Back to Journals » International Journal of General Medicine » Volume 16
New Insights into the Role of HMGB2 in ST-Segment Elevation Myocardial Infarction
Authors Qin H, Wang W , Hu L , Yu Z, Chen Y, Zhao Y, Liao Y, Yang R
Received 9 July 2023
Accepted for publication 31 August 2023
Published 14 September 2023 Volume 2023:16 Pages 4181—4191
DOI https://doi.org/10.2147/IJGM.S429761
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Prof. Dr. Yuriy Sirenko
Hao Qin,1,* Wenjun Wang,2,* Longlong Hu,1 Zuozhong Yu,1 Yang Chen,1 Yuanbin Zhao,1 Yanhui Liao,1 Renqiang Yang1
1Department of Cardiovascular Medicine, the Second Affiliated Hospital of Nanchang University, Nanchang, Jiangxi, 330006, People’s Republic of China; 2Department of Respiratory Diseases, the Second Affiliated Hospital of Nanchang University, Nanchang, Jiangxi, 330006, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Renqiang Yang, Department of Cardiovascular Medicine, the Second Affiliated Hospital of Nanchang University, 1 Minde Road, Nanchang, Jiangxi, 330006, People’s Republic of China, Tel +86-13979117790, Fax +86-0791-87357375, Email [email protected]
Background: Ischemic heart disease is one of the leading causes of death in the world, of which ST-segment elevation myocardial infarction (STEMI) is an important type. Inappropriate activation and accumulation of platelets typically induced thrombosis, which may result in acute vessel occlusion and STEMI. Multiple cytokines have been shown to regulate platelet activation, but the relationship between HMGB2 and platelet activation has not been elucidated.
Methods: We collected peripheral blood of STEMI patients and healthy adults, and mass spectrometry analysis of platelet proteins was conducted. The “edgeR” package was used to identify the differentially expressed proteins (DEPs). The Kyoto Encyclopedia of Genes and Genomes (KEGG), Gene ontology (GO) and Gene Set Enrichment Analysis (GSEA) were used to identify the significantly changed pathways. Western blot and ELISA were used to detect the expression of a high mobility group box 2 (HMGB2). Flow cytometric analysis and platelet aggregation rate were performed to evaluate the activation of platelets.
Results: We identified ALOX5, HIST1H1B, S100A11, HMGB2, and RPS15A were the top five up-regulated proteins by differential expression analysis. Western blot verified that the relative protein expression of HMGB2 in platelet was significantly higher in STEMI patients compared with control adults, and the results of ELISA indicated that the serum HMGB2 level increased and significantly correlated with neutrophil count in STEMI patients. Further investigation showed that the platelet aggregation induced by ADP, the activation of integrin αIIbβ 3 and CD62P expression on platelet surface were all enhanced by the recombinant HMGB2 (rHMGB2).
Conclusion: In conclusion, HMGB2 may be the key molecule to regulate platelet activation in patients with STEMI, which may serve as a potential therapeutic target for STEMI.
Keywords: ST-segment elevation myocardial infarction, STEMI, proteomics analysis, platelet, HMGB2
Introduction
Cardiovascular disease is the most common disease in the world. In 2019, nearly 15 million people died of myocardial infarction and stroke.1 ST-segment elevation myocardial infarction (STEMI) is an important type of myocardial infarction and poses a serious threat to patients’ lives. Although STEMI was explored by numerous scholars, the pathophysiological mechanism has not been revealed thoroughly.
Platelets, as an important type of blood cells, were responsible for maintaining the integrity of vascular endothelium.2 Inappropriate platelet activation and accumulation at the site of injured vascular are critical steps for occlusive thrombus formation in those arterial thrombotic diseases.3,4 Enhanced platelet reactivity is associated with atherosclerosis, hyperlipidemia, and hyperglycemia.5,6 Studies have shown that there was a significant correlation between platelet hyperreactivity and cardiovascular death in STEMI patients [HR=1.78 (95% CI 1.04–3.03), p=0.036], thus the platelet activity was an important marker for long-term prognosis of STEMI.7 Multiple cytokines have been identified to regulate platelet activation. Study revealed that PCSK9 activates the p38MAPK/cPLA2/COX-1/TXA2 signaling pathways by binding to platelet CD36 and thus enhanced platelet activation. In vivo experiments indicate that PCSK9 enhances thrombosis in a FeCl3-injured mesenteric arteriole thrombosis mouse model and aggravates microvascular obstruction and promotes MI expansion post-MI.8 However, the relationship between HMGB2 and platelets hyperactivity, as well as the enhanced tendency of thrombus formation, has not been elucidated.9
DAMPs (including oxidized lipid derivatives, high mobility group box 1 (HMGB1), high mobility group box 2 (HMGB2), S100A, and so on) are typically signaling molecules of tissue damage and oxidative stress to alert the host’s innate immune system. DAMPs are recognized by pattern recognition receptors (PRRs) which are classified into 5 major families according to protein domain homology: toll-like receptors (TLRs), C-type lectin receptors, NLRs, absent in melanoma 2 (AIM2)–like receptors, and retinoic acid inducible gene-I-like receptors (RIG-I-like receptors).10,11 Platelet-derived HMGB1 could potentiate platelet activation under the stimulation of agonists (Collagen, Adenosine triphosphate (ADP), and C-reactive protein (CRP)) and further enhance the thrombus formation of mesenteric artery induced by FeCl3 in mice.12 These results indicate the important role of HMGB1 in thrombotic diseases. HMGB1 and HMGB2 share 80% amino acid sequence similarity, and both are expressed in leucocyte.13 In recent years, an increasing number of studies have focused on the relationship between HMGB2 and cardiovascular disease.14 For example, researchers observed the accumulation of reactive oxygen species (ROS) in myocardial cells by recombinant HMGB2 management, which promoted apoptosis and abnormal autophagy of myocardial cells and finally increased the area of myocardial infraction.15 Moreover, previous studies showed that the serum HMGB2 level was positively correlated with the incidence of in-stent restenosis, which hints at the possible role of HMGB2 in thrombosis.16
In this study, we explored the changes of platelet proteomics in patients with STEMI, and bioinformatics analysis and clinical sample detection were conducted to verify the differential expression proteins. Through analyzing the correlation between serum HMGB2 levels and blood cell counts, we further explored the source of serum HMGB2. Eventually, we found that HMGB2 directly enhanced platelet activation, which may be a new mechanism of platelet hyperreactivity in STEMI.
Materials and Methods
Platelet Proteomics Mass Spectrometry Analysis
According to the diagnostic criteria of STEMI,17 6 patients with acute chest pain who were sent to the emergency department of The Second Affiliated Hospital of Nanchang University were enrolled between October 2022 and January 2023. At the same time, 6 healthy controls were recruited. Patients were included in this study if ST-elevation >0.1mV in at least two contiguous leads within 24 h, and the age was older than 18 years. Exclusion criteria included patients with active inflammatory or autoimmune diseases, severe heart failure, hemodynamic instability, myocarditis or pericarditis, diseases of the hematopoietic system, severe kidney or liver disease, malignancy, therapy of immunosuppressant agents, and previous coronary artery bypass graft surgery.18 The study was performed in accordance with the Declaration of Helsinki and was approved by the Ethics Committee of the Second Affiliated Hospital of Nanchang University. All patients gave written informed consent.
In order to find the key molecules participated in the pathogenesis of STEMI, adults matched with baseline information, such as sex, age, and history of past illness, were selected. Platelets were purified from peripheral venous blood by gradient centrifugation,19 and the expression of proteins was detected by liquid chromatography-tandem mass spectrometry.
Differential Expression Analysis
The “edgeR” package was used to screen the differentially expressed proteins (DEPs) in RStudio software 4.1.1 with the threshold of log |Fold Change|>0 and p value<0.05.20 Volcano plot was presented to visualize the DEPs.
Enrichment Analysis
Kyoto Encyclopedia of Genes and Genomes (KEGG), Gene Ontology (GO), and Gene Set Enrichment Analysis (GSEA) databases were used to identify the significantly changed pathways. Enrichment analysis and visualization of KEGG and GO functional annotation were carried out by ClueGo v2.5.8 and CluePedia v1.5.8 in Cytoscape v3.7.2 software.21 GSEA was performed in RStudio software 4.1.1, and the GSEA data sets, C2: curated gene sets, derived from Human MSigDB Collections in BROAD website.22,23 According to the criterion of FDR<0.05, the top pathways were screened for further analysis.
Proteins Preparation and Western Blot
According to the above criteria, 5 STEMI patients and 3 control adults were recruited. Then, 10 mL of peripheral venous blood was collected to the sodium citrate anticoagulant tube. Platelets were precipitated by gradient centrifugation and then lysed by RIPA buffer (Wuhan Boster Biological Technology Co., Ltd, Wuhan, China) to extract total platelet protein. The concentration of total platelet protein was determined using the bicinchoninic acid assay (Pierce™ BCA Protein Assay Kit, Thermo Scientific). Each protein sample was sonicated, diluted with 6× standard SDS loading buffer, and heated at 100°C for 10 minutes. Platelet protein (20 μg) was separated via 10% SDS-PAGE and subsequently transferred to 0.22 µm nitrocellulose membrane for 120 min at 260 mA. Membranes were blocked for 1 h in 5% non-fat dry milk and then rinsed three times by Tris-buffered saline pH 7.5 with 0.1% Tween-20 (TBS-T). Then, membranes were incubated with HMGB2 primary antibody (Goat anti-Rabbit IgG antibody, dilution ratio 1:1000, Cell Signaling) overnight at 4°C, washed with TBST, and incubated with secondary antibody for 30min at 37°C. The protein bands were imaged by the Bio-Rad Chemidoc system (Bio-Rad Laboratories, California, USA) and analyzed by Image Lab software (version 6.1).
ELISAs
According to the above criteria, 26 serum samples of STEMI patients and 29 control serum samples were collected. The samples were centrifuged for 20 min at 1000×g, then the levels of serum HMGB2 were routinely measured using a commercial HMGB2 ELISA kit (Aachen, antibodies, Germany) according to the manufacturer’s instruction.
Flow Cytometric Analysis
After pretreated with rHMGB2 (500ng/mL) for 40 min,16 washed platelet sample was analysed using a Beckman Coulter FC-500 flow cytometer (BeckmanCoulter, Hialeah, FL, USA). The activation of platelet integrin αIIbβ3 detected by PAC-1 binding and the release of P-selectin from α granules detected by CD62P expression on platelet surface were reported as mean fluorescence intensity (MFI).
Platelet Aggregation Rate
Platelet aggregation in response to ADP (10μM) was measured using platelet function analyzer (SINNOWA, Jiangsu, China) at room temperature. Briefly, the washed platelets were pretreated with rHMGB2 for 40 min. 250 μL platelet suspension was placed into the detecting hole, and ADP was added to induce platelet aggregation. Platelet aggregation rate (PAR) was obtained by calculating the changes of platelet counts. PAR analyses were repeated three times at least.
Statistics
All data sets were analyzed for normality by Shapiro–Wilk test. The data are presented as mean ± standard deviation (SD) for data exhibiting a normal distribution. Student’s t-test was used to compare the differences between the two groups. A one-way ANOVA test with Bonferroni post-test was applied to compare the differences of multiple groups. Rank-sum test was utilized for non-normally distributed data. The categorical data was analyzed with chi-square test or Fisher’s exact test. Statistical analyses were performed by SPSS software (version 25.0), and figures were created by GraphPad Prism software (version 8.0). P<0.05 was considered as statistically significant.
Results
Differential Expression Analysis of Proteomic
In order to investigate the key proteins regulating platelet functions in STEMI patient, 6 STEMI patients and 6 control adults were enrolled. The gender, age, history of smoking and past illness, cTnT peak, and platelet count of these patients were included. The results showed that there was no statistical difference in baseline information except that the cTnT peak in STEMI group was significantly higher than that in the control group (Table 1). Difference analysis of platelet proteomic showed that 772 proteins were differentially expressed, 510 of which were up regulated, and 262 proteins were down regulated in STEMI group (Figure 1A). ALOX5, HIST1H1B, S100A11, HMGB2, and RPS15A were the top five up-regulated genes. We further assessed the protein level of HMGB2 in both platelet and serum by Western blot and ELISA, respectively, of STEMI patients and healthy controls. The table details the clinical characteristics of both groups (Table 2 and 3). The result indicated enhanced expression levels of HMGB2 in platelets and plasma in STEMI patients (Figure 1B and C).
 |
Table 1 Subject Characteristics for Identifying the Platelet Proteomics |
 |
Table 2 Subject Characteristics for Identifying the Platelet HMGB2 Level |
 |
Table 3 Subject Characteristics for Identifying the Plasma HMGB2 Level |
The Correlation of Serum HMGB2 Levels with Blood Cell Count
To seek the ectopic source of HMGB2 protein in platelet, we assessed the correlation of serum HMGB2 levels with blood cell count. The result showed that serum HMGB2 in STEMI patients was positively correlated with neutrophil count (R2=0.3245, P=0.001) (Figure 2A) and leucocyte count (R2=0.1742, P=0.019) (Figure 2D). But there was no correlation between serum HMGB2 levels and platelet count, monocyte count, erythrocyte count, and lymphocyte count (Figure 2B, C, E and F).
Functional Enrichment
To further investigate the related pathways of these differentially expressed platelet proteins, GO, KEGG, and GSEA analysis were performed. The result of GO-BP (Biological Process) analysis showed that the platelet biological function was significantly changed including vesicle-mediated transport, cellular response to chemical stimulus, immune system process, biological adhesion, catabolic process, cellular component biogenesis, regulation of protein metabolic process, nucleic acid metabolism process, and peptide metabolic process (Figure 3A). The GO-CC (Cell Components) analysis indicated that the enriched cell components were ESCRT complex, cytoplasmic vesicle, cytosolic ribosome, side of membrane, nucleoplasm, and nuclear protein-containing complex (Figure 3B). The GO-MF (Molecular Function) that displayed the enriched cellular functions were regulation of molecular function, protein-containing complex binding, enzyme binding, signaling receptor binding, identical protein binding, carbohydrate derivative binding, hydrolase activity acting on acid anhydrides and catalytic activity (Figure 3C). The result of KEGG analysis presented that the cellular functions including platelet activation, phagosome, endocytosis, neutrophil extracellular trap formation, lipid, and atherosclerosis and regulation of actin cytoskeleton were significantly changed (Figure 4A). GSEA enrichment analysis showed that the up-regulated pathways were Nod-like receptor signaling pathway, Toll-like receptor signaling pathway, IL-1 signaling pathway, NF- κ B signaling pathway, and pentose phosphate pathway (Figure 4B). The down-regulated pathway included calcium signaling pathway, PPAR signaling pathway, G protein signaling pathway, platelet calcium homeostasis, and platelet homeostasis (Figure 4C).
HMGB2 Involve in Platelet Aggregation and Activation
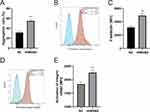
To explore the effect of serum HMGB2 on platelet function, the washed platelet was pretreated with recombinant HMGB2 (rHMGB2). We found that the rHMGB2 potentiated platelet aggregation induced by ADP (Figure 5A), and the activation of platelet integrin αIIbβ3 and CD62P expression on platelet surface were both enhanced by rHMGB2 (Figure 5B-E).
Discussion
Nowadays, atherosclerosis-related cardiovascular and cerebrovascular diseases seriously threaten people’s life and health.1 Recent studies have shown that platelet played an important role in the occurrence and development of atherosclerotic diseases.24 This study explored the relationship between HMGB2 and platelets, and there were few relevant studies focusing on this aspect. Our study found that the level of HMGB2 in both platelet and serum was significantly increased in STEMI patients compared with healthy control. Previous studies indicated that HMGB family were chaperone molecule of the transcription factor, which strongly bound with the small grooves of DNA and involved in DNA bending so as to enhance the local flexibility of the double helix and stabilize looping.25 Recently, studies have found that HMGB2 played an important role in the occurrence and development of atherosclerosis. For instance, knocking down of HMGB2 by siRNA in macrophage foam cells significantly reduced lipophagy, which attenuated the cholesterol efflux and resulted in the accumulation of oxidized lipids and foam cells in plaque.26 Another study found that endothelium-derived particles aggravated the inflammation and hypertrophy of vascular smooth muscle cells after co-incubation, and these damages could be rescued by HMGB1/HMGB2 inhibitor insulin (25 µ mol/L).27 Although the above studies presented inconsistent results on the role of HMGB2 in atherosclerosis, which did not preclude a potentially important role of HMGB2 in atherosclerosis. Analyzing these inconsistent results may give us some hints for exploring HMGB2. Our study explored the relationship between HMGB2 and platelet function. Considering limited protein synthesis in platelet, the transfer of protein to platelet represented another mechanism for protein enrichment.28 So, the level of serum HMGB2 was evaluated, and we found that the serum concentration of HMGB2 in STEMI patients was increased and positively correlated with neutrophil count. These findings revealed that the serum HMGB2 may be released by neutrophils and further acted on platelet.
Previous study showed that the serum HMGB2 levels were not only increased in STEMI patient but also associated with the coronary artery in-stent restenosis.16 Compared with 298 patients without in-stent restenosis (ISR), the serum HMGB2 level of 262 ISR patients was significantly increased and was positively related to the severity of ISR.16 Further investigations indicated that HMGB2 was involved in enhancing the proliferation and migration of human aortic smooth muscle cells and neointimal hyperplasia in C57Bl/6 mice by inducing ROS accumulation.16 However, such changes were attenuated when the receptor of advanced glycation end products (RAGE) was knocked out.16 Moreover, local injection of rHMGB2 in mice with acute myocardial infarction (AMI) could enlarge the infarct area and decrease left ventricular ejection fraction.15 Further study indicated that the potential mechanism was cardiomyocyte ROS accumulation, apoptosis, and inflammation induced by HMGB2.15 Our study identified for the first time that platelet function could be regulated by HMGB2. After HMGB2 management, platelet aggregation induced by ADP was enhanced, and the activation of platelet integrin αIIbβ3 and CD62P expression on platelet surface was increased, which may accelerate thrombosis and aggregate ischemia-reperfusion injury. Interestingly, GSEA analysis revealed the activation of Toll-like receptor pathway (Figure 4B), so we speculated that HMGB2 may regulate platelet activation through RAGE or Toll-like receptor.
Numerous studies showed the role of platelet in atherosclerosis and AMI. Although antiplatelet drug therapy could greatly improve the prognosis of patient with myocardial infarction, the existence of drug resistance still limits the clinical effectiveness of antiplatelet drug. This study found that HMGB2 may be the key molecule involved in regulating platelet activation. Further research was necessary to clarify the potential mechanisms of platelet activation induced by HMGB2 and excavate potential therapeutic targets for STEMI. However, there are still some limitations in this manuscript. The sample size of this study is small and some important baseline information such as comorbidities of patients, pharmacological history, and anthropometric features were lost. In the future, we will increase the sample size and collect more detailed baseline characteristics for subgroup analysis.
Data Sharing Statement
The data used to support the results of this paper are available from the corresponding author upon request.
Ethics Approval and Consent to Participate
The study was performed in accordance with the Declaration of Helsinki and was approved by the Ethics Committee of the Second Affiliated Hospital of Nanchang University. All patients gave written informed consent.
Acknowledgment
Hao Qin and Wenjun Wang are co-first authors for this study. The authors would like to thank Dr Longlong Hu and Zuozhong Yu for their technical help. In addition, many thanks go to Yang Chen and Yuanbin Zhao for their advice while drafting the manuscript. Finally, the authors would like to thank Yanhui Liao for donating antibodies for this study.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
This work was supported by the National Natural Science Foundation of China (No. 81960081).
Disclosure
The authors declare that there are no conflicts of interest in this study.
References
1. Anderson JL, Morrow DA. Acute myocardial infarction. N Engl J Med. 2017;376(21):2053–2064. doi:10.1056/NEJMra1606915
2. van der Meijden PEJ, Heemskerk JWM. Platelet biology and functions: new concepts and clinical perspectives. Nat Rev Cardiol. 2019;16(3):166–179. doi:10.1038/s41569-018-0110-0
3. Ruggeri ZM. Platelets in atherothrombosis. Nat Med. 2002;8(11):1227–1234. doi:10.1038/nm1102-1227
4. Gurbel PA, Jeong YH, Navarese EP, Tantry US. Platelet-mediated thrombosis: from bench to bedside. Circ Res. 2016;118(9):1380–1391. doi:10.1161/CIRCRESAHA.115.307016
5. Murphy AJ, Tall AR. Disordered haematopoiesis and athero-thrombosis. Eur Heart J. 2016;37(14):1113–1121. doi:10.1093/eurheartj/ehv718
6. Hu L, Zhang Y, Zhang Y, et al. Platelets Express Activated P2Y12 Receptor in Patients With Diabetes Mellitus. Circulation. 2017;136(9):817–833. doi:10.1161/CIRCULATIONAHA.116.026995
7. Marcucci R, Valente S, Gori AM, et al. Global platelet hyperreactivity and elevated C-reactive protein levels predict long term mortality in STEMI patients. Thromb Res. 2014;134(4):884–888. doi:10.1016/j.thromres.2014.07.020
8. Qi Z, Zhang J, Yang W, et al. PCSK9 Enhances Platelet Activation, Thrombosis, and Myocardial Infarct Expansion by Binding to Platelet CD36. Circulation. 2021;143(1):45–61. doi:10.1161/CIRCULATIONAHA.120.046290
9. Jennings LK. Mechanisms of platelet activation: need for new strategies to protect against platelet-mediated atherothrombosis. Thromb Haemost. 2009;102(08):248–257. doi:10.1160/TH09-03-0192
10. De Martinis M, Ginaldi L, Sirufo MM, et al. Alarmins in Osteoporosis, RAGE, IL-1, and IL-33 Pathways: a Literature Review. Medicina. 2020;56(3). doi:10.3390/medicina56030138
11. Olsen MB, Gregersen I, Sandanger O, et al. Targeting the inflammasome in cardiovascular disease. JACC Basic Transl Sci. 2022;7(1):84–98. doi:10.1016/j.jacbts.2021.08.006
12. Vogel S, Bodenstein R, Chen Q, et al. Platelet-derived HMGB1 is a critical mediator of thrombosis. J Clin Invest. 2015;125(12):4638–4654. doi:10.1172/JCI81660
13. Barreiro-Alonso A, Lamas-Maceiras M, Rodriguez-Belmonte E, Vizoso-Vazquez A, Quindos M, Cerdan ME. High mobility group b proteins, their partners, and other redox sensors in ovarian and prostate cancer. Oxid Med Cell Longev. 2016;2016:5845061. doi:10.1155/2016/5845061
14. Jo HR, Jeong JH. MicroRNA-Mediated Downregulation of HMGB2 Contributes to Cellular Senescence in Microvascular Endothelial Cells. Cells. 2022;11(3):584. doi:10.3390/cells11030584
15. Liu ZH, Dai DP, Ding FH, et al. Association of serum HMGB2 level with MACE at 1 mo of myocardial infarction: aggravation of myocardial ischemic injury in rats by HMGB2 via ROS. Am J Physiol Heart Circ Physiol. 2017;312(3):H422–H436. doi:10.1152/ajpheart.00249.2016
16. He YH, Wang XQ, Zhang J, et al. Association of Serum HMGB2 Levels With In-Stent Restenosis: HMGB2 Promotes Neointimal Hyperplasia in Mice With Femoral Artery Injury and Proliferation and Migration of VSMCs. Arterioscler Thromb Vasc Biol. 2017;37(4):717–729. doi:10.1161/ATVBAHA.116.308210
17. Ibanez B, James S, Agewall S, et al. ESC Guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation: the Task Force for the management of acute myocardial infarction in patients presenting with ST-segment elevation of the European Society of Cardiology (ESC). Eur Heart J. 2017;39(2):119–177. doi:10.1093/eurheartj/ehx393
18. Zhang X, Cheng M, Gao N, et al. Utility of S100A12 as an Early Biomarker in Patients With ST-Segment Elevation Myocardial Infarction. Front Cardiovasc Med. 2021;8:747511. doi:10.3389/fcvm.2021.747511
19. Falker K, Klarstrom-Engstrom K, Bengtsson T, Lindahl TL, Grenegard M. The toll-like receptor 2/1 (TLR2/1) complex initiates human platelet activation via the src/Syk/LAT/PLCgamma2 signalling cascade. Cell Signal. 2014;26(2):279–286. doi:10.1016/j.cellsig.2013.11.011
20. Rabbani G, Baig MH, Ahmad K, Choi I. Protein-protein Interactions and their Role in Various Diseases and their Prediction Techniques. Curr Protein Pept Sci. 2018;19(10):948–957. doi:10.2174/1389203718666170828122927
21. Liu S, Wang Z, Zhu R, Wang F, Cheng Y, Liu Y. Three Differential Expression Analysis Methods for RNA Sequencing: limma, EdgeR, DESeq2. J Vis Exp. 2021;175. doi:10.3791/62528
22. Bindea G, Mlecnik B, Hackl H, et al. ClueGO: a Cytoscape plug-in to decipher functionally grouped gene ontology and pathway annotation networks. Bioinformatics. 2009;25(8):1091–1093. doi:10.1093/bioinformatics/btp101
23. Bindea G, Galon J, Mlecnik B. CluePedia Cytoscape plugin: pathway insights using integrated experimental and in silico data. Bioinformatics. 2013;29(5):661–663. doi:10.1093/bioinformatics/btt019
24. Giovanni Davi MD, Carlo Patrono MD. Platelet activation and atherothrombosis. N Eng J Med. 2007;357(24):2482.
25. McCauley MJ, Rueter EM, Rouzina I, Maher LJ 3rd, Williams MC. Single-molecule kinetics reveal microscopic mechanism by which High-Mobility Group B proteins alter DNA flexibility. Nucleic Acids Res. 2013;41(1):167–181. doi:10.1093/nar/gks1031
26. Robichaud S, Fairman G, Vijithakumar V, et al. Identification of novel lipid droplet factors that regulate lipophagy and cholesterol efflux in macrophage foam cells. Autophagy. 2021;17(11):3671–3689. doi:10.1080/15548627.2021.1886839
27. Boyer MJ, Kimura Y, Akiyama T, et al. Endothelial cell-derived extracellular vesicles alter vascular smooth muscle cell phenotype through high-mobility group box proteins. J Extracell Vesicles. 2020;9(1):1781427. doi:10.1080/20013078.2020.1781427
28. Joshi A, Schmidt LE, Burnap SA, et al. Neutrophil-Derived Protein S100A8/A9 Alters the Platelet Proteome in Acute Myocardial Infarction and Is Associated With Changes in Platelet Reactivity. Arterioscler Thromb Vasc Biol. 2022;42(1):49–62. doi:10.1161/ATVBAHA.121.317113
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.