 2015;162:155–162. | ||
Bao ZX, Zhao GP, Sun W, Chen BJ. [Clinical curative effects of kaixin powder on depression with mild or moderate degree]. Chin Arch Tradit Chin Med. 2011;28(5):987–988. Chinese. | ||
Dang H, Sun L, Liu X, et al. Preventive action of kai xin san aqueous extract on depressive-like symptoms and cognition deficit induced by chronic mild stress. Exp Biol Med (Maywood). 2009;234(7):785–793. | ||
Zhu KY, Mao QQ, Ip SP, et al. A standardized Chinese herbal decoction, kai-xin-san, restores decreased levels of neurotransmitters and neurotrophic factors in the brain of chronic stress-induced depressive rats. Evid Based Complement Alternat Med. 2012;2012:149256. | ||
Dong XZ, Li ZL, Zheng XL, Mu LH, Zhang GQ, Liu P. A representative prescription for emotional disease, ding-zhi-xiao-wan restores 5-HT system deficit through interfering the synthesis and transshipment in chronic mild stress-induced depressive rats. J Ethnopharmacol. 2013;150(3):1053–1061. | ||
Cai C, Qian GQ, Zhao GP, Peng X, Liang XB. [Study on regulatory effect of kaixin san on endogenous melatonin biosynthesis in rat depression model]. Zhongguo Zhong Yao Za Zhi. 2012;37(11):1638–1641. Chinese. | ||
Huang YL, Liang XB, Qian LQ, et al. Effects of kaixin powder on melatonin receptor expression and 125I-Mel binding affinity in a rat model of depression. Chin J Integr Med. 2015;21(7):507–515. | ||
Mu LH, Huang ZX, Liu P, Hu Y, Gao Y. Acute and subchronic oral toxicity assessment of the herbal formula kai-xin-san. J Ethnopharmacol. 2011;138(2):351–357. | ||
Qiao HY, Luo R, Wu J, et al. [UPLC-MS fingerprint of shugan jieyu capsules]. Zhong Cao Yao. 2014;45(2):208–213. Chinese. | ||
Huang L, Chen LJ, Liu LL, et al. [A systematic review of therapeutic efficacy and safety of shugan jieyu capsules in the treatment of mild to moderate depression]. China Pharm. 2013;24(32):3043–3046. Chinese. | ||
Zhang X, Kang D, Zhang L, Peng L. Shuganjieyu capsule for major depressive disorder (MDD) in adults: a systematic review. Aging Ment Health. 2014;18(8):941–953. | ||
Xie M, Jiang W, Yang H. Efficacy and safety of the Chinese herbal medicine shuganjieyu with and without adjunctive repetitive transcranial magnetic stimulation (rTMS) for geriatric depression: a randomized controlled trial. Shanghai Arch Psychiatry. 2015;27(2):103–110. | ||
Fu JH, Liu Y, Wang QY, Zhao JP. [Effect of shuganjieyu capsules on neuronal apoptosis in hippocampal CA3 area and the expression of caspase-3 in the brain of rat depression model]. Zhong Nan Da Xue Xue Bao Yi Xue Ban. 2012;37(12):1198–1204. Chinese. | ||
Fu J, Zhang Y, Wu R, et al. Shuganjieyu capsule increases neurotrophic factor expression in a rat model of depression. Neural Regen Res. 2014;9(5):489–497. | ||
Yao J, Li QW, Ji JL, et al. [Efficacy and safety of different doses of shuganjieyu capsules in treating mild or moderate depressive disorder: a multicenter, random, open and controlled trial]. Zhongguo Xin Yao Yu Lin Chuan Za Zhi. 2014;33(8):568–572. Chinese. | ||
Guo R, Shu Y, Zhang L, Cao Y, Ding A, Yao W. [Analysis of chemical composition of sini san by ultra-performance liquid chromatography-electrospray ionization-mass spectrometry]. Zhongguo Zhong Yao Za Zhi. 2011;36(22):3114–3118. Chinese. | ||
Qiao Y, Wen J, Song Y, Lu X, Xiong Z, Li F. [Study on in vitro and in vivo material base of Sini San by UPLC-PDA-MS/MS]. Zhongguo Zhong Yao Za Zhi. 2012;37(11):1672–1676. Chinese. | ||
Yan Z, Chen Y, Li T, Zhang J, Yang X. Identification of metabolites of si-ni-san, a traditional Chinese medicine formula, in rat plasma and urine using liquid chromatography/diode array detection/triple-quadrupole spectrometry. J Chromatogr B Analyt Technol Biomed Life Sci. 2012;885–886:73–82. | ||
Wang YT, Tan QR, Sun LL, et al. Possible therapeutic effect of a traditional Chinese medicine, sinisan, on chronic restraint stress related disorders. Neurosci Lett. 2009;449(3):215–219. | ||
Li Y, Sun Y, Ma X, et al. Effects of sini san used alone and in combination with fluoxetine on central and peripheral 5-HT levels in a rat model of depression. J Tradit Chin Med. 2013;33(5):674–681. | ||
Qin L. [Study on the antidepressant mechanism of sini san]. Dang Dai Yi Xue. 2010;16(14):29–30. Chinese. | ||
Yi LT, Li J, Liu BB, Li CF. Screening of the antidepressant-like effect of the traditional Chinese medicinal formula si-ni-san and their possible mechanism of action in mice. Pharmacognosy Res. 2013;5(1):36–42. | ||
Wang HH, Zhang BX, Ye XT, He SB, Zhang YL, Wang Y. [Study on mechanism for anti-depression efficacy of sini san through auxiliary mechanism elucidation system for Chinese medicine]. Zhongguo Zhong Yao Za Zhi. 2015;40(19):3723–3728. Chinese. | ||
Lu JX, Zhu M, Chen Y, Zhang P, Fang L. [HPLC specific chromatogram of chemical constituents of wuling capsules]. Yao Wu Fen Xi Za Zhi. 2011;4:764–767. Chinese. | ||
Chen Y, Lu J, Zhu M, Luo L. [Determination of multiple constituents in wuling capsules by HPLC simultaneously]. Zhongguo Zhong Yao Za Zhi. 2012;37(2):218–221. Chinese. | ||
Lu JX, Lei L, Chen Y, Chen J, Zhu M. [Chemical constituents of wuling fermentative powder]. Zhongguo Xian Dai Ying Yong Yao Xue. 2014;31(5):541–543. Chinese. | ||
He XR, Liu P. [Determination of 14 kinds of amino acids in wuling capsule by HPLC-FLD]. Chin Tradit Pat Med. 2010;32(8):1358–1361. Chinese. | ||
Peng L, Zhang X, Kang DY, Liu XT, Hong Q. Effectiveness and safety of wuling capsule for post stroke depression: a systematic review. Complement Ther Med. 2014;22(3):549–566. | ||
Wang XJ, Li J, Zou QD, Jin L. [Wuling capsule for climacteric patients with depression and anxiety state: a randomized, positive parallel controlled trial]. Zhong Xi Yi Jie He Xue Bao. 2009;7(11):1042–1046. Chinese. | ||
Peng WF, Wang X, Hong Z, et al. The anti-depression effect of Xylaria nigripes in patients with epilepsy: a multicenter randomized double-blind study. Seizure. 2015;29:26–33. | ||
Li DQ, Li XJ, Duan JF, Cai W. Wuling capsule promotes hippocampal neurogenesis by improving expression of connexin 43 in rats exposed to chronic unpredictable mild stress. Zhong Xi Yi Jie He Xue Bao. 2010;8(7):662–669. | ||
Li DQ, Li XJ, Duan JF, Cai W. Effects of wuling capsule on hippocampal dependent cognitive changes in post-stroke depression rats. Int J Pharmacol. 2011;7(1):50–57. | ||
Li D, Zheng J, Wang M, et al. Wuling powder prevents the depression-like behavior in learned helplessness mice model through improving the TSPO mediated-mitophagy. J Ethnopharmacol. 2016;186:181–188. | ||
Tan YF, Liao ZL, Qiu YJ, Zhu JP, Yu EY. Possible involvement of L-arginine-nitric oxide (NO)-cyclic guanosine monophosphate (cGMP) signaling pathway in the antidepressant-like effect of wuling mycelia powder in rat. Biomed Pharmacother. 2016;78:60–65. | ||
Pharmacopoeia Commission of the People’s Republic of China. Pharmacopoeia of the People’s Republic of China. Beijing: China Medical Science Press; 2015. | ||
Liao ML, Yu J. [Wuling capsule]. Zhongguo Xin Yao Za Zhi. 2000;9(11):797. Chinese. | ||
Han J, Gao SQ. [The clinical applications and adverse effects of wuling capsule]. China Pharm. 2007;18(15):1184–1186. Chinese. | ||
Gao XX, Cui J, Zheng XY, et al. An investigation of the antidepressant action of xiaoyaosan in rats using ultra performance liquid chromatography-mass spectrometry combined with metabonomics. Phytother Res. 2013;27(7):1074–1085. | ||
Zhou Y, Ren Y, Ma Z, et al. Identification and quantification of the major volatile constituents in antidepressant active fraction of xiaoyaosan by gas chromatography-mass spectrometry. J Ethnopharmacol. 2012;141(1):187–192. | ||
Zhu X, Xia O, Han W, et al. Xiao yao san improves depressive-like behavior in rats through modulation of β-arrestin 2-mediated pathways in hippocampus. Evid Based Complement Alternat Med. 2014;2014:902516. | ||
Yin SH, Wang CC, Cheng TJ, et al. Room-temperature super-extraction system (RTSES) optimizes the anxiolytic- and antidepressant-like behavioral effects of traditional xiao-yao-san in mice. Chin Med. 2012;7(1):24. | ||
Zhang Y, Han M, Liu Z, Wang J, He Q, Liu J. Chinese herbal formula xiao yao san for treatment of depression: a systematic review of randomized controlled trials. Evid Based Complement Alternat Med. 2012;2012:931636. | ||
Zhou Y, Lu L, Li Z, et al. Antidepressant-like effects of the fractions of xiaoyaosan on rat model of chronic unpredictable mild stress. J Ethnopharmacol. 2011;137(1):236–244. | ||
Liang Y, Guo XL, Chen JX, Yue GX. Effects of the Chinese traditional prescription xiaoyaosan decoction on chronic immobilization stress-induced changes in behavior and ultrastructure in rat hippocampus. Evid Based Complement Alternat Med. 2013;2013:984797. | ||
Ding XF, Zhao XH, Tao Y, et al. Xiao yao san improves depressive-like behaviors in rats with chronic immobilization stress through modulation of locus coeruleus-norepinephrine system. Evid Based Complement Alternat Med. 2014;2014:605914. | ||
Li P, Tang XD, Cai ZX, et al. CNP signal pathway up-regulated in rectum of depressed rats and the interventional effect of xiaoyaosan. World J Gastroenterol. 2015;21(5):1518–1530. | ||
Tian JS, Peng GJ, Gao XX, et al. Dynamic analysis of the endogenous metabolites in depressed patients treated with TCM formula xiaoyaosan using urinary 1H NMR-based metabolomics. J Ethnopharmacol. 2014;158 Pt A:1–10. | ||
Liu CC, Wu YF, Feng GM, et al. Plasma-metabolite-biomarkers for the therapeutic response in depressed patients by the traditional Chinese medicine formula xiaoyaosan: a 1H NMR-based metabolomics approach. J Affect Disord. 2015;185:156–163. | ||
Tian JS, Peng GJ, Wu YF, et al. A GC-MS urinary quantitative metabolomics analysis in depressed patients treated with TCM formula of xiaoyaosan. J Chromatogr B Analyt Technol Biomed Life Sci. 2016;1026:227–235. | ||
Wang YH, Li B, Cai GX, Yang H. [Comparative study on effect of three preparations of xiaoyao formula on soothing liver and strengthening spleen]. Zhongguo Zhong Yao Za Zhi. 2012;37(19):2951–2955. Chinese. | ||
Zhu ZF, Zhang SY, Zhou GZ. [Identification of Gardenia jasminoides Ellis and its six Chinese patent medicine by TLC]. Chin Tradit Pat Med. 1985(12):31–32. Chinese. | ||
Di WF, Wei XH, Zhang SX, Wang ZT. [HPLC determination of ligustilide, α-cyperone and atrctylodin in YJ-XCC1Z3 extract of yueju pills]. Yao Wu Fen Xi Za Zhi. 2009;29(5):693–696. Chinese. | ||
Wei XH, Chen Y, Xia GX, Shen JS. [Antidepressant effect of “yueju pills” ethanol extract and water extract in mice models of depression]. Shang Hai Zhong Yi Yao Za Zhi. 2006;40(8):69–70. Chinese. | ||
Wei XH, Cheng XM, Shen JS, Wang ZT. Antidepressant effect of yueju-wan ethanol extract and its fractions in mice models of despair. J Ethnopharmacol. 2008;117(2):339–344. | ||
Wei XH, Chang HS, Zhai WF, Wang ZT. [Experimental studies on treatment of depression with YJ-XCC1Z3 in mouse models]. Zhongguo Zhong Yao Za Zhi. 2007;32(24):2628–2631. Chinese. | ||
Xue W, Zhou X, Yi N, et al. Yueju pill rapidly induces antidepressant-like effects and acutely enhances BDNF expression in mouse brain. Evid Based Complement Alternat Med. 2013;2013:184367. | ||
Tang J, Xue W, Xia B, et al. Involvement of normalized NMDA receptor and mTOR-related signaling in rapid antidepressant effects of yueju and ketamine on chronically stressed mice. Sci Rep. 2015;5:13573. | ||
Xia B, Zhang H, Xue W, et al. Instant and lasting down-regulation of NR1 expression in the hippocampus is associated temporally with antidepressant activity after acute yueju. Cell Mol Neurobiol. 2016;36(7):1189–1196. | ||
Xue W, Wang W, Gong T, et al. PKA-CREB-BDNF signaling regulated long lasting antidepressant activities of yueju but not ketamine. Sci Rep. 2016;6:26331. | ||
Willner P, Scheel-Krüger J, Belzung C. The neurobiology of depression and antidepressant action. Neurosci Biobehav Rev. 2013;37(10 Pt 1):2331–2371. | ||
Cuijpers P, Vogelzangs N, Twisk J, Kleiboer A, Li J, Penninx BW. Comprehensive meta-analysis of excess mortality in depression in the general community versus patients with specific illnesses. Am J Psychiatry. 2014;171(4):453–462. | ||
McGirr A, Berlim MT, Bond DJ, Fleck MP, Yatham LN, Lam RW. A systematic review and meta-analysis of randomized, double-blind, placebo-controlled trials of ketamine in the rapid treatment of major depressive episodes. Psychol Med. 2015;45(4):693–704. | ||
Hasselmann HW. Ketamine as antidepressant? Current state and future perspectives. Curr Neuropharmacol. 2014;12(1):57–70. | ||
Mathews DC, Henter ID, Zarate CA. Targeting the glutamatergic system to treat major depressive disorder: rationale and progress to date. Drugs. 2012;72(10):1313–1333. | ||
Chan WM, Liang Y, Wai MS, Hung AS, Yew DT. Cardiotoxicity induced in mice by long term ketamine and ketamine plus alcohol treatment. Toxicol Lett. 2011;207(2):191–196. | ||
Morgan CJ, Muetzelfeldt L, Curran HV. Consequences of chronic ketamine self-administration upon neurocognitive function and psychological wellbeing: a 1-year longitudinal study. Addiction. 2010;105(1):121–133. | ||
Yeung WF, Chung KF, Poon MM, et al. Chinese herbal medicine for insomnia: a systematic review of randomized controlled trials. Sleep Med Rev. 2012;16(6):497–507. | ||
Wang L, Zhou GB, Liu P, et al. Dissection of mechanisms of Chinese medicinal formula Realgar-Indigo naturalis as an effective treatment for promyelocytic leukemia. Proc Natl Acad Sci U S A. 2008;105(12):4826–4831. | ||
Qiu J. ‘Back to the future’ for Chinese herbal medicines. Nat Rev Drug Discov. 2007;6(7):506–507. | ||
Wu L, Wang Y, Li Z, Zhang B, Cheng Y, Fan X. Identifying roles of “jun-chen-zuo-shi” component herbs of qishenyiqi formula in treating acute myocardial ischemia by network pharmacology. Chin Med. 2014;9:24. | ||
Ung CY, Li H, Cao ZW, Li YX, Chen YZ. Are herb-pairs of traditional Chinese medicine distinguishable from others? Pattern analysis and artificial intelligence classification study of traditionally defined herbal properties. J Ethnopharmacol. 2007;111(2):371–377. | ||
Dong X, Fan Y. [Internal relations between seven methods in prescription compatibility and toxicity and therapeutic effects of complex prescription]. Zhong Hua Zhong Yi Yao Xue Kan. 2008;26(3):618–619. Chinese. | ||
Chang X, Jia H, Zhou C, et al. Role of bai-shao towards the antidepressant effect of chaihu-shu-gan-san using metabonomics integrated with chemical fingerprinting. J Chromatogr B Analyt Technol Biomed Life Sci. 2015;1006:16–29. | ||
Lv H, Sun H, Sun W, et al. Pharmacokinetic studies of a Chinese triple herbal drug formula. Phytomedicine. 2008;15(11):993–1001. | ||
Zheng Q, Yue PF, Wu B, Hu PY, Wu ZF, Yang M. Pharmacokinetics comparative study of a novel Chinese traditional herbal formula and its compatibility. J Ethnopharmacol. 2011;137(1):221–225. | ||
Zheng M, Zhou H, Wan H, Chen YL, He Y. Effects of herbal drugs in mahuang decoction and their main components on intestinal transport characteristics of Ephedra alkaloids evaluated by a Caco-2 cell monolayer model. J Ethnopharmacol. 2015;164:22–29. | ||
Pharmacopoeia Commission of the People’s Republic of China. Pharmacopoeia of the People’s Republic of China. Beijing: Chemical Industry Press; 2005. | ||
Lin HJ, Kao ST, Siao YM, Yeh CC. The Chinese medicine sini-san inhibits HBx-induced migration and invasiveness of human hepatocellular carcinoma cells. BMC Complement Altern Med. 2015;15:348. | ||
Li X, Wu L, Liu W, et al. A network pharmacology study of Chinese medicine qishenyiqi to reveal its underlying multi-compound, multi-target, multi-pathway mode of action. PLoS One. 2014;9(5):e95004. | ||
Sun C, Geng C, Yin X, Huang X, Chen J. Natural products as antidepressants documented in Chinese patents from 1992 to 2013. J Asian Nat Prod Res. 2015;17(2):188–198. | ||
Liu LY, Feng B, Chen J, et al. Herbal medicine for hospitalized patients with severe depressive episode: a retrospective controlled study. J Affect Disord. 2015;170:71–77. | ||
Saki K, Bahmani M, Rafieian-Kopaei M. The effect of most important medicinal plants on two important psychiatric disorders (anxiety and depression): a review. Asian Pac J Trop Med. 2014;7 Suppl 1:S34–S42. | ||
Li S, Zhang B. Traditional Chinese medicine network pharmacology: theory, methodology and application. Chin J Nat Med. 2013;11(2):110–120. | ||
Jun JH, Choi TY, Lee JA, Yun KJ, Lee MS. Herbal medicine (gan mai da zao decoction) for depression: a systematic review and meta-analysis of randomized controlled trials. Maturitas. 2014;79(4):370–380. |
 © 2016 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2016 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.


