Back to Journals » Journal of Inflammation Research » Volume 15
New Insights in the Treatment of SAPHO Syndrome and Medication Recommendations
Authors Cheng W, Li F, Tian J, Xie X , Chen JW, Peng XF, Tang Q, Ge Y
Received 11 December 2021
Accepted for publication 25 March 2022
Published 13 April 2022 Volume 2022:15 Pages 2365—2380
DOI https://doi.org/10.2147/JIR.S353539
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Ning Quan
Wei Cheng, Fen Li, Jing Tian, Xi Xie, Jin-Wei Chen, Xiao-Fei Peng, Qi Tang, Yan Ge
Department of Rheumatology and Immunology, The Second Xiangya Hospital, Central South University, Changsha, 410011, Hunan, People’s Republic of China
Correspondence: Yan Ge, Department of Rheumatology and Immunology, The Second Xiangya Hospital, Central South University, Changsha, 410011, Hunan, People’s Republic of China, Email [email protected]
Abstract: Synovitis, acne, pustulosis, hyperostosis, osteitis (SAPHO) syndrome is a rare autoinflammatory disease characterized by dermatological disorders and osteoarticular inflammatory lesions. This article reviews the application of biologics and other treatments based on the therapeutic target and the size of molecules in SAPHO syndrome. We found that drugs, especially biologics, have different effects on bone, joint, and skin damage. This may relate to the different inflammatory pathways involved in the osteoarticular and cutaneous symptoms in SAPHO patients. In this study, we provide stratified medication recommendations for SAPHO syndrome. Patients with osteoarticular symptoms can consider tumor necrosis factor blockers, JAK inhibitor, interleukin (IL)-1 inhibitor, and IL-17 inhibitor. Patients with cutaneous symptoms should consider IL-17 and JAK inhibitors. Apremilast, Tripterygium wilfordii Hook F, and bisphosphonates are other effective treatments.
Keywords: SAPHO, osteoarticular symptoms, cutaneous symptoms, immune pathway pathways, biologics, targeted small molecule compounds
Introduction
Synovitis, acne, pustulosis, hyperostosis, osteitis (SAPHO) syndrome is a rare autoinflammatory disease characterized by dermatological disorders and osteoarticular inflammatory lesions. There were no validated diagnostic criteria until 1988.1 The presence of only 1 of the 4 inclusion criteria is sufficient to arrive at a diagnosis of SAPHO syndrome: (1) Osteo-articular manifestations of acne conglobata, acne fulminans, or hidradenitis suppurativa. (2) Osteo-articular manifestations of PPP.(3) Hyperostosis (of the anterior chest wall, limbs or spine) with or without dermatosis. (4) CRMO involving the axial or peripheral skeleton with or without dermatosis. In 1994, Kahn MF presented the diagnostic standards, which emphasized biopsy-proven aseptic osteitis limited clinical application. Therefore, it was revised again in 2003 as follows:2 bone–joint involvement associated with isolated palmoplantar pustulosis (PPP); bone–joint involvement associated with severe acne; isolated or multifocal sterile hyperostosis/osteitis (adults); chronic recurrent multifocal osteomyelitis (children).
Most related drugs come from case reports or single-center cohort studies because of the low incidence of SAPHO, and no consensus has been reached regarding the treatment of SAPHO syndrome. The treatment strategy is based on seronegative spondyloarthropathy. Non-steroid anti-inflammatory drugs (NSAIDs) are the first-line treatment in most patients; however, they are ineffective in some cases. Intra-articular injections or systemic oral glucocorticoids are effective in most patients. However, the chronic long-term adverse effects cannot be ignored, including relapse after dose reduction or withdrawal. Disease-modifying antirheumatic drugs (DMARDs), such as methotrexate, sulfasalazine, cyclosporine A, cyclophosphamide, and thalidomide are used as second-line treatment; however, a significant percentage of patients fail to achieve remission.3,4 After understanding the pathogenesis better, biologics were suggested for several cases refractory to conventional treatment and achieved good results. TNF blockers were the first choice; however, improvements in the cutaneous symptoms were unsatisfactory. For example, for patients unresponsive to TNF blockers, IL-1 inhibitors and biologics targeting the IL-17/IL-23 axis could be used. The latest case reports show JAK inhibitor therapy as a promising treatment strategy for SAPHO syndrome. This article reviews the application of biologics and other treatments based on the therapeutic targets and the size of molecules in SAPHO syndrome until September 2021 and summarizes the immune pathways involved in SAPHO syndrome pathogenesis and new drug treatments. We found that drugs, especially biologics, have different effects on bone, joint, and skin damage. This might relate to the different inflammatory pathways involved in osteoarticular and cutaneous symptoms in SAPHO patients. Based on known pathogenesis, we provide stratified medication recommendations for SAPHO syndrome. Bone and skin damage should be evaluated first, followed by appropriate drug selection.
Etiopathogenesis
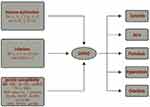
The precise etiopathogenesis of SAPHO remains unclear; however, it is considered an autoinflammatory syndrome related to various etiologies, such as immune dysfunction,4 infection,5 and genetic susceptibility.6 In patients with SAPHO, elevation in proinflammatory cytokine tumor necrosis factor (TNF)-α, interleukin (IL)-1, IL-8, IL-17, IL-18,7,8 and IL-23–helper T cells and (Th) 17 axis,9,10 imbalance in Th17 and regulatory T (Treg) cells,11 increase in the prevalence of autoantibodies12 and levels of receptor activator nuclear factor kappa-Β ligand (RANKL),13 and reduction in peripheral natural killer (NK) cells11 leads to immune system instability. Moreover, treatment targeting TNF-α, IL-1, or IL-17-IL-23 also supports the underlying inflammation-mediated pathogenesis in SAPHO patients14 (Figure 1).
Therapeutic Drug Progress
We reviewed the therapeutic drug progress for SAPHO syndrome and summarized the recently published drug-related case reports or open studies, including TNF blockers (infliximab, adalimumab, etanercept, and certolizumab pegol), IL-1 inhibition (anakinra), IL-6 inhibition (tocilizumab), IL-17 inhibition (secukinumab), IL-12/23 inhibition (ustekinumab), small molecule compounds, such as JAK inhibitors (tofacitinib) and phosphodiesterase 4 (PDE-4) inhibitors (apremilast) and Tripterygium wilfordii Hook F (TwHF), bisphosphonates. We found the different effects of these drugs, and the main difference is between bone and skin damage. Therefore, in this article, we have listed and focused on the progress of therapeutics in SAPHO syndrome and observing the drug response to osteoarticular and cutaneous symptoms (Table 1).
 |  |  |  |
Table 1 Treatment of SAPHO Syndrome |
We used the keywords SAPHO in English publications to search for case reports or case series in PubMed from 2002.1 to 2021.9 combined, including the following: antitumor necrosis factor-α (anti-TNF), infliximab, etanercept, adalimumab, certolizumab pegol, IL-1, anakinra, IL-6, tocilizumab (TCZ), IL-23, ustekinumab, IL-17, secukinumab, JAK inhibitors, tofacitinib, PDE-4 inhibitors, apremilast, abatacept, and rituximab. After removing duplicates, the abstracts of these articles were assessed to identify studies related to the inclusion criteria and therapeutic drugs, and 58 articles remained. We also reviewed literatures on TwHF, bisphosphonates at the same time.
Biologics in SAPHO Syndrome
TNF Blockers
Antitumor necrosis factor-α (anti-TNF) agents are widely used biologics for SAPHO syndrome treatment because of the following reasons: The high expression of TNF-α in the bone biopsy and abnormal expressions of IL-8 and IL-18 in the serum of SAPHO syndrome patients. High expression of these cytokines can change the neutrophil response and upregulate the expression of TNF-α and related products. Currently, the short-term efficacy of anti-TNF has been acknowledged. Monoclonal antibodies are frequently compared with receptor fusion proteins. However, maintaining treatment while avoiding potential adverse drug reactions remains inconclusive.
Infliximab
Infliximab is an anti-TNF commonly used in SAPHO treatment. Data indicate that it is fast acting and highly effective, especially in the inflammation of the bone and joint manifestations.14 Osteoarticular symptoms significantly improved in 90.3% (28/31) of patients with refractory SAPHO syndrome receiving infliximab. However, only 57.1% (12/21) saw improvement in PPP symptoms; moreover, it aggravated skin lesions (Table 1). Massara et al19 reported four patients treated with infliximab responding positively regarding bone/joint manifestations; however, 2/3 patients with skin involvement exhibited exacerbation or relapse. Fruehauf et al22 reported a patient with SAPHO syndrome and collagenous colitis. After infliximab treatment, there were rapid and partial remissions of the osteoarticular symptoms and skin lesions, respectively. However, after ten months of continuous therapy, a bone scan uncovered new active bone lesions. Ben Abdelghani et al23 reported four patients with SAPHO treated with infliximab. Improvements were temporary after infusions and chest wall pain recurred in two cases. Skin lesions healed in three of four patients and recurred or worsened in two patients. Anić et al28 reported infliximab treatment leading to complete regression of osteoarticular symptoms; however, cutaneous lesions were temporarily aggravated. The skin lesions disappeared within several weeks following the fifth application of infliximab. Mateo et al72 showed that treatment with infliximab improved clinical symptoms and radiological abnormalities and normalized ESR and CRP; however, plantar pustulosis persisted. Li et al29 reported that seven (17.1%) of the 41 patients receiving anti-TNF therapies developed new skin lesions during treatment.
Etanercept
Etanercept is a fusion protein TNF-α inhibitor. Currently, case reports related to its use in SAPHO showed good responses in osteoarticular symptoms in patients (95%, 19/20). However, some cases with cutaneous symptoms achieved unsatisfactory results (60%, 9/15) (Table 1). Li et al29 found that etanercept was effective in treating osteoarticular manifestations in five patients except for one refractory case. However, these patients developed new psoriasiform skin lesions on the trunk and limbs, and PPP worsened in two. Zhang et al39 reported that a 58-year-old woman with SAPHO developed paradoxical psoriasiform lesions, and the primary palmoplantar pustulosis were exacerbated after seven weeks of etanercept treatment. She then received Tripterygium wilfordii Hook F (TwHF) treatment, which resulted in rapid and remarkable improvement in her skin lesions and osteoarticular pain.
Adalimumab
Adalimumab is a fully-humanized monoclonal antibody. There are 15 case reports related to the use of adalimumab in patients with SAPHO (Table 1). It is effective because musculoskeletal and skin manifestations respond well in most patients. The osteoarticular and cutaneous symptoms improved in 88.2% (15/17) and 78.6% (11/14) of patients, respectively (Table 1). Henriques et al43 reported a case of SAPHO syndrome, in which the pain only slightly improved and osteitis persisted after treatment with adalimumab. Hess et al44 reported that a 17-year-old girl with SAPHO syndrome received adalimumab for over four weeks, joint and skin symptoms improved; however, life-threatening disseminated tuberculosis occurred. Cianci et al49 reported two cases with SAPHO syndrome. One showed worsening of cutaneous lesion and deterioration on MRI and X-ray. The other suffered recurring pustular lesions on the gluteal skin after administration of 40 mg adalimumab every other week. Vekic48 reported a good clinical response for adalimumab combined with methotrexate in SAPHO syndrome with concomitant hidradenitis suppuration. Marrani et al50 reported that cutaneous and osteoarticular manifestations improved with adalimumab treatment in two cases of SAPHO syndrome after failed treatment with infliximab, which occurred in two girls with Crohn’s disease and ulcerative colitis.
Certolizumab Pegol
Certolizumab pegol (Cimzia®) is a PEGylated, Fab-only recombinant humanized antibody against TNF-α.73 There are two reported use of certolizumab pegol in patients with SAPHO. Kamata et al53 reported that monotherapy with certolizumab pegol was extremely effective for osteoarticular lesions and palmoplantar cysts.
IL-1 Inhibition
Data related to IL-1 inhibition in SAPHO are encouraging, with most patients exhibiting a significant response in osteoarticular symptoms (8/10, 80%). In contrast, IL-1 inhibition seems ineffective in controlling skin disease. Only 2/7 (28.6%) patients exhibited improvement in skin manifestations; however, without deterioration in other cases (Table 1). In 2010, Colina et al55 first proposed that the abnormal regulation of the P2X7-IL-1β inflammatory axis is related to SAPHO syndrome. They used anakinra for treatment and achieved a specific effect. After the label treatment with anakinra 100 mg/day, the painful osteoarticular symptomatology, cutaneous lesions, and systemic symptoms disappeared. In 2012, Wendling et al56 used anakinra to treat six SAPHO syndrome patients, and the pain scores and inflammation levels significantly improved in five. Anakinra was effective in two patients who were unresponsive to TNF blockers. There are two additional cases of successful treatment with anakinra in patients with CRMO.57,58 However, Eleftheriou et al reported that anakinra treatment alleviated the symptoms of one child with CRMO at 6 weeks, but no sustained response with costochondritis and psoriasis-like rash after 1-year follow-up.24 It has a short half-period compared with TNF-α antagonists and requires daily injections, limiting its clinical application.
IL-6 Inhibition
Tocilizumab is an anti-IL-6 receptor monoclonal antibody. Sato et al60 reported that tocilizumab (TCZ) might be an effective therapy for muscle inflammation in CRMO, one of the criteria for SAPHO syndrome. However, current data demonstrate that TCZ is not ideal for treating SAPHO syndrome (2/5 efficacy) (Table 1). Fujita et al59 administered TCZ for SAPHO syndrome and AA amyloidosis in a 78-year-old man. However, he developed an aseptic subcutaneous abscess in the anterior chest 3 weeks after the first administration. TCZ treatment increases serum IL-6 levels, and a transient increase in the serum levels of IL-6 immediately after IL-6 receptor blockade might induce aseptic abscesses in SAPHO syndrome. Sun et al61 reported two cases of SAPHO syndrome with disease progression and unexpected neutropenia after treatment with tocilizumab (TCZ), which might be associated with the inhibition of IL-6 in recruiting neutrophils into the peripheral blood.
IL-23/IL-17 Axis
Data regarding the use of newer biologics targeting the IL-23/IL-17 axis in patients with SAPHO are limited. There are only five and eight cases treated with ustekinumab and secukinumab, respectively; the efficacy of IL-23/IL-17 inhibitors is inconclusive. Ustekinumab is an antibody against the p40 subunit of IL-12 and IL-23.74 Response rates with ustekinumab for both osteoarticular and cutaneous symptoms were 60% (3/5). Secukinumab (Cosentyx®) is a first-in-class fully human monoclonal antibody against interleukin-17A.69 After receiving secukinumab, 87.5% (7/8) of patients with cutaneous symptoms achieved satisfactory results; however, only 66.7% (6/9) with osteoarticular symptoms improved (Table 1). Cornillier et al62 reported a 44-year-old patient suffering from SAPHO syndrome for ten years. After initiation of ustekinumab, the skin lesions disappeared and joint pain improved. Wendling et al10 reported the results of six courses of IL-12/IL-23 and IL-17 targeted therapies (3 ustekinumab and 3 secukinumab), skin symptoms improved in three cases, one improvement with secukinumab, and two remissions (one each with secukinumab and ustekinumab). Regarding the rheumatic symptoms, no significant improvement was observed under any of the six treatment courses. Firinu et al63 reported that subcutaneous monotherapy using ustekinumab 90 mg significantly improved skin and osteoarticular symptoms after two years of treatment, without adverse effects. Wang et al64 reported a case series of four patients with SAPHO syndrome to clarify the efficacy of secukinumab. PPP was alleviated, and the BME on MRI showed alleviation or complete resolution after the treatment. No deterioration, new lesions, or severe adverse events were observed. Sun et al65 reported a 31-year-old male patient with a 9-year history of SAPHO syndrome. He was successively treated with pamidronate, tofacitinib, and adalimumab between 2017 and 2019 without achieving long-term remission. After secukinumab was prescribed in October 2019, MRI revealed remarkable remission, and his jaw pain, mouth opening limitation, and inflammatory indicators improved significantly. This case suggested that IL-17A blockade might be a potential treatment option for refractory SAPHO syndrome with mandibular lesions. Considering the increasing evidence supporting the role of IL-17/TH17 in SAPHO and the successful application of IL-12/23 and IL-17 inhibitors in psoriasis and psoriatic arthritis, an increase in the available data is expected in the future.
However, there are no published data related to the use of abatacept or rituximab in patients with SAPHO.
Beyond Biologics
Other drugs have been used to treat SAPHO in recent years, including targeted small molecule compounds, such as JAK inhibitors, PDE-4, arthralgia‐dispelling herbs, and bisphosphonates.
JAK Inhibitors
Tofacitinib, the first rheumatologic JAK inhibitor, is a small-molecule oral selective inhibitor of JAK1/JAK3, JAK2 to a lesser extent, and TYK2 to the least extent.75,76 Tofacitinib is effective in patients with poor response and tolerance to traditional drugs or biological agents. In 2018, Yang et al67 reported a case of SAPHO treated with traditional DMARDs, a combination of methotrexate, glucocorticoid, etanercept, and other treatments; however, the effect was unsatisfactory. After switching to tofacitinib, the clinical symptoms of synovitis, synovial hypertrophy, joint effusion, skin symptoms, and other symptoms improved, and the inflammatory indicators and MRI showed improvements. Liu et al68 presented a patient with SAPHO syndrome complicated by lymphangioleiomyomatosis whose arthralgia and pulmonary function improved after tofacitinib treatment. Li et al69 reported a 62-year-old female patient presenting with swelling and pain at the sternoclavicular joints, back pain that limited her activities, arthralgia in the right knee, and cutaneous lesions. A combination oral treatment with tofacitinib 5 mg twice daily with methotrexate and bisphosphonates was administered. The patient reported that her pain symptoms were relieved after 3 days, cutaneous lesions reduced after 4 weeks, and vertebral lesions improved after six months. No serious adverse effects were noted. Li et al70 first showed the effectiveness of tofacitinib in patients with SAPHO syndrome, evidenced by alleviation of pain and rash, decreased systemic inflammation, improved quality of life, and remission on MRI. Nine patients (75.0%) exhibited MRI response, including six (50.0%) with moderate and three (25.0%) with mild. Skin lesions were alleviated in seven of the eight patients. Six patients (50%) complained of upper respiratory tract infections (without antibiotics prescription) during the treatment. No other adverse events were reported. Li et al71 reported 13 patients with SAPHO syndrome accompanied by nail lesions and active palmoplantar pustulosis receiving tofacitinib 5 mg twice daily for 12 weeks. In this pilot study, tofacitinib yielded significant remission in nail lesions, palmoplantar psoriasis, and improved the quality of life. The patient, was a 48–year-old female, a case of SAPHO syndrome combined with Sjogren’s syndrome diagnosed by the Department of Rheumatology and Immunology of the Second Xiangya Hospital of Central South University. After being treated with traditional DMARDs combined with adalimumab (three cycles), she discontinued because of palmoplantar pustulosis (PPP) exacerbation and urinary tract infection. She was switched to tofacitinib combined with glucocorticoid, the patient responded well. Her rash, pain, and laboratory indicators significantly improved and remained symptom-free at a dose of 5 mg twice a day.
PDE-4 Inhibitor
Apremilast (Otezla®) is an orally administered, small-molecule inhibitor of phosphodiesterase 4 (PDE-4). It is well tolerated in patients with psoriasis and psoriatic arthritis, which have overlapping features with SAPHO syndrome.49 Adamo et al66 described, for the first time, the efficacy of apremilast in a patient with SAPHO syndrome, resulting in stabilization of the skin and joint symptoms without side effects. The patient was treated with 45 mg ustekinumab first; however, it exacerbated joint pain. The patient was switched to adalimumab, which exacerbated the disease. Finally, the patient was switched to secukinumab, which improved skin and joint symptoms significantly; however, it was associated with a pustular hypersensitivity reaction.
The novel use of apremilast and JAK inhibitors provided a therapeutic modulation involving a novel class of drugs, targeting a wide spectrum of cytokines and cells. This provides a promising new treatment for SAPHO syndrome that deserves further studies.
Others
TwHF is an arthralgia‐dispelling herb with the benefits of activating blood circulation and collaterals, resisting rheumatism, and relieving pain and swelling.78 A clinical trial recruited 30 eligible SAPHO patients to this single-center trial to receive a 12-week TwHF treatment. The patients achieved significant changes from baseline in ASDAS, visual analogue scale in global osteoarticular pain, bath Ankylosing Spondylitis Disease Activity Index, and other efficacy measures.79 Remission of osteoarticular are reported by Li et al.80 Three case reports showed osteoarticular or cutaneous symptoms of SAPHO patients improved in response to TwHF.39,80,81 Bisphosphonates inhibit bone resorption and have some anti-inflammatory properties.82 Although good effectiveness of bisphosphonates for osteoarticular symptoms are showed in some reports,83–89 there are also reports of its ineffectiveness for skin lesions.16,23,83,85,90,91 In a retrospective observational study, Eighteen SAPHO patients applied bisphosphonates, symptoms were improved in 16 patients, but it difficult to estimate the effectiveness because of a combination of other drugs. The author also reviewed of literature from 1996 to 2019 and showed 48.9% (68/139) patients received bisphosphonates and reached complete remission, 75% (43/57) patients received a combination of NSAIDs and bisphosphonates and the treatment was effective.40
After treating with TNF blockers, patients with osteoarticular symptoms significantly improved; however, the improvement in cutaneous symptoms was unsatisfactory. There was a risk of inducing new psoriasiform lesions and other infections. Data related to IL-1 inhibition in SAPHO are encouraging with most patients exhibiting a significant response in musculoskeletal manifestations. In contrast, IL-1 inhibition seems ineffective against skin diseases. IL-6 inhibition might be an effective therapy for muscle inflammation in CRMO. Side effects like aseptic subcutaneous abscess and neutropenia suggest that tocilizumab might not be an ideal option for treating SAPHO syndrome. The efficacy of IL-23/IL-17 inhibitors in SAPHO is still inconclusive. Despite limited cases, cutaneous symptoms in most patients improved after secukinumab treatment. The JAK inhibitor tofacitinib is effective in patients with poor responses and tolerance to traditional and biological agents. Apremilast, TwHF showed the efficacy although limited cases. Bisphosphonates is recommended as a combination medication, especially in patients with osteoarticular symptoms.
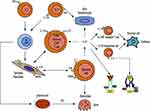
TNF blockers act with the synovial fibroblasts and osteoclasts, keratinocytes were also involved, which showed a good response to early inflammation. The efficacy of osteoarticular symptoms has been acknowledged. Moreover, TNF activates the keratinocytes in the skin, leading to an inflammatory process. IL-1 also participates in the inflammatory osteoarticular processes. Moreover, it does not affect keratinocytes directly, which explains the excellent effects of osteoarticular symptoms; however, the improvements to the cutaneous symptoms were unsatisfactory. IL‑6 contributes to local inflammation. However, it does not regulate keratinocytes, and mainly affects the stromal cells and not the osteoclast. In the joints, TH17 cells, the CD4+ memory cells, CCR6+ T cells, and IL-23R+ resident T cells produce cytokines, such as IL‑17A and IL‑17F (Figure 2). Direct cell–cell interaction and cytokine-driven activation of tissue-specific cells (FLS, resident macrophages, and myeloid cells) boost cytokine production. Enthesitis is strongly IL‑23-mediated, involving resident IL‑23R+ T cells and IL‑23-responsive cells, with IL‑17A, IL‑22, and IL‑6 (Figure 2). Etiopathogenesis of SAPHO involves different pathways, and there is a differentiation of therapeutic effects. Recent studies revealed that blocking the JAKs can directly or indirectly block the action of several cytokines, including γ-chain cytokines, IL-1, IL-6, IL-7, IL-12, IL-15, IL-17, IL-22, IL-23, and TNF-α.92 Inhibition of the JAK/signal transducer and activator of transcription (STAT) pathway could help in regulating the expression of inflammatory factors, such as IL-6 or IL-23 (Figure 2). JAKs modulate the inflammatory process by activating intracytoplasmic transcription factors called signal transducer and activator of transcription (STAT). The efficacy of tofacitinib strongly suggested the role of a JAK-STAT signaling pathway in the pathogenesis of SAPHO syndrome. Moreover, tofacitinib suppresses osteoclast-mediated structural damage in arthritic joints by inhibiting the receptor activator for the nuclear factor kB ligand (RANKL) pathway.93 The effectiveness of tofacitinib might be associated with its potent and broad suppression of cytokine networks in direct and indirect manners; however, further investigations are needed because of design and sample size limitations.
Our study has several limitations. The main limitation of the reported cases is the relatively small sample size. The efficacy of drugs for SAPHO syndrome needs a larger population. Moreover, most research types are case reports; therefore, the evaluation criteria might be inconsistent.
Conclusions
In conclusion, Patients with osteoarticular symptoms can consider tumor necrosis factor blockers, JAK inhibitor, interleukin (IL)-1 inhibitor, and IL-17 inhibitor (Table 2). Patients with cutaneous symptoms should consider IL-17 and JAK inhibitors (Table 2). Apremilast, Tripterygium wilfordii Hook F, and bisphosphonates are other effective treatments options.
 |
Table 2 Stratified Medication Recommendations for SAPHO Syndrome |
Data Sharing Statement
Data are presented in the manuscript and there is no conflict of interest with any entity involved with the research or acquisition of data in this manuscript.
Funding
The study was supported by the National Natural Science Foundation of China (Grant No.81701622) and research grant (2018JJ2588, 2018JJ3724) from the Natural Science Foundation of Hunan Province.
Disclosure
The authors declare no relationships with any companies whose products or services may be related to the subject matter of the article. The authors report no conflicts of interest in this work.
References
1. Benhamou CL, Chamot AM, Kahn MF. Synovitis-acne-pustulosis hyperostosis-osteomyelitis syndrome (SAPHO). A new syndrome among the spondyloarthropathies? Clin Exp Rheumatol. 1988;6(2):109–112.
2. Hayem G. [SAPHO syndrome]. Rev Prat. 2004;54(15):1635–1636. Swedish.
3. Hayem G, Bouchaud-Chabot A, Benali K, et al. SAPHO syndrome: a long-term follow-up study of 120 cases. Semin Arthritis Rheum. 1999;29(3):159–171. doi:10.1016/S0049-0172(99)80027-4
4. Hayama K, Inadomi T, Fujisawa D, Terui T. A pilot study of medium-dose cyclosporine for the treatment of palmoplantar pustulosis complicated with pustulotic arthro-osteitis. Eur J Dermatol. 2010;20(6):758–762. doi:10.1684/ejd.2010.1109
5. Rozin AP. SAPHO syndrome: is a range of pathogen-associated rheumatic diseases extended? Arthritis Res Ther. 2009;11(6):131. doi:10.1186/ar2837
6. Golla A, Jansson A, Ramser J, et al. Chronic recurrent multifocal osteomyelitis (CRMO): evidence for a susceptibility gene located on chromosome 18q21.3-18q22. Eur J Hum Genet. 2002;10(3):217–221. doi:10.1038/sj.ejhg.5200789
7. Hurtado-Nedelec M, Chollet-Martin S, Nicaise-Roland P, et al. Characterization of the immune response in the synovitis, acne, pustulosis, hyperostosis, osteitis (SAPHO) syndrome. Rheumatology. 2008;47(8):1160–1167. doi:10.1093/rheumatology/ken185
8. Berthelot JM, Corvec S, Hayem G. SAPHO, autophagy, IL-1, FoxO1, and Propionibacterium (Cutibacterium) acnes. Joint Bone Spine. 2018;85(2):171–176. doi:10.1016/j.jbspin.2017.04.010
9. Firinu D, Barca MP, Lorrai MM, et al. TH17 cells are increased in the peripheral blood of patients with SAPHO syndrome. Autoimmunity. 2014;47(6):389–394. doi:10.3109/08916934.2014.906582
10. Wendling D, Aubin F, Verhoeven F, Prati C. IL-23/Th17 targeted therapies in SAPHO syndrome. A case series. Joint Bone Spine. 2017;84(6):733–735. doi:10.1016/j.jbspin.2017.05.016
11. Xu D, Liu X, Lu C, et al. Reduction of peripheral natural killer cells in patients with SAPHO syndrome. Clin Exp Rheumatol. 2019;37(1):12–18.
12. Grosjean C, Hurtado-Nedelec M, Nicaise-Roland P, et al. Prevalence of autoantibodies in SAPHO syndrome: a single-center study of 90 patients. J Rheumatol. 2010;37(3):639–643. doi:10.3899/jrheum.090863
13. Zhang S, Li C, Zhang S, et al. Serum levels of proinflammatory, anti-inflammatory cytokines, and RANKL/OPG in synovitis, acne, pustulosis, hyperostosis, and osteitis (SAPHO) syndrome. Mod Rheumatol. 2019;29(3):523–530. doi:10.1080/14397595.2018.1469580
14. Daoussis D, Konstantopoulou G, Kraniotis P, Sakkas L, Liossis SN. Biologics in SAPHO syndrome: a systematic review. Semin Arthritis Rheum. 2019;48(4):618–625. doi:10.1016/j.semarthrit.2018.04.003
15. Olivieri I, Padula A, Ciancio G, Salvarani C, Niccoli L, Cantini F. Successful treatment of SAPHO syndrome with infliximab: report of two cases. Ann Rheum Dis. 2002;61(4):375–376.
16. Wagner AD, Andresen J, Jendro MC, Hulsemann JL, Zeidler H. Sustained response to tumor necrosis factor alpha-blocking agents in two patients with SAPHO syndrome. Arthritis Rheum. 2002;46(7):1965–1968.
17. Iqbal M, Kolodney MS. Acne fulminans with synovitis-acne-pustulosis-hyperostosis-osteitis (SAPHO) syndrome treated with infliximab. J Am Acad Dermatol. 2005;52(5 Suppl 1):S118–120.
18. Deutschmann A, Mache CJ, Bodo K, Zebedin D, Ring E. Successful treatment of chronic recurrent multifocal osteomyelitis with tumor necrosis factor-alpha blockage. Pediatrics. 2005;116(5):1231–1233.
19. Massara A, Cavazzini PL, Trotta F. In SAPHO syndrome anti-TNF-alpha therapy may induce persistent amelioration of osteoarticular complaints, but may exacerbate cutaneous manifestations. Rheumatology (Oxford). 2006;45(6):730–733.
20. Moll C, Hernandez MV, Canete JD, et al. Ilium osteitis as the main manifestation of the SAPHO syndrome: response to infliximab therapy and review of the literature. Semin Arthritis Rheum. 2008;37(5):299–306.
21. Sabugo F, Liberman C, Niedmann JP, Soto L, Cuchacovich M. Infliximab can induce a prolonged clinical remission and a decrease in thyroid hormonal requirements in a patient with SAPHO syndrome and hypothyroidism. Clin Rheumatol. 2008;27(4):533–535.
22. Fruehauf J, Cierny-Modre B, Caelen Lel S, Schwarz T, Weinke R, Aberer E. Response to infliximab in SAPHO syndrome. BMJ Case Rep. 2009;2009.
23. Ben Abdelghani K, Dran DG, Gottenberg JE, Morel J, Sibilia J, Combe B. Tumor necrosis factor-alpha blockers in SAPHO syndrome. J Rheumatol. 2010;37(8):1699–1704.
24. Eleftheriou D, Gerschman T, Sebire N, Woo P, Pilkington CA, Brogan PA. Biologic therapy in refractory chronic non-bacterial osteomyelitis of childhood. Rheumatology (Oxford). 2010;49(8):1505–1512.
25. De Souza A, Solomon GE, Strober BE. SAPHO syndrome associated with hidradenitis suppurativa successfully treated with infliximab and methotrexate. Bull NYU Hosp Jt Dis. 2011;69(2):185–187.
26. Burgemeister LT, Baeten DL, Tas SW. Biologics for rare inflammatory diseases: TNF blockade in the SA PHO syndrome. Neth J Med. 2012;70(10):444–449.
27. Hampton SL, Youssef H. Successful treatment of resistant SAPHO syndrome with anti-TNF therapy. BMJ Case Rep. 2013;2013.
28. Anić B, Padjen I, Baresic M, Tezak S. The lobster sign in SAPHO syndrome: unusually extensive osteitis of the anterior chest wall partially responsive to infliximab. Rheumatol Int. 2014;34(2):281–282.
29. Li C, Wu X, Cao Y, et al. Paradoxical skin lesions induced by anti-TNF-alpha agents in SAPHO syndrome. Clin Rheumatol. 2019;38(1):53–61.
30. Borok S, Flusser G, Elkayam O. Clinical Images: marked inflammation in a patient with cervical vertebral SAPHO complicated by vertebral body collapse and severe kyphosis. Isr Med Assoc J. 2017;19(11):725–726.
31. Asano T, Furuya MY, Fujita Y, et al. Diagnostic value of ultrasonography in synovitis-acne-pustulosis-hyperostosis-osteitis (SAPHO) syndrome: a case report. Medicine (Baltimore). 2018;97(41):e12725.
32. Vilar-Alejo J, Dehesa L, de la Rosa-del Rey P, et al. SAPHO syndrome with unusual cutaneous manifestations treated successfully with etanercept. Acta Derm Venereol. 2010;90(5):531–532.
33. Zhang LL, Zhao JX, Liu XY. Successful treatment of SAPHO syndrome with severe spinal disorder using entercept: a case study. Rheumatol Int. 2012;32(7):1963–1965.
34. Su YS, Chang CH. SAPHO syndrome associated with acne conglobata successfully treated with etanercept. J Formos Med Assoc. 2015;114(6):562–564.
35. Abourazzak FE, Hachimi H, Kadi N, Berrada K, Tizniti S, Harzy T. Etanercept in the treatment of SAPHO syndrome: which place? Eur J Rheumatol. 2014;1(3):125–128.
36. Marí A, Morla A, Melero M, Schiavone R, Rodriguez J. Diffuse sclerosing osteomyelitis (DSO) of the mandible in SAPHO syndrome: a novel approach with anti-TNF therapy. Systematic review. J Craniomaxillofac Surg. 2014;42(8):1990–1996.
37. Sàez-Martin LC, Gomez-Castro S, Roman-Curto C, Palacios-Alvarez I, Fernandez-Lopez E. Etanercept in the treatment of SAPHO syndrome. Int J Dermatol. 2015;54(6):e206–208.
38. Zhang L, Gao Z. Etanercept in the treatment of refractory SAPHO syndrome. Am J Clin Exp Immunol. 2016;5(4):62–66.
39. Zhang X, Wu X, Li C. Successful treatment of synovitis, acne, pustulosis, hyperostosis, and osteitis and paradoxical skin lesions by Tripterygium wilfordii hook f: a case report. J Int Med Res. 2020;48(9):300060520949100.
40. Huang H, Zhang Z, Zhao J, Hao Y, Zhou W. The effectiveness of treatments for patients with SAPHO syndrome: a follow-up study of 24 cases from a single center and review of literature. Clin Rheumatol. 2021;40(3):1131–1139.
41. Arias-Santiago S, Sanchez-Cano D, Callejas-Rubio JL, Fernandez-Pugnaire MA, Ortego-Centeno N. Adalimumab treatment for SAPHO syndrome. Acta Derm Venereol. 2010;90(3):301–302.
42. Castellví I, Bonet M, Narvaez JA, Molina-Hinojosa JC. Successful treatment of SAPHO syndrome with Adalimumab: a case report. Clin Rheumatol. 2010;29(10):1205–1207.
43. Henriques CC, Sousa M, Panarra A, Riso N. The dark side of SAPHO syndrome. BMJ Case Rep. 2011;2011.
44. Hess S, Hospach T, Nossal R, Dannecker G, Magdorf K, Uhlemann F. Life-threatening disseminated tuberculosis as a complication of TNF-alpha blockade in an adolescent. Eur J Pediatr. 2011;170(10):1337–1342.
45. Garcovich S, Amelia R, Magarelli N, Valenza V, Amerio P. Long-term treatment of severe SAPHO syndrome with adalimumab: case report and a review of the literature. Am J Clin Dermatol. 2012;13(1):55–59.
46. Cotti E, Careddu R, Schirru E, et al. A case of SAPHO syndrome with endodontic implications and treatment with biologic drugs. J Endod. 2015;41(9):1565–1570.
47. Chou A, Schulman JM, Gross AJ, Jordan RC, Ramos DM. Gingival pustules and sterile diffuse sclerosing osteomyelitis as a feature of synovitis, acne, pustulosis, hyperostosis, osteitis (SAPHO) syndrome. Oral Surg Oral Med Oral Pathol Oral Radiol. 2016;121(5):e116–122.
48. Vekic DA, Woods J, Lin P, Cains GD. SAPHO syndrome associated with hidradenitis suppurativa and pyoderma gangrenosum successfully treated with adalimumab and methotrexate: a case report and review of the literature. Int J Dermatol. 2018;57(1):10–18.
49. Cianci F, Zoli A, Gremese E, Ferraccioli G. Clinical heterogeneity of SAPHO syndrome: challenging diagnose and treatment. Clin Rheumatol. 2017;36(9):2151–2158.
50. Marrani E, Belli G, Simonini G, Trapani S, Caproni M, Lionetti P. SAPHO syndrome in pediatric patients with inflammatory bowel disease treated with infliximab. Dig Liver Dis. 2018;50(11):1249–1251.
51. Genovese G, Caorsi R, Moltrasio C, Marzano AV. Successful treatment of co-existent SAPHO syndrome and hidradenitis suppurativa with adalimumab and methotrexate. J Eur Acad Dermatol Venereol. 2019;33(Suppl 6):40–41.
52. Luzzati M, Simonini G, Filippeschi C, Giani T, Trapani S. SAPHO syndrome: the supposed trigger by isotretinoin, the efficacy of adalimumab and the specter of depressive disorder: a case report. Ital J Pediatr. 2020;46(1):169.
53. Kamata Y, Minota S. Successful treatment of a patient with SAPHO syndrome with certolizumab pegol. Rheumatol Int. 2015;35(9):1607–1608.
54. Liew J, Xu TH, Chu CQ. Synovitis acne pustulosis hyperostosis osteitis (SAPHO) - paradoxical reactions and different responses to tumour necrosis factor inhibitors. Rheumatology (Oxford). 2017;56(12):2239–2241.
55. Colina M, Pizzirani C, Khodeir M, et al. Dysregulation of P2X7 receptor-inflammasome axis in SAPHO syndrome: successful treatment with anakinra. Rheumatology (Oxford). 2010;49(7):1416–1418.
56. Wendling D, Prati C, Aubin F. Anakinra treatment of SAPHO syndrome: short-term results of an open study. Ann Rheum Dis. 2012;71(6):1098–1100.
57. Rech J, Manger B, Lang B, Schett G, Wilhelm M, Birkmann J. Adult-onset Still’s disease and chronic recurrent multifocal osteomyelitis: a hitherto undescribed manifestation of autoinflammation. Rheumatol Int. 2012;32(6):1827–1829.
58. Sakran W, Shalev SA, Sakran W, et al. Chronic recurrent multifocal osteomyelitis and deficiency of interleukin-1-receptor antagonist. Pediatr Infect Dis J. 2013;32(1):94.
59. Fujita S, Kosaka N, Mito T, Hayashi H, Morita Y. Development of aseptic subcutaneous abscess after tocilizumab therapy in a patient with SAPHO syndrome complicated by amyloid A amyloidosis. Int J Rheum Dis. 2015;18(4):476–479.
60. Sato H, Wada Y, Hasegawa E, et al. Adult-onset chronic recurrent multifocal osteomyelitis with high intensity of muscles detected by magnetic resonance imaging, successfully controlled with tocilizumab. Intern Med. 2017;56(17):2353–2360.
61. Sun XC, Liu S, Li C, et al. Failure of tocilizumab in treating two patients with refractory SAPHO syndrome: a case report. J Int Med Res. 2018;46(12):5309–5315.
62. Cornillier H, Kervarrec T, Tabareau-Delalande F, Mammou S, Jonville Bera AP, Machet L. Interstitial granulomatous dermatitis occurring in a patient with SAPHO syndrome one month after starting leflunomide, and subsequently disappearing with ustekinumab. Eur J Dermatol. 2016;26(6):614–615.
63. Firinu D, Garcia-Larsen V, Manconi PE, Del Giacco SR. SAPHO syndrome: current developments and approaches to clinical treatment. Curr Rheumatol Rep. 2016;18(6):35.
64. Wang L, Sun B, Li C. Clinical and radiological remission of osteoarticular and cutaneous lesions in SAPHO patients treated with secukinumab: a case series. J Rheumatol. 2021;48(6):953–955.
65. Sun B, Cao Y, Wang L, Wang M, Li C. Successful treatment of refractory mandibular lesions in SAPHO syndrome with secukinumab. Rheumatology (Oxford). 2021;60(1):473–474.
66. Adamo S, Nilsson J, Krebs A, et al. Successful treatment of SAPHO syndrome with apremilast. Br J Dermatol. 2018;179(4):959–962.
67. Yang Q, Zhao Y, Li C, Luo Y, Hao W, Zhang W. Case report: successful treatment of refractory SAPHO syndrome with the JAK inhibitor tofacitinib. Medicine (Baltimore). 2018;97(25):e11149.
68. Liu S, Li C, Tang MW, et al. Improvement of lymphangioleiomyomatosis following successful tofacitinib treatment for refractory synovitis, acne, pustulosis, hyperostosis, and osteitis syndrome. Chin Med J (Engl). 2019;132(19):2378–2379.
69. Li B, Li GW, Xue L, Chen YY. Rapid remission of refractory synovitis, acne, pustulosis, hyperostosis, and osteitis syndrome in response to the Janus kinase inhibitor tofacitinib: a case report. World J Clin Cases. 2020;8(19):4527–4534.
70. Li Y, Huo J, Cao Y, et al. Efficacy of tofacitinib in synovitis, acne, pustulosis, hyperostosis and osteitis syndrome: a pilot study with clinical and MRI evaluation. Ann Rheum Dis. 2020;79(9):1255–1257.
71. Li C, Li Z, Cao Y, et al. Tofacitinib for the treatment of nail lesions and palmoplantar pustulosis in synovitis, acne, pustulosis, hyperostosis, and osteitis syndrome. JAMA Dermatol. 2021;157(1):74–78.
72. Mateo L, Sanint J, Rodriguez Muguruza S, Martinez Morillo M, Perez Andres R, Domenech Puigcerver S. SAPHO syndrome presenting as an osteolytic lesion of the neck. Reumatol Clin. 2017;13(1):44–47.
73. Lee A, Scott LJ. Certolizumab pegol: a review in moderate to severe plaque psoriasis. BioDrugs. 2020;34(2):235–244.
74. Gottlieb A, Narang K. Ustekinumab in the treatment of psoriatic arthritis: latest findings and clinical potential. Ther Adv Musculoskelet Dis. 2013;5(5):277–285.
75. Singh JA, Saag KG, Bridges SL Jr, et al. 2015 American College of Rheumatology Guideline for the Treatment of Rheumatoid Arthritis. Arthritis Rheumatol. 2016;68(1):1–26.
76. Smolen JS, Landewe RBM, Bijlsma JWJ, et al. EULAR recommendations for the management of rheumatoid arthritis with synthetic and biological disease-modifying antirheumatic drugs: 2019 update. Ann Rheum Dis. 2020;79(6):685–699.
77. Keating GM. Apremilast: a review in psoriasis and psoriatic arthritis. Drugs. 2017;77(4):459–472.
78. Zhang Y, Mao X, Li W, et al. Tripterygium wilfordii: an inspiring resource for rheumatoid arthritis treatment. Med Res Rev. 2021;41(3):1337–1374.
79. Wang L, Gong L, Zhang X, et al. Tripterygium wilfordii Hook F. in the treatment of synovitis, acne, pustulosis, hyperostosis, and osteitis syndrome: a clinical trial. Clin Rheumatol. 2021;40(6):2427–2438.
80. Li C, Sun X, Cao Y, Xu W, Zhang W, Dong Z. Case report: remarkable remission of SAPHO syndrome in response to Tripterygium wilfordii hook f treatment. Medicine (Baltimore). 2017;96(47):e8903.
81. Gong L, Wang L, Cao Y, Li C. Rapid induction of clinical remission in SAPHO syndrome using high-dose Tripterygium glycosides: a case report. Medicine (Baltimore). 2020;99(27):e21102.
82. Nguyen MT, Borchers A, Selmi C, Naguwa SM, Cheema G, Gershwin ME. The SAPHO syndrome. Semin Arthritis Rheum. 2012;42(3):254–265.
83. Aljuhani F, Tournadre A, Tatar Z, et al. The SAPHO syndrome: a single-center study of 41 adult patients. J Rheumatol. 2015;42(2):329–334.
84. Galadari H, Bishop AG, Venna SS, Sultan E, Do D, Zeltser R. Synovitis, acne, pustulosis, hyperostosis, and osteitis syndrome treated with a combination of isotretinoin and pamidronate. J Am Acad Dermatol. 2009;61(1):123–125.
85. Soyfoo MS, Gangji V, Margaux J. Successful treatment of SAPHO syndrome with ibandronate. J Clin Rheumatol. 2010;16(5):253.
86. Just A, Adams S, Brinkmeier T, et al. Successful treatment of primary chronic osteomyelitis in SAPHO syndrome with bisphosphonates. J Dtsch Dermatol Ges. 2008;6(8):657–660.
87. Spyridonidis T, Giannakenas C, Papandrianos N, Barla P, Apostolopoulos DJ. Two cases of synovitis, acne, pustulosis, osteitis–SAPHO syndrome. Hell J Nucl Med. 2007;10(2):109–112.
88. Amital H, Applbaum YH, Aamar S, Daniel N, Rubinow A. SAPHO syndrome treated with pamidronate: an open-label study of 10 patients. Rheumatology (Oxford). 2004;43(5):658–661.
89. Courtney PA, Hosking DJ, Fairbairn KJ, Deighton CM. Treatment of SAPHO with pamidronate. Rheumatology (Oxford). 2002;41(10):1196–1198.
90. Susanto CR, Nanayakkara PW. The skin is the clue. Eur J Intern Med. 2003;14(8):493–494.
91. Kim CH, Kadhim S, Julien C. Treatment of pain in SAPHO (synovitis, acne, pustulosis, hyperostosis, and osteitis) syndrome. PM R. 2014;6(1):92–95.
92. Ucciferri C, Vecchiet J, Falasca K. Role of monoclonal antibody drugs in the treatment of COVID-19. World J Clin Cases. 2020;8(19):4280–4285.
93. LaBranche TP, Jesson MI, Radi ZA, et al. JAK inhibition with tofacitinib suppresses arthritic joint structural damage through decreased RANKL production. Arthritis Rheum. 2012;64(11):3531–3542.
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.