Back to Journals » OncoTargets and Therapy » Volume 13
Next Generation Sequencing Reveals a Synchronous Trilateral Lung Adenocarcinoma Case with Distinct Driver Alterations of EGFR 19 Deletion or EGFR 20 Insertion or EZR-ROS1 Fusion
Authors Zhang X, Feng J, Su X, Lei Y, Wu W , Cheng X
Received 23 September 2020
Accepted for publication 21 November 2020
Published 9 December 2020 Volume 2020:13 Pages 12667—12671
DOI https://doi.org/10.2147/OTT.S283617
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Prof. Dr. Nicola Silvestris
Xuhui Zhang,1,* Jiemei Feng,2,* Xiaoxing Su,3 Yan Lei,3 Wendy Wu,3 Xiangyang Cheng4
1Department of Oncology, Guangdong Second Provincial General Hospital, Guangzhou, People’s Republic of China; 2Department of Respiratory and ICU, Guigang People’s Hospital, Guigang, 537100, People’s Republic of China; 3Clinical Research Division, Berry Oncology Corporation, Fuzhou, 350200, People’s Republic of China; 4Department of Thoracic Surgery, The First Affiliated Hospital of Guangzhou Medical University, Guangzhou, 510120, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Wendy Wu; Xiangyang Cheng Email [email protected] [email protected]
Objective: Synchronous multiple primary lung cancer (SMPLC) has a reported occurrence from 0.5% to 2% in lung cancer, and the surgical treatment and prognosis were quite diverse. With the discovery of driver mutations in lung adenocarcinoma (ADC), next-generation sequencing (NGS) would provide an explicit answer to the key question, whether individual tumors represent intrapulmonary metastases or independent tumors. Here, we reported a 64-year-old female diagnosed with a synchronous trilateral early-stage ADC with distinct driver alterations.
Materials and Methods: NGS test targeting 31 cancer-relevant genes and amplification RNA sequencing (if gene fusion was found on DNA level) were performed on the surgical tumor tissue.
Results: A 64-year-old Chinese female never smoker was found with one nodule in the right upper lobe and two nodules in the right middle lobe through chest computed tomography. The lesions were resected through video-assisted thoracic surgery and diagnosed with stage IA ADC, T1N0M0, in the postoperative pathology. NGS detected three independent driver mutations in three primary sites, respectively, EGFR 19del, EGFR 20ins and ROS1 fusion.
Conclusion: This is the first report of a synchronous trilateral early-stage ADC with distinct driver alterations. All individual tumors were independent identified by NGS methodology, which had provided a clear answer to the key question of SMPLC in this case and should be used as a routine genetic test to explore fully pathological diagnosis and more comprehensive oncogenesis information in the early-stage ADC clinical prevention.
Keywords: synchronous multiple primary lung cancer, lung adenocarcinoma, NGS, EGFR mutation, ROS1 fusion
Introduction
The incidence of adenocarcinoma (ADC) accounts for nearly 50% of all lung cancer, which remains the leading cause of cancer death (1.8 million, 18.4%) and occupies the highest number of new cancer cases (2.1 million, 11.6%) worldwide.1 Synchronous multiple primary lung cancer (SMPLC) has a reported occurrence from 0.5% to 2% in lung cancer patients,2 which has increased recently due to the development of early detection techniques. Based on the established clinicopathological criteria, the surgical outcome and prognosis of SMPLC patients were quite different even if all the lesions were ADC.3 With the discovery of driver mutations in ADC, such as epidermal growth factor receptor (EGFR) mutation, ALK receptor tyrosine kinase (ALK) and c-ros oncogene 1 (receptor tyrosine kinase, ROS1) rearrangement,4 next-generation sequencing (NGS) would provide a more clear answer to the key question, whether individual tumors represent intrapulmonary metastases or independent tumors. Liu et al had profiled genomic heterogeneity of 6 SMPLC patients and suggested that different lung cancer in the same patient may have distinct genomic profiles and could be driven by distinct molecular events.5 There were 2 pN0 trilateral cases in this study, one patient had EGFR p.L858R mutation in two nidi and PIK3CA mutations in the third nidus, while another patient had EGFR p.L858R mutation in all lesions. Luo et al reported a double ALK rearrangement bilateral lung adenocarcinoma case in recent, one nodule with PRKCB-ALK fusion and the other one with EML4-ALK fusion.6
EGFR and ROS1 are both well-established driver alterations with 10~35% and 1% prevalence, respectively, in non-small cell lung cancer (NSCLC) patients, and preferentially affecting non-smokers.4,7 Compound EGFR mutations are defined as double or multiple mutations in tyrosine kinase domain of EGFR, which are frequently detected in advanced lung cancer. In a retrospective study of 3000 treatment-naïve Chinese advanced NSCLC patients,8 compound EGFR mutations were found in 1.2% EGFR mutated patients; none EGFR exon 19 deletion (19del) cases was observed in the compound EGFR dataset; ROS1 fusion had not been detected in all 1266 EGFR mutation patients (42.2% of the whole cohort). ROS1 rearrangement had been identified with several 5ʹ fusion gene partners, such as Ezrin (EZR), and ROS1 fusion is mostly exclusive to EGFR, KRAS, or ALK mutations.7 The concurrent ROS1 rearrangement with EGFR mutation in a single tumor was only observed in 6 cases, all were advanced ADC patients, and one of them was EZR-ROS1 fusion with EGFR 19del.9–12
Here we described an extremely rare case that a 64 years old female never-smoker early-stage ADC patient had three synchronous primary adenocarcinoma nodules with distinct driver mutations of EGFR 19del (stage IA, T1N0M0) or EGFR exon 20 insertion (20ins, stage IA, T1N0M0) or EZR-ROS1 fusion (stage IA, T1N0M0). All individual tumors were independent. NGS had provided a clear differentiation in this case and offered an explicit answer to the key question of SMPLC, whether individual tumors represent intrapulmonary metastases or independent tumors. NGS technique should be used as a routine genetic test to explore fully pathological diagnosis and more comprehensive tumorigenesis information for ADC patients.
Materials and Methods
The patient underwent resection of the right middle lobe and wedge resection of the right upper lobe and en bloc resection of the associated hilar and mediastinal lymph nodes by video-assisted thoracic surgery. Genomic DNA (gDNA) was extracted from fresh surgical tumor tissue samples using DNeasy Blood & Tissue Kit (Qiagen, USA) according to the manufacturer’s instructions, and the paired white blood cell samples using DNA Blood Midi/Mini kit (Qiagen, USA). An in-house designed panel targeting 31 cancer-relevant genes (Berry Oncology) was used to generate sequencing libraries, which was sequenced by NextSeq CN500 (Berry Genomics, China & Illumina, USA) with 150PE mode. Amplification RNA sequencing (if gene fusion was found on DNA level) was performed on the surgical tumor tissue.
Case Presentation
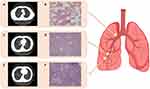
A 64-year-old non-smoking female was diagnosed as multiple pulmonary nodules in lung through physical examination and visited our hospital for follow-up with no abnormality in June, 2019. Chest computed tomography (CT) revealed one lesion in the right upper lobe and two lesions in the right middle lobe on June 16, 2019 (Figure 1A, C and E). The subsequent surgical resection had been performed through video-assisted thoracic surgery (VATS). The post-operative course was uneventful and the patient recovered quickly. Immunohistochemical staining in the post-operative pathology indicated that three nodules of the patient all was clinically diagnosed with stage IA minimally invasive lung adenocarcinoma, T1N0M0 (Figure 1B, D and F).
The surgical tumor tissues were subjected to target capture-based clinical next-generation sequencing (NGS) test targeting 31 cancer-relevant genes (Berry Oncology), and three independent driver mutations had been found in three nidi, respectively (Figure 2). EGFR exon 19 deletion (c.2240–2260delinsCAAGAG) (Figure 2A) was detected at a mutant allele frequency (MAF) of 1.13% in the nidus (0.5 cm in diameter) of the right upper lobe (Figure 1A). EGFR exon 20 insertion (c.2311–2312delinsGGGTT) (Figure 2A) was identified at a MAF of 5.13% in one nodule (0.4 cm in diameter) in the right middle lobe (Figure 1C). And EZR exon 10-ROS1 exon 34 (EZR-ROS1) rearrangement (Figure 2B) was detected at DNA level in the other nodule (0.6 cm in diameter) in the right middle lobe (Figure 1E) and confirmed by amplification RNA sequencing with 7574 mutation fragments (Figure 2C). The fusion gene contained the N-terminal coiled-coil domain of EZR and the C-terminal tyrosine kinase domain of ROS1. All sequencing reads were examined on Integrative Genomic Viewer (IGV) software.
Up to the 9 months post-surgery follow-up (until March 2020), no recurrence or metastasis was observed in the patient.
Discussion
EGFR gene is the most frequent driver mutation in ADC,4 and most alterations are located at the tyrosine kinase domain in exons 19~21. Short in-frame deletion in exon 19 and insertion in exon 20 are both typical mutations and have not been simultaneously found in two independent primary nidi of one pN0 lung adenocarcinoma patient in the literatures. ROS1 gene belongs to the insulin receptor family and the rearrangement with other gene partners would stimulate the downstream mitogen-activated protein kinases pathway, enhancing cell growth and proliferation. EZR-ROS1 fusion results in a not-so-small deletion of the long arm of chromosome 6,7 which could keep the full ROS1 kinase domains and lead to constitutive kinase activity in tumor formation.
Previous investigations indicated that ROS1 rearrangement is hardly concurring with EGFR, KRAS, or ALK mutations. Until now, ROS1 fusion was only observed to co-occur with EGFR alteration in 6 advanced ADC patients,9–12 all happened in a single tumor, one of them was the synchronous EZR-ROS1 fusion and EGFR 19del in the same lesion. Although compound EGFR mutations are frequently found in advanced ADC cases, one of the most prevalent EGFR mutations, EGFR 19del, is mostly exclusive.8 The concurrence of ROS1 rearrangement and EGFR mutation has been detected in the same nidus of only one early-stage ADC patient,10 neither in the differential nidi. Here, we reported a scarce case carrying three primary tumors each with an independent driver mutation. It is the first time to discover this phenomenon in such an early-stage ADC case (stage IA, T1N0M0), which expanded our cognition of tumorigenesis.
This is the first report of a synchronous trilateral early-stage lung adenocarcinoma with distinct driver alterations of EGFR 19del or EGFR 20ins or EZR-ROS1 fusion. Three primary minimally invasive adenocarcinoma were surgical resected through VATS, no metastasis was found. All oncogenic mutations were identified by NGS methodology, DNA copy number variation (CNV) analysis was also performed but no amplification/deletion was found. Three nodules in this pN0 ADC patient have EGFR 19del, EGFR 20ins and EZR-ROS1 fusion, respectively, with sufficient genomic evidence. SMPLC only occurs 0.5% to 2% in lung cancer patients even with the worldwide use of high-resolution imaging systems, and the surgical treatment and prognosis of SMPLC are all related to accurate pathological diagnosis with molecular and genomics methods. In previous studies of synchronous primary lung adenocarcinoma, few cases with multiple tumors (n≥3) were confirmed by NGS technology that they were independent tumors with distinct driver mutations. Our report identified that the patient had three different well-established genomic alterations in three separate nodules of two right lobes with NGS methodology. It could be concluded that three lesions with differential driver mutations were all independent tumors not intrapulmonary metastases. There is no abnormality presented on this patient from VATS surgery until now. EGFR tyrosine kinase inhibitors (EGFR-TKI) and crizotinib, the well-established Anaplastic Lymphoma Kinase (ALK) inhibitor sensitive to ROS1 rearrangement, should be considered as the drug treatment when this case recurs.
Since EGFR mutations are more frequent in lung adenocarcinoma with recurrent/metastatic disease,13 follow-up monitoring on this patient is needed with close attention. NGS technique had clearly differentiated three independent tumors in this SMPLC case and provided valuable insights into oncogenesis and early-stage cancer prevention in clinic. It suggested that differential malignant lung tumors in the same individual could have distinct genomic profiles and be driven by distinct molecular events under the identical genetic background. NGS detection should be used as a routine genetic test to explore fully molecular diagnosis and more comprehensive tumorigenesis information. We recommended that the genomic profiles of each resected nodule should be addressed through NGS method, which could expand the existing clinical practice solution for better outcome and precisely dynamics monitoring for ADC patients.
Abbreviations
SMPLC, synchronous multiple primary lung cancer; ADC, lung adenocarcinoma; NGS, next-generation sequencing; CT, computed tomography; VATS, video-assisted thoracic surgery; EGFR, epidermal growth factor receptor; 19del, exon 19 deletion; 20ins, exon 20 insertion; ROS1, c-ros oncogene 1; EZR, Ezrin.
Ethics Statement
The study was approved by the Ethical Committee of Medical Research, The First Affiliated Hospital of Guangzhou Medical University.
Consent for Publication
Written informed consent was obtained from the patient for publication of this case report and any accompanying images.
Acknowledgments
We thank Dr Lin Wu for her critical comments on the preparation of this manuscript.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
This work was partly supported by grants from National Natural Science Foundation of China (No.81973718).
Disclosure
Xiaoxing Su, Yan Lei and Wendy Wu are employees of Berry Oncology Corporation. The authors report no other potential conflicts of interest for this work.
References
1. Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394–424. doi:10.3322/caac.21492
2. Synchronous Bilateral Lung Cancer With Discordant Histology. Available from: https://www.cancernetwork.com/lung-cancer/synchronous-bilateral-lung-cancer-discordant-histology.
3. Loukeri AA, Kampolis CF, Ntokou A, Tsoukalas G, Syrigos K. Metachronous and synchronous primary lung cancers: diagnostic aspects, surgical treatment, and prognosis. Clin Lung Cancer. 2015;16(1):15–23. doi:10.1016/j.cllc.2014.07.001
4. Black RC, Khurshid H. NSCLC: an update of driver mutations, their role in pathogenesis and clinical significance. R I Med J. 2015;98(10):25–28.
5. Liu Y, Zhang J, Li L, et al. Genomic heterogeneity of multiple synchronous lung cancer. Nat Commun. 2016;7:13200.
6. Luo J, Gu D, Lu H, Liu S, Kong J. Coexistence of a novel PRKCB-ALK, EML4-ALK double-fusion in a lung adenocarcinoma patient and response to crizotinib. J Thorac Oncol. 2019;14(12):e266–e268. doi:10.1016/j.jtho.2019.07.021
7. Takeuchi K, Soda M, Togashi Y, et al. RET, ROS1 and ALK fusions in lung cancer. Nat Med. 2012;18(3):378–381. doi:10.1038/nm.2658
8. Li M, Zhou CZ, Yang JJ, et al. The in cis compound EGFR mutations in Chinese advanced non-small cell lung cancer patients. Cancer Biol Ther. 2019;20(8):1097–1104.
9. Facchinetti F, Loriot Y, Kuo MS, et al. Crizotinib-resistant ROS1 mutations reveal a predictive kinase inhibitor sensitivity model for ROS1- and ALK-rearranged lung cancers. Clin Cancer Res. 2016;22(24):5983–5991. doi:10.1158/1078-0432.CCR-16-0917
10. Zhu YC, Xu CW, Ye XQ, et al. Lung cancer with concurrent EGFR mutation and ROS1 rearrangement: a case report and review of the literature. Onco Targets Ther. 2016;9:4301–4305.
11. Ju L, Han M, Zhao C, Li X. EGFR, KRAS and ROS1 variants coexist in a lung adenocarcinoma patient. Lung Cancer. 2016;95:94–97. doi:10.1016/j.lungcan.2016.03.005
12. Mao Y, Wu S. ALK and ROS1 concurrent with EGFR mutation in patients with lung adenocarcinoma. Onco Targets Ther. 2017;10:3399–3404. doi:10.2147/OTT.S133349
13. Jordan EJ, Kim HR, Arcila ME, et al. Prospective comprehensive molecular characterization of lung adenocarcinomas for efficient patient matching to approved and emerging therapies. Cancer Discov. 2017;7(6):596–609. doi:10.1158/2159-8290.CD-16-1337
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.