Back to Journals » Open Access Rheumatology: Research and Reviews » Volume 13
New Perspectives on Diagnosing Psoriatic Arthritis by Imaging Techniques
Authors Sarbu MI, Sarbu N, Cristea Ene D, Corche D, Baz R, Negru D, Nechita A, Fotea S, Anghel L , Tatu AL
Received 30 July 2021
Accepted for publication 22 November 2021
Published 22 December 2021 Volume 2021:13 Pages 343—352
DOI https://doi.org/10.2147/OARRR.S331859
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Chuan-Ju Liu
Mihaela Ionela Sarbu,1,* Nicolae Sarbu,2 Doriana Cristea Ene,3 Daniela Corche,3 Radu Baz,4,* Dragos Negru,5,* Aurel Nechita,1,6 Silvia Fotea,1,6 Lucretia Anghel,1,3 Alin Laurentiu Tatu7,8
1Faculty of Medicine and Pharmacy, “Dunarea de Jos” University, Galati, Romania; 2Faculty of Medicine and Pharmacy, “Dunarea de Jos” University, Al. I. Cuza No 35, Galati, Romania; 3Sf Apostol Andrei Clinical County Emergency Hospital, Galati, Romania; 4Department of Radiology and Medical Imaging, Clinical County Emergency Hospital Constanta, “Ovidius” University, Constanta, Romania; 5Department of Radiology – Imaging, University Hospital “Sf. Spiridon”, University of Medicine and Pharmacy “Grigore T. Popa”, Iasi, Romania; 6Department of Pediatrics, “Sf. Ioan” Clinical Hospital for Children, Galati, Romania; 7Faculty of Medicine and Pharmacy, Clinical Department, Medical and Pharmaceutical Research Unit/Competitive, Interdisciplinary Research Integrated Platform’, ReForm-UDJG, “Dunarea de Jos” University, Galati, Romania; 8Clinical Hospital St Parascheva of Infectious Diseases, Dermatology Department, Galati, Romania
*These authors contributed equally to this work
Correspondence: Nicolae Sarbu
Faculty of Medicine and Pharmacy, “Dunarea de Jos” University, Al. I. Cuza No 35, Galati, Romania
Tel +40728301044
Email [email protected]
Abstract: Psoriatic arthritis is a chronic inflammatory condition that can lead to severe functional impairment and irreversible damage. The diagnosis can be difficult in early cases where the clinical exam is often scarce. The lack of a serological biomarker can lead to a considerable delay in diagnosis. In this review, we discuss the existent imaging methods that have improved the diagnosis of psoriatic arthritis (PsA). The degree and type of musculoskeletal involvement cannot be assessed by only one imaging method. We think that a combination of methods is the best approach to evaluate both structural damage and inflammatory lesions and that ultrasound (US) could be the best tool to screen a patient when considering the diagnosis of PsA. US is an accessible, non-ionizing technique that offers information regarding active inflammation in joints, entheses, and soft tissues.
Keywords: imaging, psoriatic arthritis, MRI, ultrasound
Introduction
Psoriatic arthritis (PsA) is a complex disease emerging from a combination of environmental and genetic predisposing factors, affecting 20–30% of the patients with cutaneous psoriasis.1–3 The metabolic pathways play an important role in the pathogenic process, although they are still far from being comprehended.4,5 PsA is a highly underdiagnosed chronic inflammatory arthritis that is responsible for important cardiovascular-associated morbidity of the affected patients. The triggering events are yet to be understood.6,7
The delay in diagnosis is linked to the variable and heterogeneous pattern of presentation with an inflammatory process that can extend from the synovial membrane, entheseal apparatus, juxta-articular bone, and tendon sheaths to the profound structures of the spine and sacroiliac joints.8 Another possible reason for the late diagnosis is the absence of a serological biomarker, hence the importance of the imaging techniques. The severity of the disease, which may range from limited and non-destructive forms to extensive and destructive ones, could be responsible for the diagnostic conundrum. The articular manifestations usually occur after the diagnosis of cutaneous psoriasis has been established. However, it appears that in up to 10% of cases psoriatic arthritis can precede the cutaneous manifestations or occur simultaneously.1,2 Dactylitis is a hallmark of PsA and has been linked to a worse outcome, more erosive, and severe articular manifestations.
In this context, imaging methods are more important than ever because they offer a method of quantification of the inflammatory process, guiding the diagnosis, monitoring the progression of the disease, and the treatment. Rapid and cost-effective methods like musculoskeletal ultrasound (US) could become the surrogate for the laboratory biomarkers used in other inflammatory arthritis (like the anti-citrullinated protein antibody or the rheumatoid factor for rheumatoid arthritis (RA)). Ultrasound could prove useful in the identification of early PsA in patients affected by cutaneous psoriasis.
Moll and Wright were the first to classify PsA into 5 categories according to the clinical characteristics: (a) predominant involvement of distal interphalangeal (DIP) joints, (b) arthritis mutilans, (c) symmetric polyarthritis (d) oligoarthritis, and (e) predominant axial involvement.2 Classification criteria for psoriatic arthritis (CASPAR) were later on developed9 and are currently largely used in studies and clinical practice because of their high sensitivity and specificity. Another significant advantage is their easy application making them suitable for clinical scenarios. To be classified as PsA, a patient must have an inflammatory articular disease (joint, spine, or entheseal) and three or more of the following features: current, past or familial psoriasis, psoriatic onychopathy, negative rheumatoid factor, dactylitis recorded by a rheumatologist and new bone formation such as periostitis (excluding osteophytes). The Group for Research and Assessment of Psoriasis and Psoriatic Arthritis (GRAPPA) has classified the patients into 6 clinical domains (peripheral arthritis, axial disease, enthesitis, dactylitis, skin disease, and nail disease). The main purpose of this classification is to guide the choice of treatment.10
Inflammatory manifestations of the musculoskeletal system can often lead to diagnostic difficulties, particularly because of the absence of a specific laboratory biomarker. Musculoskeletal pain is a frequent complaint in the general population, making inflammatory manifestations of PsA harder to demonstrate exclusively on clinical grounds. Nevertheless, progress has been made in recent years, and the refinement of imaging methods together with the growing interest in understanding this disease is improving the diagnostic process. Imaging has provided a more standardized approach and homogeneous results in studies allowing for the development of definitions and a better classification of the patients affected by PsA.
Methods
We conducted PubMed literature searches using the terms “psoriatic arthritis imaging”, “ psoriatic arthritis conventional radiography”, “psoriatic arthritis ultrasound”, and “psoriatic arthritis magnetic resonance imaging” focusing on the publications from the last 10 years. Articles were deemed relevant if they provided insights into the diagnosis and monitoring of PsA through imaging methods.
Results
Ionizing Imaging Techniques
Conventional radiography (CR) is a classic imaging method used as a standard initial evaluation for any kind of articular or periarticular symptoms particularly when a chronic inflammatory disease is suspected. Its value resides in the detection of structural lesions important in the process of diagnosis and monitoring.11 It is a rapid, accessible, and validated imaging method. Even though it is a 2-dimensional technique, it offers valuable information regarding the structural damage and progression of the disease. CR has nevertheless little value for soft tissues or early lesions. The ionizing radiation is also a disadvantage even though with the new technology available and for the peripheral sites, the values are very low. Structural damage is irreversible, has prognostic value and includes erosion, new bone formation, subluxation, and ankylosis. CR is used to monitor structural changes such as new bone formation. For the axial involvement, CR should not be repeated more than every second year due to the radiation exposure and its limited sensitivity to change.12
The erosions from PsA differ from RA by the fact that they combine osteoproliferative with osteodestructive lesions. The proliferative lesions are described as ill-defined juxta-articular ossifications, while the destructive lesions are represented by « pencil in cup » deformity, subluxations, erosions, and joint space narrowing (JSN) regarded as an indirect measure of cartilage loss.13,14 New bone formation or enthesitis is present at the site of attachment to the bone of tendons, ligaments, or capsules. The radiographic changes appear late in the course of the disease and do not help in the evaluation of the inflammatory process that could potentially be halted by a certain therapeutic approach.15
Feced-Olmos et al16 studied 76 PsA patients and found that structural alterations of the dominant hand assessed by conventional X-ray are associated with loss of strength measured by dynamometry. The structural lesions taken into account were erosions and JSN. Simon et al17 studied the radiographs of 101 patients with PsA comparing them with the radiographs of 55 patients with psoriasis and 47 healthy controls. They found that bone erosions are age-dependent and more frequent in long-standing PsA. Enthesiophytes were not dependent on age but influenced by disease duration. Enthesiophytes seem to have an impact on physical function in PsA making therapeutic interventions necessary possibly early in the disease.
Radiographic Scoring Methods
The protean clinical manifestations of PsA have made the assessment of disease activity in both clinical settings and trials a big challenge. CR has been used in clinical trials for the evaluation of damage and progression for more than 20 years.18 Scoring systems originally designed for RA assessment18 have been modified for research purposes and used in clinical trials to evaluate the radiographic progression of peripheral joints in patients with PsA.18,19 These scoring methods take into account erosions, JSN in hands, wrists, and feet, pencil-in-cup deformity, osteolysis, and ankylosis. The modified Steinbrocker global scoring method includes 42 joints (hands, wrists, and feet) assessing the extent of erosions and JSN (range 0–4) arriving at a total score between 0 and 168.18 The PsA-modified Sharp method allows for the assessment of distal interphalangeal joints besides the ones in the modified Steinbrocker scoring method with a total score ranging between 0 and 470. Erosions (scale from 0 to 5) and JSN (scale from 0 to 4) are measured separately.
The Sharp-van der Heijde score modified for PsA (total score between 0 and 528) evaluates the same joints as the PsA-modified Sharp method. Erosions are scored from 0 to 5 in the hands and from 0 to 10 in the feet. JSN is scored from 0 to 4 in both hands and feet. Significant osteolysis and pencil-in-cup deformity are scored separately and given the highest score for erosion and JSN for the affected joint. The Psoriatic Arthritis Ratingen score (PARS) is a scoring method designed for PsA (total score from 0 to 360). It scores the destructive lesions from 0 to 5 and the proliferative lesions from 0 to 4. This assesses 30 joints in the hands and 10 joints in the feet.
Computed tomography (CT) is considered a reference technique to detect structural damage such as erosions, JSN, and ankylosis.20 Despite having a better resolution than CR, its use is limited by the higher level of ionizing radiation and the lack of information regarding soft tissue. EULAR recommends this only in cases where MRI is contraindicated and the radiography is inconclusive.14 High-resolution CT and micro CT are being investigated as methods for detecting structural damage, having shown that the morphology of the erosion is different in PsA Ω-shaped compared to U-shaped in RA patients.13
Recent progress in imaging technology could make methods like low dose CT an alternative in the future for axial radiography. Studies have shown14,15,20,21 that these methods demonstrate up to 50% more bone formation than CR. CT iodine mapping is another emerging method that allows the visualization of inflammation on CT.22
High-resolution Positron Emission Tomography PET/CT imaging is being investigated in the evaluation of arthritis.23 It is a method that uses fluorine 18 fluorodeoxyglucose (18-FDG) based on the fact that it accumulates in the inflammatory tissue. Commonly performed for the assessment of malignant processes and particularly the search of metastatic lesions, PET/CT proves useful in inflammatory articular diseases allowing the evaluation of the whole body and the extra-articular manifestations. The uptake of 18-FDG is nonspecific, this being a functional exam, its usefulness residing in the detection of whole-body inflammation proving helpful in the diagnostic process and the choice of treatment. However, PET/CT is an expensive method making it less available for wide use.
Non-Ionizing Imaging Techniques
US is a rapid, non-invasive and inexpensive method that allows the assessment of the peripheral skeleton. The most important advantages of US are represented by the fact that it is a rapid, painless, repeatable technique that can detect early inflammatory changes in the joints, synovial sheaths, or entheses.24 The grayscale assessment evaluates structural anomalies such as synovial hypertrophy, effusion, and tenosynovitis, while the Power Doppler mode or the Color Doppler mode can quantify active inflammation.
US has been increasingly used for the guidance of synovial biopsies allowing an accurate diagnosis. US is also used for aspiration and guided injections. Helliwell et al25 compared the data from the Tight Control in Psoriatic Arthritis (TICOPA) trial and found no difference between the inflammation scores detected by MRI versus US.
For optimal results, US parameters should be adjusted for the grayscale to obtain maximum contrast. The power Doppler settings should be adjusted to the lowest possible pulse repetition frequency, the lowest possible persistence, and the lowest possible wall filter.25,26
MRI is the method of choice for the study of inflammatory processes of the profound musculoskeletal system such as the axial segment (spine and sacroiliacs) and the pelvic structures.27,28 Furthermore, MRI is the only method that allows the visualization of bone marrow oedema (BMO). In psoriatic arthritis, the MRI exam can identify the following patterns:
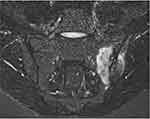
a) Inflammatory lesions are described as high signal intensity on Short Tau Inversion Recovery sequence (STIR) or T2 sequence with fat suppression. Active inflammatory lesions (Figure 1) are defined as BMO, capsulitis, enthesitis, and synovitis. BMO, which is highly suggestive of spondyloarthritis, is subchondral and in the inferior portion of the sacroiliac joint, visible in more than 2 consecutive slices.12,28,29 In PsA, BMO is located close to the entheses, in RA close to the capsular attachments, and in osteoarthritis (OA) close to the subchondral bone, although, in clinical practice, the differentiation may be difficult, particularly between PSA and OA.28 Synovitis is characterized as increased post-contrast enhancement in a thickened synovial membrane.
b) Structural lesions comprise sclerosis, erosions, fat deposition, bony bridges/ankylosis. Structural lesions are visualized on T1 sequences: erosions are described as the full-thickness loss of the dark signal of the cortical bone, while enthesiophytes and ankylosis are seen as new bone formation in the periarticular region in the form of a bright signal on T1 (Figure 2). Fat infiltration appears as a bright signal on T1.
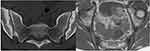 |
Figure 2 Sacroiliitis on CT. Axial CT (computer tomography; left side of the figure) and T1-weighted sequence (right side of the figure) demonstrates subchondral sclerosis and erosions. |
A new MRI technique has been described for the quantitative evaluation of the fat infiltration in the sacroiliac joints in patients suffering from ankylosing spondylitis (AS), although further studies are necessary. The IDEAL sequence (iterative decomposition of water and fat with echo asymmetry and least square estimation) provides information on the spatial distribution of the fat infiltration, being correlated with changes after treatment.30,31 Whole-body MRI is another non-ionizing method used to investigate the occurrence of inflammatory changes. It has been proved useful in oncology for the detection of metastases and the assessment of treatment of bone lesions.31
Fluorescence optical imaging (FOI) is a method that studies the microcirculation of the skin, nails or joints after intravenous injection of a fluorescent dye called indocyanine green. After injection, a special camera detects the emissions of the fluorescent dye corresponding to the micro-vascularisation thus showing different patterns of inflammation.32–35 Schmidt et al33 applied this method to patients with psoriasis, PsA, RA and healthy controls and found that PsA and psoriasis patients have a specific FOI pattern that can be differentiated from healthy controls or even RA patients. Werner et al32 performed a study where they demonstrated a correlation between the findings obtained by FOI, MRI, and US, while Wiemann et al35 were able to differentiate between PsA and RA using this method.
Utility of Imaging Techniques in Diagnosing Clinical Forms of PsA
Enthesitis
The entheses consist of unique fibrocartilaginous structures that connect ligaments, tendons, and capsules to the bone. The entheseal organ allows the subtle transfer of mechanical forces from the muscle through the tendon and ligaments to the bone while also having a stabilizing role for the joint.36,37 Enthesitis is defined by pain and tenderness at the site of attachment of the tendon, ligament, or capsule to the bone and is recognized as a feature of all spondylarthritis (SpA).24,37,38 The most commonly affected sites in PsA are the Achilles tendon, the plantar fascia, and the common extensor tendon insertion in the lateral epicondyle of the elbow. Clinical assessment of the enthesitis is based on applying a pressure of 4 kg/cm2 which can be difficult to reproduce between examiners,37 meaning that the burden of enthesitis could be higher if imaging techniques were employed to detect it. Enthesitis has been identified as an outcome domain by the ASAS and GRAPPA experts for determining disease activity and response to treatment for both spondyloarthritis and psoriatic arthritis.10,39–41 On CR, enthesitis appears late in the course of the disease, as bone cortex irregularities (erosions), new bone formation at the insertion of the tendon, ligament, or capsule (enthesiophytes), and intra- or peri-tendinous calcifications.37
US can detect abnormalities in grayscale, such as loss of fibrillar pattern, hypoechoic tendon, increased thickness, intralesional focal changes, erosions, or enthesiophytes.24 Involvement of the adjacent bursae and distal tendons can be present.42 The Doppler mode detects active inflammation in the form of abnormal vascularisation at the insertion of the tendon. US remains an excellent technique for diagnosing enthesitis that is non-ionizing, non-invasive, cheap, and offers a dynamic view from different angles of the examined structure. Additionally, US is a very good method for monitoring the treatment response.15,24,42–44 The OMERACT (Outcome Measures in Rheumatology) ultrasound group gave the following definition for enthesitis:
hypoechoic and/or thickened insertion of the tendon close to the bone (within 2 mm from the bony cortex), which exhibits Doppler signal if active and that may show erosions, enthesiophytes/calcifications as a sign of structural damage.
24,44 However, other pathologies can be responsible for enthesitis, the most common one being mechanical stress. Differentiation between mechanical and inflammatory enthesitis is extremely important since the treatment of the inflammatory condition often involves systemic immunosuppressive drugs.
Enthesitis can also be assessed by MRI and can be visualized as soft-tissue inflammatory changes outside the joint capsule,29,37,38,45 increase in thickness of the tendon, loss of fibrillar pattern, focal thickening, and bone marrow edema at the site of the bone attachment. None of these MRI aspects are specific to PsA – enthesitis appearing in many other conditions.29,38,45 MRI has certain limitations regarding enthesitis: cost, availability, limited visualization of the enthesis, particularly in sites where the water accumulation is low.
Dactylitis
Dactylitis, defined as the uniform and diffuse swelling of one digit, is considered a hallmark of psoriatic arthritis. It affects up to 49% of patients with PsA at one point in the course of the disease.46 PsA dactylitis has a predilection for the feet and is often asymmetrical. Sometimes it can involve multiple digits at the same time, and it can be the sole feature for months or even years.47 Dactylitis is associated with erosive disease and a worse prognosis in terms of disability and complications.17,48 The differential diagnosis of psoriatic dactylitis includes gout, syphilis, tuberculosis, flexor sheath infections, sickle cell disease, sarcoidosis, and reactive arthritis. The clinical presentation can be acute or chronic. Interestingly, chronic dactylitis can be asymptomatic, leading to delay in diagnosis. It is considered to be a deep Koebner phenomenon, with different studies having demonstrated the occurrence of dactylitis in previously injured joints in patients with a genetic predisposition.49
Even if dactylitis is often evident on clinical examination, there are cases where imaging methods prove helpful in the diagnosis such as in less clear clinical pictures or overweight patients. Dactylitis appears in US as a combination of flexor tendon tenosynovitis, synovitis, extracapsular inflammatory changes, abnormalities of the accessory pulleys, and subcutaneous oedema.10,47,50,51 Girolimetto et al51 found that extracapsular inflammation in dactylitis like flexor tenosynovitis and soft tissue edema are more frequent in early disease. In their study, the patients had a high prevalence of joint synovitis at the proximal interphalangeal level in the chronic form of dactylitis. Furlan et al52 studied 228 patients with early arthritis and found that thickening of flexor tendons pulleys is a distinctive ultrasonographical sign in psoriatic arthritis patients. MRI of PsA-related dactylitis shows flexor tendon tenosynovitis, synovitis, and soft tissue oedema.12,29
Onicopathy
Nail involvement is an important feature of psoriatic disease and most importantly a risk factor for developing PsA. Some studies have found it to be a prognostic factor for erosive disease.53,54 The US evaluation of the nail complex shows a very high variability of parameters, making the comparison of the obtained data very difficult.55–58 Naredo et al58 studied the US of the nail complex in patients with psoriasis, psoriatic arthritis, and healthy controls. They analyzed the score for the integrity of the nail plate and the color Doppler scores for both nail bed and matrix together with the thickness of the nail bed and nail plate. Interestingly, they found that not the percentage occupied by the Doppler signal is important but the number of signals that were in contact with the ventral plate. The thickness of both the nail bed and nail matrix were significantly higher, as expected. Mondal et al59 studied the difference between the nail plates in 45 PsA patients versus 45 healthy controls. They found that loosening of the ventral nail plate border was the most common US anomaly in the PsA group (51.79%) but nail bed thickness and nail matrix thickness were also present even in normal-appearing nails of the PsA group. Antony et al studied a group of 134 patients with established PsA and found that psoriatic onycholysis is associated with erosive disease at the affected DIP joint.54
Arthritis
The articular pattern in PsA can be extremely heterogeneous, ranging from exclusive involvement of distal interphalangeal joints, asymmetric oligoarticular pattern (four or fewer joints), polyarticular pattern (more than five) to arthritis mutilans (a severe destructive form). In 2011, Naredo et al54 were the first to show that almost 50% of the patients with psoriasis had occult synovitis observed by US, findings that have been confirmed by other authors.42,60
Axial PsA
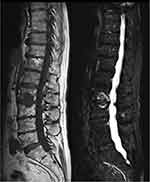
Clinical manifestations of axial spondyloarthritis are represented by chronic inflammatory back pain.61,62 Imaging techniques could detect ankylosing spondylitis or non-radiographic spondyloarthritis.63 Vertebral body corner oedema, fat infiltration, posterior ligaments enthesitis, aseptic spondylodiscitis, and syndesmophytes in final phases are the main findings on MRI (Figure 3). PsA is part of the SpA group of diseases with psoriasis being a criterion in the ASAS classification. The term ankylosing spondylitis refers to patients who fulfill the New York classification criteria. The AS associated with psoriasis has a different pattern from the classical form. The clinical characteristics of psoriatic SpA are asymmetrical sacroiliitis, even though symmetrical involvement can also occur, and the presence of marginal and paramarginal syndesmophytes that progress randomly unlike classical AS where the pattern of progression is more caudal to cranial.64–67 There is a high prevalence of asymptomatic axial involvement in psoriatic arthritis.63,64
Jadon et al63 found that in psoriatic SpA, spondylitis without sacroiliitis was common and usually asymptomatic. The burden of the disease in psoriatic SpA is similar to AS, despite being less severe radiographically. Psoriatic SpA could represent a different phenotype of disease depending on the presence of certain other factors like psoriasis, psoriatic onychopathy, or HLA-B*27 variants. Furthermore, the EULAR imaging recommendations for the diagnosis of sacroiliitis include CR, followed by MRI if CR is inconclusive12 (Table 1).
 |
Table 1 Imaging Techniques in Psoriatic Arthritis |
Conclusions
Imaging techniques represent powerful tools for diagnosing psoriatic arthritis. Early therapeutic intervention can halt the inflammatory destructive process leading to improved long-term prognosis and quality of life. There is no perfect tool for determining both structural and inflammatory lesions. Every method has advantages and disadvantages. We think that the combination of methods could provide the best information depending on the clinical details. US is the best tool for screening because it can detect synovitis, tenosynovitis, dactylitis, and enthesitis. It is fast, non-expensive, non-ionizing, and can be repeated as many times as needed to enrich the clinical examination or to follow the response to treatment. CR is the standard and also the most available technique used to detect structural damage. MRI is the best tool to assess the inflammatory process of the profound musculoskeletal structures like the spine or the sacroiliac joints. MRI and US allow for an early diagnosis before the irreversible structural damage has set in.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Michalek IM, Loring B, John SM. A systematic review of worldwide epidemiology of psoriasis. J Eur Acad Dermatol Venereol. 2017;31:205–212. doi:10.1111/jdv.13854
2. Moll JM, Wright V. Psoriatic arthritis. Semin Arthritis Rheum. 1973;3:55–78. doi:10.1016/0049-0172(73)90035-8
3. Veale DJ, Fearon U. The pathogenesis of psoriatic arthritis. Lancet. 2018;391(10136):2273–2284. doi:10.1016/S0140-6736(18)30830-4 Epub 2018 Jun 1. PMID: 29893226.
4. Ciccia F, Triolo G, Rizzo A. Psoriatic Arthritis. N Engl J Med. 2017;376(21):2094–2095. doi:10.1056/NEJMc1704342 PMID: 28541014.
5. Menter A. Psoriasis and psoriatic arthritis overview. Am J Manag Care. 2016;22(8Suppl):s216–24. PMID: 27356193.
6. Nwabudike LC. Tatu AL. Using complementary and alternative medicine for the treatment of psoriasis: a step in the right direction. JAMA Dermatol. 2019;1:155.
7. Tatu AL, Nwabudike LC. Metoprolol-associated onset of psoriatic arthropathy. Am J Ther. 2017;24:e370–e371. doi:10.1097/MJT.0000000000000560
8. Gudu T, Gossec L. Quality of life in psoriatic arthritis. Expert Rev Clin Immunol. 2018;14(5):405–417. doi:10.1080/1744666X.2018.1468252 Epub 2018 Apr 30. PMID: 29681202.
9. Taylor W, Gladman D, Helliwell P, et al.; CASPAR Study Group. classification criteria for psoriatic arthritis: development of new criteria from a large international study. Arthritis Rheum. 2006;54:2665–2673.
10. Mease PJ, Garg A, Helliwell PS, et al. Development of criteria to distinguish inflammatory from noninflammatory arthritis, enthesitis, dactylitis, and spondylitis: a report from the GRAPPA 2013 Annual Meeting. J Rheumatol. 2014;41(6):1249–1251. doi:10.3899/jrheum.140182
11. van der Heijde D. How to read radiographs according to the Sharp/van der Heijde meth- od. J Rheumatol. 1999;26:743–745.
12. Mandl P, Navarro-Compán V, Terslev L, et al. European League Against Rheumatism (EULAR). EULAR recommendations for the use of imaging in the diagnosis and management of spondyloarthritis in clinical practice. Ann Rheum Dis. 2015;74:1327–1339. doi:10.1136/annrheumdis-2014-206971
13. Finzel S, Englbrecht M, Engelke K, et al. A comparative study of periarticular bone lesions in rheumatoid arthritis and psoriatic arthritis. Ann Rheum Dis. 2011;70(1):122–127. doi:10.1136/ard.2010.132423
14. Diekhoff T, Hermann KG, Greese J, et al. Comparison of MRI with radiography for detecting structural lesions of the sacroiliac joint using CT as standard of reference: results from the SIMACT study. Ann Rheum Dis. 2017;76:1502–1508. doi:10.1136/annrheumdis-2016-210640
15. Kaeley GS, Eder L, Aydin SZ, et al. Enthesitis: a hallmark of psoriatic arthritis. Semin Arthritis Rheum. 2018;48(1):35–43. doi:10.1016/j.semarthrit.2017.12.008
16. Feced Olmos CM, Alvarez-Calderon O, Hervás Marín D, et al. Relationship between structural damage with loss of strength and functional disability in psoriatic arthritis patients. Clin Biomech. 2019;68:169–174. doi:10.1016/j.clinbiomech.2019.06.009
17. Simon D, Kleyer A, Faustini F, et al. Simultaneous quantification of bone erosions and enthesiophytes in the joints of patients with psoriasis or psoriatic arthritis - effects of age and disease duration. Arthritis Res Ther. 2018;20:203. doi:10.1186/s13075-018-1691-z
18. Wassenberg S. Radiographic scoring methods in psoriatic arthritis. Clin Exp Rheumatol. 2015;33:55–59.
19. van der Heijde D, Sharp J, Wassenberg S, et al. Psoriatic arthritis imaging: a review of scoring methods. Ann Rheum Dis. 2005;64:ii61– ii64. doi:10.1136/ard.2004.030809
20. De Bruin F, De Koning A, van den Berg R. Development of the CT Syndesmophyte Score (CTSS) in patients with ankylosing spondylitis: data from the SIAS cohort. Ann Rheum Dis. 2018;77(3):371–377. doi:10.1136/annrheumdis-2017-212553
21. de Koning A, de Bruin F, van den Berg R, et al. Low-dose CT detects more progression of bone formation in comparison to conventional radiography in patients with ankylosing spondylitis: results from the SIAS cohort. Ann Rheum Dis. 2018;77:293–299. doi:10.1136/annrheumdis-2017-211989
22. Fukuda T, Umezawa Y, Asahina A, et al. Dual energy CT iodine map for delineating inflammation of inflammatory arthritis. Eur Radiol. 2017;27(12):5034–5040. doi:10.1007/s00330-017-4931-8
23. Hotta M, Minamimoto R, Kaneko H, et al. Fluorodeoxyglucose PET/CT of Arthritis in Rheumatic Diseases: a Pictorial Review. Radiographics. 2020;40:223–240. doi:10.1148/rg.2020190047
24. D’Agostino MA. Enthesitis detection by ultrasound: where are we now? Clin Exp Rheumatol. 2018;36:127–130.
25. Helliwell PS, Coates LC, Chew NS, et al. Comparing psoriatic arthritis low-field magnetic resonance imaging, ultrasound, and clinical outcomes: data from the TICOPA trial. J Rheumatol. 2020;47:1338–1343. doi:10.3899/jrheum.181385
26. Ammitzbøll-Danielsen M, Glinatsi D, Torp-Pedersen S, et al. Tenosynovitis Evaluation Using Image Fusion and B-Flow - A Pilot Study on New Imaging Techniques in Rheumatoid Arthritis Patients. Ultraschall Med. 2017;38:285–293. doi:10.1055/s-0043-101228
27. Sewerin P, Ostendorf B, Schleich C. MRI diagnostics in inflammatory joint and spinal diseases: protocols and special sequences: when and for what? Z Rheumatol. 2018;77:538–548. doi:10.1007/s00393-018-0497-3
28. Sudoł-Szopińska I, Pracoń G. Diagnostic imaging of psoriatic arthritis. Part II: magnetic resonance imaging and ultrasonography. J Ultrason. 2016;16:163–174. doi:10.15557/JoU.2016.0018
29. Guo RM, Lin WS, Liu WM, et al. Quantification of fat infiltration in the sacroiliac joints with ankylosing spondylitis using IDEAL sequence. Clin Radiol. 2018;73:231–236. doi:10.1016/j.crad.2017.10.015
30. Ren C, Zhu Q, Yuan H. Mono-exponential and bi-exponential model-based diffusion-weighted MR imaging and IDEAL-IQ sequence for quantitative evaluation of sacroiliitis in patients with ankylosing spondylitis. Clin Rheumatol. 2018;37:3069–3076. doi:10.1007/s10067-018-4321-x
31. Stecco A, Trisoglio A, Soligo E, et al. Whole-Body MRI with Diffusion-Weighted Imaging in Bone Metastases: a Narrative Review. Diagnostics. 2018;8:45.
32. Werner SG, Langer HE, Ohrndorf S, et al. Inflammation assessment in patients with arthritis using a novel in vivo fluorescence optical imaging technology. Ann Rheum Dis. 2012;71:504–510. doi:10.1136/annrheumdis-2010-148288
33. Schmidt A, Glimm AM, Haugen IK, et al. Detection of subclinical skin manifestation in patients with psoriasis and psoriatic arthritis by fluorescence optical imaging. Arthritis Res Ther. 2020;22:192. doi:10.1186/s13075-020-02277-x
34. Erdmann-Keding M, Ohrndorf S, Werner SG, et al. Fluorescence optical imaging for the detection of potential psoriatic arthritis in comparison to musculoskeletal ultrasound. J Dtsch Dermatol Ges. 2019;17:913–921. doi:10.1111/ddg.13931
35. Wiemann A, Werner SG, Langer HE, et al. The “green nail” phenomenon in ICG-enhanced fluorescence optical imaging - a potential tool for the differential diagnosis of psoriatic arthritis. J Dtsch Dermatol Ges. 2019;17:138–147.
36. Sunar I, Ataman S, Nas K, et al. Enthesitis and its relationship with disease activity, functional status, and quality of life in psoriatic arthritis: a multi-center study. Rheumatol Int. 2020;40:283–294. doi:10.1007/s00296-019-04480-9
37. Mathew AJ, Krabbe S, Eshed I, et al. The OMERACT MRI in Enthesitis Initiative: definitions of Key Pathologies, Suggested MRI Sequences, and a Novel Heel Enthesitis Scoring System. J Rheumatol. 2019;46:1232–1238. doi:10.3899/jrheum.181093
38. Palominos PE, de Campos APB, Ribeiro SLE, et al. Correlation of enthesitis indices with disease activity and function in axial and peripheral spondyloarthritis: a cross-sectional study comparing MASES, SPARCC, and LEI. Adv Rheumatol. 2019;59:23. doi:10.1186/s42358-019-0066-8
39. Molto A, Sieper J. Peripheral spondyloarthritis: concept, diagnosis, and treatment. Best Pract Res Clin Rheumatol. 2018;32:357–368. doi:10.1016/j.berh.2019.02.010
40. Sarbu MI, Sarbu N. Musculoskeletal clinical and imaging manifestations in inflammatory bowel diseases. Open Med. 2019;14:75–84. doi:10.1515/med-2019-0011
41. Balint PV, Terslev L, Aegerter P, et al. Reliability of a consensus-based ultrasound definition and scoring for enthesitis in spondyloarthritis and psoriatic arthritis: an OMERACT US initiative. Ann Rheum Dis. 2018;77:1730–1735. doi:10.1136/annrheumdis-2018-213609
42. Lai KL. Ultrasonography greatly improves the ability of clinicians to diagnose psoriatic arthritis. J Med Ultrasound. 2019;27:167–168. doi:10.4103/JMU.JMU_52_19
43. Bruyn GA, Iagnocco A, Naredo E, et al. OMERACT definitions for ultrasonographic pathologies and elementary lesions of rheumatic disorders 15 years on. J Rheumatol. 2019;46:1388–1393. doi:10.3899/jrheum.181095
44. Felbo SK, Terslev L, Østergaard M. Imaging in peripheral and axial psoriatic arthritis: contributions to diagnosis, follow-up, prognosis, and knowledge of pathogenesis. Clin Exp Rheumatol. 2018;36:24–34.
45. Bagel J, Schwartzman S. Enthesitis and Dactylitis in Psoriatic Disease: a Guide for Dermatologists. Am J Clin Dermatol. 2018;19:839–852. doi:10.1007/s40257-018-0377-2
46. Kaeley GS, Eder L, Aydin SZ, et al. Dactylitis: a hallmark of psoriatic arthritis. Semin Arthritis Rheum. 2018;48:263–273. doi:10.1016/j.semarthrit.2018.02.002
47. Choy E, Baraliakos X, Behrens F, et al. The need for comparative data in spondyloarthritis. Arthritis Res Ther. 2019;21:32. doi:10.1186/s13075-019-1812-3
48. Tinazzi I, McGonagle D, Aydin SZ, et al. ‘Deep Koebner’ phenomenon of the flexor tendon-associated accessory pulleys as a novel factor in tenosynovitis and dactylitis in psoriatic arthritis. Ann Rheum Dis. 2018;77:922–925. doi:10.1136/annrheumdis-2017-212681
49. Girolimetto N, Costa L, Mancarella L, et al. Symptomatic psoriatic dactylitis is associated with ultrasound determined extra-synovial inflammatory features and shorter disease duration. Clin Rheumatol. 2019;38:903–911. doi:10.1007/s10067-018-4400-z
50. Tinazzi I, McGonagle D, Zabotti A, et al. Comprehensive evaluation of finger flexor tendon entheseal soft tissue and bone changes by ultrasound can differentiate psoriatic arthritis and rheumatoid arthritis. Clin Exp Rheumatol. 2018;36:785–790.
51. Girolimetto N, Macchioni P, Tinazzi I, et al. Ultrasonographic evidence of predominance of acute extracapsular and chronic intrasynovial patterns in 100 cases of psoriatic hand dactylitis. J Rheumatol. 2020;47:227–233. doi:10.3899/jrheum.190046
52. Furlan A, Stramare R. The thickening of flexor tendons pulleys: a useful ultrasonographical sign in the diagnosis of psoriatic arthritis. J Ultrasound. 2018;21:309–314. doi:10.1007/s40477-018-0325-2
53. Antony AS, Allard A, Rambojun A, et al. Psoriatic Nail Dystrophy Is Associated with Erosive Disease in the Distal Interphalangeal Joints in Psoriatic Arthritis: a Retrospective Cohort Study. J Rheumatol. 2019;46:1097–1102. doi:10.3899/jrheum.180796
54. Naredo E, Möller I, de Miguel E, et al. High prevalence of ultrasonographic synovitis and enthesopathy in patients with psoriasis without psoriatic arthritis: a prospective case-control study. Rheumatology. 2011;50:1838–1848. doi:10.1093/rheumatology/ker078
55. Krajewska-Włodarczyk M, Owczarczyk-Saczonek A, Placek W, et al. Ultrasound assessment of changes in nails in psoriasis and psoriatic arthritis. J Biomed Res Int. 2018;9:25.
56. Mondal S, Dutta S, Lahiri D, et al. Assessment of nail unit structures by ultrasound in patients with psoriatic arthritis and their correlations with disease activity indices: a case-control study. A Rheumatol Int. 2018;38:2087–2093. doi:10.1007/s00296-018-4160-8
57. Mendonça JA, Aydin SZ, D’Agostino MA. The use of ultrasonography in the diagnosis of nail disease among patients with psoriasis and psoriatic arthritis: a systematic review. Adv Rheumatol. 2019;59:41. doi:10.1186/s42358-019-0081-9
58. Naredo E, Janta I, Baniandrés-Rodríguez O, et al. To what extend is nail ultrasound discriminative between psoriasis, psoriatic arthritis and healthy subjects? Rheumatol Int. 2019;39:697–705. doi:10.1007/s00296-018-4222-y
59. Mondal S, Dutta S, Lahiri D, et al. Assessment of nail unit structures by ultrasound in patients with psoriatic arthritis and their correlations with disease activity indices: a case-control study. Rheumatol Int. 2018;38:2087–2093.
60. Tang Y, Cheng S, Yang Y, et al. Ultrasound assessment in psoriatic arthritis (PsA) and psoriasis vulgaris (non-PsA): which sites are most commonly involved and what features are more important in PsA? Quant Imaging Med Surg. 2020;10:86–95. doi:10.21037/qims.2019.08.09
61. Yap KS, Ye JY, Li S, et al. Back pain in psoriatic arthritis: defining prevalence, characteristics and performance of inflammatory back pain criteria in psoriatic arthritis. Ann Rheum Dis. 2018;77:1573–1577. doi:10.1136/annrheumdis-2018-213334
62. Rusman T, John MB, van der Weijden MAC, et al. Presence of active MRI lesions in patients suspected of non-radiographic axial spondyloarthritis with high disease activity and chance at conversion after a 6-month follow-up period. Clin Rheumatol. 2020;39:1521–1529. doi:10.1007/s10067-019-04885-8
63. Jadon DR, Sengupta R, Nightingale A, et al. Axial Disease in Psoriatic Arthritis study: defining the clinical and radiographic phenotype of psoriatic spondyloarthritis. Ann Rheum Dis. 2017;76:701–707. doi:10.1136/annrheumdis-2016-209853
64. Feld J, Chandran V, Haroon N, et al. Axial disease in psoriatic arthritis and ankylosing spondylitis: a critical comparison. Nat Rev Rheumatol. 2018;14:363–371. doi:10.1038/s41584-018-0006-8
65. Helliwell PS, Hickling P, Wright V. Do the radiological changes of classic ankylosing spondylitis differ from the changes found in the spondylitis associated with inflammatory bowel disease, psoriasis, and reactive arthritis? Ann Rheum Dis. 1998;57:135–140. doi:10.1136/ard.57.3.135
66. Pérez Alamino R, Maldonado Cocco JA, Citera G, et al. Differential features between primary ankylosing spondylitis and spondylitis associated with psoriasis and inflammatory bowel disease. J Rheumatol. 2011;38:1656–1660. doi:10.3899/jrheum.101049
67. Salaffi F, Carotti M, Di Donato E, et al. Preliminary validation of the Simplified Psoriatic Arthritis Radiographic Score (SPARS). Skeletal Radiol. 2019;48:1033–1041. doi:10.1007/s00256-018-3124-0
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.