Back to Journals » Infection and Drug Resistance » Volume 16
New Developments and Challenges in Antibody-Based Therapies for the Respiratory Syncytial Virus
Authors Diethelm-Varela B, Soto JA, Riedel CA, Bueno SM, Kalergis AM
Received 3 February 2023
Accepted for publication 29 March 2023
Published 8 April 2023 Volume 2023:16 Pages 2061—2074
DOI https://doi.org/10.2147/IDR.S379660
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Professor Suresh Antony
Benjamín Diethelm-Varela,1 Jorge A Soto,1,2 Claudia A Riedel,2 Susan M Bueno,1 Alexis M Kalergis1,3
1Millennium Institute of Immunology and Immunotherapy, Departamento de Genética Molecular y Microbiología, Facultad de Ciencias Biológicas, Pontificia Universidad Católica de Chile, Santiago, Chile; 2Millennium Institute on Immunology and Immunotherapy, Departamento de Ciencias Biológicas, Facultad de Ciencias de la Vida, Universidad Andrés Bello, Santiago, Chile; 3Departamento de Endocrinología, Facultad de Medicina, Pontificia Universidad Católica de Chile, Santiago, Chile
Correspondence: Alexis M Kalergis, Email [email protected]
Abstract: Since the discovery of the human respiratory syncytial virus (hRSV), multiple research efforts have been conducted to develop vaccines and treatments capable of reducing the risk of severe disease, hospitalization, long-term sequelae, and death from this pathogen in susceptible populations. In this sense, therapies specifically directed against hRSV are mainly based on monoclonal and polyclonal antibodies such as intravenous IgG (IVIG)-RSV and the monoclonal antibody palivizumab. However, these therapies are associated with significant limitations, including the need for the recruitment of a high number of convalescent volunteers who donate blood to procure IVIG-RSV and the costs associated with the need for repeated administrations of palivizumab. These limitations render this product not cost-effective for populations other than high-risk patients. These problems have underscored that it is still necessary to identify new safe and effective therapies for human use. However, these new therapies must benefit from a comparatively cheap production cost and the opportunity to be available to the high-risk population and anyone who requires treatment. Here, we review the different antibodies used to prevent the pathology caused by hRSV infection, highlighting therapies currently approved for human use and their clinical value. Also, the new, most promising candidates based on preclinical studies and clinical trial results are revised.
Keywords: prevention, treatment, antibodies, respiratory syncytial virus
Introduction
The human respiratory syncytial virus (hRSV) or human orthopneumovirus, based on its reclassification in 2016,1,2 is a pathogen of primary global concern.3–8 This infectious agent is responsible for seasonal outbreaks associated with significant morbidity and mortality.3,5,7,9,10 High-risk populations for severe outcomes following hRSV infections consist of preterm children, especially those with biomedical complications such as bronchopulmonary dysplasia,11 as well as the elderly.3,7,10,12–15 In these high-risk populations, hRSV infection has a significantly higher likelihood of causing lower respiratory tract disease, leading to life-threatening pneumonia in many cases.16,17 Nevertheless, it is important to note that healthy infants born at term are also at risk for severe hRSV pneumonia (albeit to a lesser degree than preterm infants), and due to the ubiquity of the virus, make up most cases.4,12,13,18 Additionally, substantial evidence has emerged that infection with this virus, including infections acquired during infancy, is associated with long-lasting chronic sequelae, including neuropsychiatric alterations,19–24 asthma, airway dysfunction, and susceptibility to allergies. These latter three consequences are associated with the highly inflammatory immune response that hRSV elicits. Therefore, it is a public health priority to prevent the severe health consequences brought by the immunopathology of hRSV infection, particularly in at-risk populations, either through active immunization, immunoprophylaxis, or early treatment.21,22,25
No vaccines against hRSV have been approved.25–28 Furthermore, only a single prophylactic antibody product, palivizumab, is licensed in a limited selection of cases.17,29,30 In this sense, in most cases of hRSV infection, the primary management strategies are prevention, symptomatic management, and supportive therapy.31 To understand the lack of vaccines against hRSV, the immune response that the organism mounts against this virus during infancy offers critical insights. During early life, the immune response tends to be polarized toward a Th2 profile.32,33 Thus, upon infection, hRSV promotes infants to develop an allergy-like Th2-biased immune response.34,35 This response includes considerable lung and airway inflammation, and the establishment of ineffective immune memory leaves the individual susceptible to future reinfections.8,18,36 A vaccine candidate once seen as promising, consisting of formalin-inactivated viral particles (FI-hRSV), was found to elicit this type of response in children37,38 and led to vaccine-enhanced disease (VED) in case of re-exposure to the pathogen.39 Natural hRSV infection after vaccination tragically resulted in severe illness in trial participants and two infant deaths.40,41 Research since that event has emphasized the need to develop vaccine prototypes that could shift the adaptive response towards a Th1-biased immune response with efficacious antiviral capabilities that ideally would include the production of neutralizing antibodies and hRSV-specific T and B cells.25,42–45 Preclinical research has suggested that this approach could be feasible in the immune system of newborns.46,47
Notwithstanding the troubled history of hRSV vaccine development, intense research efforts in the past decades have led to several vaccine candidates in various stages of preclinical and clinical research, including subunit vaccines,48,49 viral vector vaccines,50–52 DNA-based vaccines,53,54 and recombinant Mycobacterium bovis Bacillus Calmette-Guérin (BCG)-based vaccines,55–58 among others.48,49,56–64 As with other areas of drug development, current efforts have tapped into the now extensive structural knowledge of hRSV to identify novel pharmacological targets. However, despite these efforts, none of these vaccine candidates have been approved for clinical use yet. This fact highlights the importance of exploring and developing other prophylactic and therapeutic strategies against this pathogen as the search for a safe and effective vaccine continues.
Protection against the disease caused by hRSV has already been evaluated through therapeutic venues different from vaccination.25,65,66 The only licensed pharmaceutical products against hRSV consist of a monoclonal antibody against the Fusion protein of the virus (F-hRSV), palivizumab (Synagis, MEDI-493),29,67 and ribavirin,68,69 a broad-spectrum antiviral which does not act through a mechanism of action explicitly targeting hRSV structural components. Both products have only limited effectiveness in preventing severe disease by hRSV. Additionally, polyclonal immunoglobulin products, such as intravenous immune globulin (IVIG), have been approved for use against hRSV. Still, the advent of palivizumab caused the FDA to suspend polyclonal immunoglobulin use in favor of the more modern and effective monoclonal antibody.70
The present review explores the use of antibody products against hRSV, discussing their history of development and the types of products in clinical use and highlighting key advantages and weaknesses. Next, we discuss the clinical value of antibody products in clinical use against hRSV, focusing on anti-infective effectiveness and other critical clinical outcomes such as hospitalization and death. Finally, we provide a brief overview of novel antibody products undergoing preclinical or clinical development.
History of Antibody Use Against hRSV
HRSV was first isolated during the 50s from a colony of chimpanzees and named chimpanzee coryza agent (CCA).71 This virus is classified as a single-stranded, negative-sensed RNA virus with a genome consisting of 10 genes, coding for 11 proteins (9 structural and 2 non-structural).36,72 Elucidation of hRSV structure, gathered through decades of research, has provided numerous potential pharmacological targets as structural and nonstructural proteins. Among the structural proteins, the ones that have elicited the most interest as possible pharmacological targets are the fusion glycoprotein (F-hRSV), the attachment glycoprotein (G-hRSV), the nucleoprotein (N-hRSV), and the membrane protein (M-hRSV).18
After failing to demonstrate the safety of the FI-hRSV vaccine in the immunized pediatric population, new therapies were explored to control the severe prognosis induced by hRSV infection in the risk population.18,36,39–41 However, to date, only two specific therapeutic drugs have been approved against hRSV, both immunoglobulin-based prophylactic agents.25 These are polyclonal immunoglobulin isolated from convalescent sera (so-called intravenous immunoglobulin; IVIG)73,74 and the humanized monoclonal antibody palivizumab.17,29,30,67,74 Of the two, only palivizumab remains in clinical use, owing to IVIG’s unreliability of production, batch-to-batch variability, cost, and lack of superiority.75–78
Types of Antibodies Against hRSV in Current Clinical Use
As previously mentioned, there are two immunoglobulin-based products approved for clinical use against RSV: polyclonal antibodies formally referred to as respiratory syncytial virus intravenous immune globulin (RSV-IVIG, RespiGam®)73,74 and the humanized monoclonal anti-F antibody palivizumab.17,29,30,67,79 However, no antibodies targeting other proteins of hRSV, either structural or non-structural, have been approved for clinical use (Table 1).
 |
Table 1 The Landscape of Approved, Clinical, and Preclinical Investigational Immunoglobulins Against hRSV |
RSV-IVIG was the first antibody-based product approved against hRSV. However, its effectiveness and cost-effectiveness were questioned shortly after its approval.76 Furthermore, as a polyclonal product obtained from convalescent patients, RSV-IVIG is associated with high batch-to-batch variability, thus making its effectiveness, pharmacokinetic profile, and adverse effects unpredictable. Therefore, IVIG was discontinued after the approval of palivizumab, which showed clear superiority and greater consistency.75 RSV-IVIG is thus no longer used in standard clinical practice.
In contrast, palivizumab is an anti-F-hRSV humanized monoclonal antibody that acts as a neutralizing agent against hRSV.29,80 It is indicated for immunoprophylaxis in high-risk preterm infants.17,30,67,79,81,82 However, its high cost, the requirement for frequent administrations (as many as one per month during five months before the epidemic season), moderate effectiveness, and use limited to prophylaxis (having shown a lack of effectiveness in post-infection therapy) highlight the need for a superior agent in clinical practice.30,79,83
In general, the field of monoclonal antibodies for both prophylactic and therapeutic purposes offers promising potential.84 These agents provide exquisite target specificity and high affinity, thus potentially achieving potent therapeutic effects with comparatively limited adverse effects. Consequently, in addition to palivizumab, several antibody products against hRSV are under either preclinical or clinical development.70,84–90 Two antibodies are undergoing clinical research:80,85 motavizumab (MEDI-524), which failed in Phase III clinical trial to promote cutaneous reaction in volunteers,85,88,89,91–93 and nirsevimab (MEDI8897),86,94 which recently attained regulatory approval in the United Kingdom (UK) and in the European Union (EU).95 They are both anti-F immunoglobulins that act as neutralizing antibodies.86,88
Palivizumab, motavizumab, and nirsevimab all target F-hRSV.29,85,94,96 Despite this protein gathering most research interest as a target for antibody development,97 the generation of antibodies against G-hRSV,96,98–100 M-hRSV,101 and N-hRSV90,102 has been explored as well.
Clinical Value, Efficacy, and Outcomes of Current Antibody Products
The use of monoclonal antibodies for therapeutic and prophylactic purposes has seen an explosive increase in use during the past decades owing to their usually favorable safety and effectiveness, making for some of the most successful therapies in cancer, autoimmunity, and infectious diseases.103,104 Unfortunately, in the specific field of hRSV infections, this progress has not been as spectacular as in other areas. The two immunoglobulin products employed against hRSV thus far enjoy only limited effectiveness, and their unfavorable cost-effectiveness ratio makes their target population considerably narrow.65,73,74,78,105 An exception consists of the population of preterm infants and infants with lung complications, among whom palivizumab has been described to possess favorable cost-effectiveness.77 Conversely, even as this population could benefit from this antibody, due to the ubiquity of hRSV, most severe cases of hRSV occur in infants without biomedical complications, a population which is excluded from prophylaxis.4,12 These observations highlight the possibility for expanding the therapeutic arsenal against hRSV to a broader group of patients. As previously mentioned, only palivizumab sees current regular use due to the discontinuation of IVIG,25,30 although, because of its recent approval, nirsevimab is also expected to soon reach common use.95
Palivizumab is reserved for preterm infants with biomedical complications, most notably bronchopulmonary dysplasia.30,79,82 Efficacy is moderate: nearly 55% in preventing hospitalization.17,67,79,106–108 Because of its short half-life and lack of any active immunization effect, the therapeutic benefit of palivizumab is short-lived. Thus, it requires frequent administrations (as much as five injections over the autumn and winter). This makes it a costly therapeutic strategy for the management of hRSV. Because it acts as a neutralizing antibody, palivizumab is best used as prophylaxis to prevent the infection of many host cells. Thus, avoiding subsequent respiratory pathology and having little value as a treatment once disease, especially severe, immune-mediated disease, has been established. Additionally, escape from neutralization has been reported because of the high tendency of F-hRSV to suffer mutations,109 highlighting the need to target more conserved structures, such as N-hRSV,43 or G-hRSV, both of which have been described as possessing conserved domains.99,110,111
It is worth noting that the effectiveness of immunoglobulin products against hRSV, including palivizumab, has been questioned despite its status as an FDA-licensed product. A systematic review of the Cochrane database evaluated the use of IVIG, palivizumab, and motavizumab in clinical settings in high-income countries and found that neither therapy was different from the placebo for any of the clinical outcomes that were analyzed.78 Furthermore, immunoglobulins did not significantly reduce mortality or length of hospitalization in children.78 Additionally, studies have found that palivizumab provided no significant benefit in pulmonary outcomes, including forced expiratory volume and clearance index, in adolescent subjects born extremely prematurely.112,113 The reasons for this lack of long-term benefit remain unclear, but it could be hypothesized that, given that palivizumab acts, as indicated previously, merely as a neutralizing antibody, its usefulness might stem exclusively from early prophylaxis, having only limited therapeutic value once infection and immunopathology have been established in the respiratory tract.17,30,67,79,107,108 These results highlight that supportive therapy is the most valuable intervention for severe hRSV infections.28 Therefore, there remains an unmet need to prevent severe disease, underscoring the significant potential for developing prophylactic and therapeutic agents that may substantially improve clinical outcomes.
Novel Investigational Agents
Novel antibody products should meet several desirable criteria to develop reliable pharmacotherapies.84,104,114 First, an affinity for their intended target should be very high to avoid off-target effects at their designated doses. Also, pharmacokinetic properties need to be optimized, including a comparatively long half-life and efficient distribution to target tissues to ensure that few administrations afford the intended therapeutic effect. On that note, several modification strategies, such as glycosylation or strategic amino acid modifications, have enhanced pharmacokinetics in other antibody products.114,115 Another interesting pharmacokinetic change involves designing alternative administration routes different from injectable, such as inhalable or intranasal, which could provide enhanced local protection at the site of infection and improve patient compliance.76 On that note, proof of concept research has demonstrated that the administration of aerosolized palivizumab resulted in the successful pulmonary deposition of the antibody in an animal model, an approach which could be beneficial as a prophylaxis to prevent severe disease by controlling local viral spread in respiratory tissue after an early infection, suggesting that inhalable presentations of this antibody could be approved for clinical use in the future.116
Additionally, the use of humanized or fully human is preferred to murine or chimeric antibodies due to their lower risk of eliciting anti-immunoglobulin antibodies.104 Another critical consideration is the immunoglobulin class: while IgG and IgM antibodies can elicit complement-dependent cytotoxicity, IgA products may be better suited for local administration at viral entry points. Lastly, the pharmacological target must be designed carefully. Immunoglobulins targeting surface proteins of the virus, such as F-hRSV, G-hRSV, or M-hRSV, would be expected to act as neutralizing antibodies and thus be best suited for either prophylaxis or early therapy upon known exposure (Figure 1).
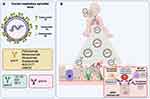 |
Figure 1 hRSV proteins as targets during the design of monoclonal antibodies against hRSV and their role during pathogenesis. (A) Schematic representation of hRSV and its proteins used as targets for developing monoclonal antibodies. (B) Role of anti-F (yellow), anti-G (green), and anti-N (Pink/purple) antibodies and their role during hRSV-induced pathogenesis in the lung epithelium. Abbreviations: ADCC, antibody-dependent cellular cytotoxicity; CDC, complement-dependent cytotoxicity. Notes: Figure 1 is adapted from the templates “Baby”, “Generic virus”, “Antibody IgG”, “Lungs”, “Natural Killer cell”, “Macrophage”, “Ciliated epithelial cell”, and “Enterocyte”, created with BioRender.com (2022). |
On the other hand, directing antibodies against either proteins found inside the viral membrane (such as N-hRSV, P-hRSV, or L-hRSV) or nonstructural proteins would potentially allow these antibodies to attach to infected cells (Figure 1). However, to date, few antibody candidates are in preclinical testing and do not include the F protein of the virus but are antibodies against the N and G proteins (Table 1). N-hRSV, unlike F-hRSV and G-hRSV, is not a surface protein but rather can be presented through the histocompatibility complex type I (MHC-I) or directly via membrane expression117 in infected cells, whereas F-hRSV and G-hRSV are available at the viral surface. Anti-N antibodies could thus serve as therapeutic agents for early infection control through effector functions of the immune system, such as complement-dependent cytotoxicity (CDC), antibody-dependent cellular cytotoxicity (ADCC), and phagocytosis.118–126 Also, these antibodies could potentially facilitate the generation of adequate immunological memory by recruiting antigen-presenting cells to the site of infection.42,55
Nirsevimab is a human immunoglobulin of the IgG1 class that works as a neutralizing antibody through binding to the prefusion conformation of F-hRSV. As a result, viral neutralization is significantly heightened as compared to immunoglobulins that bind the post-fusion conformation.127,128 Nirsevimab features targeted mutations to the Fc region of palivizumab, namely a three amino acid substitution (YTE), which has resulted in a significantly enhanced half-life compared to palivizumab when administered through the intramuscular route.94,95 Additionally, clinical studies showed a decrease of 70% in developing lower tract respiratory infections (LTRIs) associated with hospitalizations.94 This fact makes it attractive from economic and patient compliance perspectives, as fewer immunizations per person per season should be required, saving costs and helping achieve better patient compliance.86,94,129 After being granted Fast Track designation in the United States (US) in 2015, nirsevimab cleared its first Phase 1/2 clinical trial in 2016, entered Phase 3 trials in 2019, and attained its first two approvals in the EU and the UK in 2022.95
Motavizumab is an investigational anti-F-hRSV humanized monoclonal neutralizing antibody that has spent several years in the clinical development phase. This antibody was created through modifications in palivizumab’s complementarity-determining region, which drastically increased potency compared to palivizumab.85,88,89 However, the lack of clear superiority of motavizumab versus palivizumab in clinical trials,93,106 together with a high incidence of adverse effects,93 has meant that this antibody has not been able to clear clinical research and obtain approval from the US FDA.
Another anti-F-hRSV antibody that underwent clinical trials is Suptavumab (REGN2222). While it has emerged that it grants significantly more protection than palivizumab against the disease caused by hRSV,130 phase III clinical trials were not successful and Suptavumab was discontinued in 2017.131
Other neutralizing monoclonal antibodies against hRSV preclinical or early clinical phases of development include the anti-F IgA HNK20,132,133 the trimeric anti-F-hRSV antibody ALX-0171,134–137 and the anti-G-hRSV murine monoclonal antibody 131-2-G.100
A novel therapeutic approach toward hRSV infection immunoprophylaxis consists of antibodies directed against the nucleoprotein (N-hRSV).84 Anti-N-hRSV antibodies have been generated and are under preclinical investigation. Their proposed role includes diagnostic through detecting N-hRSV in infected samples90 and prophylactic and therapeutic uses against hRSV infection. As discussed previously, these agents are directed against a protein that is not present on the surface of hRSV but has been demonstrated to be expressed on the surface of infected cells. Thus, a potential use for these antibodies would be in the early control of an established infection by facilitating the selective killing of infected cells through effector functions of the immune system (Figure 1).118,123,138 Additionally, it is hypothesized that, given N-hRSV is more conserved than F-hRSV, anti-N antibodies could be more resistant to the escape variants that evade neutralization by palivizumab and nirsevimab.109,137,139–141 It is noteworthy that the development of pharmaceutical products targeting N-hRSV is still at early stages, but several preclinical products have been reported. Apart from the aforementioned anti-N antibodies used in potential diagnostic purposes,90 studies have reported the use of this protein in immunization platforms employing nanoparticles,49,60 as well as a recombinant BCG vaccine expressing N-hRSV.55–58
As mentioned in this article, the search for new therapeutic targets other than the F protein of the virus is under evaluation. Along these lines, the development of an antibody against the G protein of the virus called 131–2G, which blocks the binding of the G protein to CX3CR1, which is a receptor reportedly employed by hRSV for viral attachment and possibly for subsequent entry into lung epithelial cells (Figure 1),142–145 has shown promising protection results in mice.99,100 This antibody has since been superseded by the human monoclonal antibodies 2B11 and 3D3, both of which have shown promising data in preclinical studies, showing reductions of viral load and cellular infiltrate in lung tissue and bronchoalveolar lavage.146
Concluding Remarks
The pharmaceutical arsenal against hRSV has proven to be slow growing, and the only currently approved pharmaceutical product specific for this virus has an unsatisfactory clinical profile. Antibodies or antivirals against hRSV face the complex challenge of requiring high affinity for its intended target, either rapid neutralization or quick eliciting of effector immune functions, depending on the mechanism of action, resistance toward immune evasion by viral variants, and a prolonged half-life. Promisingly, recent developments in drug discovery and development allow for newfound optimism. Recent studies in structural biology have provided a detailed picture of the viral structure of hRSV, providing numerous potential targets for pharmacological modulation. Apart from agents in current clinical research, various others are in preclinical phases. Through mechanisms of action that involve effector functions of the immune system, it could be expected that agents directed against novel targets could provide new therapeutic strategies in the fight against hRSV. On the other hand, vaccine development against hRSV continues. The main challenge in this field involves eliciting a long-term immune response polarized toward the Th1 phenotype, to avoid the incidence of vaccine enhanced disease. Improvements in our understanding of the immune response to hRSV will likely facilitate the emergence of new promising vaccine candidates able to curb the morbidity and mortality caused by this pathogen.
Acknowledgments
This work was supported by funding from the Millennium Institute on Immunology and Immunotherapy ANID ACE 210015 (CN09_016/ICN 2021_045; former P09/016-F (AMK); CORFO grant #13CTI-21526/P4 and P5; ANID/FONDEF IDEA grant #22I10252 (AMK); PAI SA77210051 (JAS); ANID/CONICYT National Doctoral Scholarship #21221163 (BDV) and Biomedical Research Consortium CTU06 (AMK). This work was also supported by the Regional Government of Antofagasta through the Innovation Fund for Competitiveness FICR 2017 (BIP Code: 30488811-0) and FONDECYT Regular grant Nº1231866 (JAS) FONDECYT Regular grant N°T1191300 (CAR). Finally, we thank Biorender for making their templates available online, which we employed to construct the figure in this article.
Disclosure
Dr Alexis M Kalergis reports grants from Millennium Institute on Immunology and Immunotherapy CORFO, ANID, PAI, ANID, Biomedical Research Consortium, Regional Government of Antofagasta, during the conduct of the study; In addition, Dr Alexis M Kalergis has a patent (Monoclonal Antibody specific against the antigen N of the Human Respiratory Syncytial Virus (VRSH), useful for the treatment of infection, its detection and diagnosis) PCT/CL2018/050079 issued to PONTIFICIA UNIVERSIDAD CATOLICA DE CHILE. The authors report no other conflicts of interest in this work.
References
1. Afonso CL, Amarasinghe GK, Bányai K, et al. Taxonomy of the order mononegavirales: update 2016. Arch Virol. 2016;161:2351–2360. doi:10.1007/s00705-016-2880-1
2. Amarasinghe GK, Ayllón MA, Bào Y, et al. Taxonomy of the order mononegavirales: update 2019. Arch Virol. 2019;164:1967–1980. doi:10.1007/s00705-019-04247-4
3. Shi T, Denouel A, Tietjen AK, et al. Global disease burden estimates of respiratory syncytial virus–associated acute respiratory infection in older adults in 2015: a systematic review and meta-analysis. J Infect Dis. 2020;222:S577–S583. doi:10.1093/infdis/jiz059
4. World Health Organization. Respiratory syncytial virus surveillance. 2014.
5. Yoon JG, Noh JY, Choi WS, et al. Clinical characteristics and disease burden of respiratory syncytial virus infection among hospitalized adults. Sci Rep. 2020;10:12106. doi:10.1038/s41598-020-69017-8
6. Carbonell-Estrany X, Bont L, Doering G, Gouyon J-B, Lanari M. Clinical relevance of prevention of respiratory syncytial virus lower respiratory tract infection in preterm infants born between 33 and 35 weeks gestational age. Eur J Clin Microbiol Infect Dis. 2008;27:891–899. doi:10.1007/s10096-008-0520-8
7. Shi T, McAllister DA, O’Brien KL, et al. Global, regional, and national disease burden estimates of acute lower respiratory infections due to respiratory syncytial virus in young children in 2015: a systematic review and modelling study. Lancet. 2017;390:946–958. doi:10.1016/S0140-6736(17)30938-8
8. Bohmwald K, Espinoza J, Rey-Jurado E, et al. Human respiratory syncytial virus: infection and pathology. Semin Respir Crit Care Med. 2016;37:522–537. doi:10.1055/s-0036-1584799
9. Instituto de Salud Pública de Chile Vigilancia de Virus Respiratorios. Available from: https://www.ispch.gob.cl/virusrespiratorios/.
10. Stein RT, Bont LJ, Zar H, et al. Respiratory syncytial virus hospitalization and mortality: systematic review and meta‐analysis. Pediatr Pulmonol. 2017;52:556–569. doi:10.1002/ppul.23570
11. Chaw PS, Hua L, Cunningham S, et al. Respiratory syncytial virus-associated acute lower respiratory infections in children with bronchopulmonary dysplasia: systematic review and meta-analysis. J Infect Dis. 2020;222:S620–S627. doi:10.1093/infdis/jiz492
12. Gentile A, Lucion MF, Del Valle Juárez M, et al. Respiratory syncytial virus in preterm infants: 19 years of active epidemiological surveillance in a children’s hospital. Arch Argent Pediatr. 2020;118. doi:10.5546/aap.2020.eng.386
13. Azzari C, Baraldi E, Bonanni P, et al. Epidemiology and prevention of respiratory syncytial virus infections in children in Italy. Ital J Pediatr. 2021;47:198. doi:10.1186/s13052-021-01148-8
14. Simões EAF, Bont L, Manzoni P, et al. Past, present and future approaches to the prevention and treatment of respiratory syncytial virus infection in children. Infect Dis Ther. 2018;7:87–120. doi:10.1007/s40121-018-0188-z
15. Coultas JA, Smyth R, Openshaw PJ. Respiratory syncytial virus (RSV): a scourge from infancy to old age. Thorax. 2019;74:986–993. doi:10.1136/thoraxjnl-2018-212212
16. Anderson J, Do LAH, Wurzel D, et al. Severe respiratory syncytial virus disease in preterm infants: a case of innate immaturity. Thorax. 2021;76:942–950. doi:10.1136/thoraxjnl-2020-216291
17. Garegnani L, Styrmisdóttir L, Roson Rodriguez P, Escobar Liquitay CM, Esteban I, Franco JV. Palivizumab for preventing severe respiratory syncytial virus (RSV) infection in children. Cochrane Database Syst Rev. 2021;2021. doi:10.1002/14651858.CD013757.pub2
18. Collins PL, Chanock RM, Murphy BR. Respiratory Syncytial. In: Fields BN, Howley PM, Griffin DE, et al., editors. Fields Virology. Philadelphia: Lippincott Williams & Wilkins; 2001:1443–1485.
19. Bohmwald K, Andrade CA, Kalergis AM. Contribution of pro-inflammatory molecules induced by respiratory virus infections to neurological disorders. Pharmaceuticals. 2021;14:340. doi:10.3390/ph14040340
20. Andrade CA, Kalergis AM, Bohmwald K. Potential neurocognitive symptoms due to respiratory syncytial virus infection. Pathogens. 2021;11:47. doi:10.3390/pathogens11010047
21. Bohmwald K, Gálvez NMS, Ríos M, Kalergis AM. Neurologic alterations due to respiratory virus infections. Front Cell Neurosci. 2018;12. doi:10.3389/fncel.2018.00386
22. Peña M, Jara C, Flores JC, et al. Severe respiratory disease caused by human respiratory syncytial virus impairs language learning during early infancy. Sci Rep. 2020;10:22356. doi:10.1038/s41598-020-79140-1
23. Saravanos GL, King CL, Deng L, et al. Respiratory syncytial virus–associated neurologic complications in children: a systematic review and aggregated case series. J Pediatr. 2021;239:39–49.e9. doi:10.1016/j.jpeds.2021.06.045
24. Millichap JJ, Wainwright MS. Neurological complications of respiratory syncytial virus infection: case series and review of literature. J Child Neurol. 2009;24:1499–1503. doi:10.1177/0883073808331362
25. Rivera CA, Gómez RS, Díaz RA, et al. Novel therapies and vaccines against the human respiratory syncytial virus. Expert Opin Investig Drugs. 2015;24:1613–1630. doi:10.1517/13543784.2015.1099626
26. Mejias A, Rodríguez-Fernández R, Oliva S, Peeples ME, Ramilo O. The journey to a respiratory syncytial virus vaccine. Annals Allergy Asthma Immunol. 2020;125:36–46. doi:10.1016/j.anai.2020.03.017
27. Huang K, Wu H. Prevention of respiratory syncytial virus infection: from vaccine to antibody. Microbiol Spectr. 2014;2. doi:10.1128/microbiolspec.AID-0014-2014
28. Langley JM. Vaccine prevention of respiratory syncytial virus infection in older adults: the work continues. J Infect Dis. 2017;216:1334–1336. doi:10.1093/infdis/jix504
29. Subramanian S. Palivizumab. Drugs. 1999;58:312–313. doi:10.2165/00003495-199958020-00010
30. Luna MS, Manzoni P, Paes B, et al. Expert consensus on palivizumab use for respiratory syncytial virus in developed countries. Paediatr Respir Rev. 2020;33:35–44. doi:10.1016/j.prrv.2018.12.001
31. Checchia P. Identification and management of severe respiratory syncytial virus. Am J Health Syst Pharm. 2008;65:S7–S12. doi:10.2146/ajhp080439
32. Saso A, Kampmann B. Vaccine responses in newborns. Semin Immunopathol. 2017;39:627–642. doi:10.1007/s00281-017-0654-9
33. León B. Understanding the development of th2 cell-driven allergic airway disease in early life. Front Allergy. 2023;3. doi:10.3389/falgy.2022.1080153
34. Russell CD, Unger SA, Walton M, Schwarze J. The human immune response to respiratory syncytial virus infection. Clin Microbiol Rev. 2017;30:481–502. doi:10.1128/CMR.00090-16
35. Lukacs NW, Moore ML, Rudd BD, et al. Differential immune responses and pulmonary pathophysiology are induced by two different strains of respiratory syncytial virus. Am J Pathol. 2006;169:977–986. doi:10.2353/ajpath.2006.051055
36. Borchers AT, Chang C, Gershwin ME, Gershwin LJ. Respiratory syncytial virus—a comprehensive review. Clin Rev Allergy Immunol. 2013;45:331–379. doi:10.1007/s12016-013-8368-9
37. Waris ME, Tsou C, Erdman DD, Zaki SR, Anderson LJ. Respiratory synctial virus infection in BALB/c mice previously immunized with formalin-inactivated virus induces enhanced pulmonary inflammatory response with a predominant Th2-like cytokine pattern. J Virol. 1996;70:2852–2860. doi:10.1128/jvi.70.5.2852-2860.1996
38. Moghaddam A, Olszewska W, Wang B, et al. A potential molecular mechanism for hypersensitivity caused by formalin-inactivated vaccines. Nat Med. 2006;12:905–907. doi:10.1038/nm1456
39. Prince GA, Curtis SJ, Yim KC, Porter DD. Vaccine-enhanced respiratory syncytial virus disease in cotton rats following immunization with lot 100 or a newly prepared reference vaccine. J General Virol. 2001;82:2881–2888. doi:10.1099/0022-1317-82-12-2881
40. Kim HW, Canchola JG, Brandt CD, et al. Respiratory syncytial virus disease in infants despite prior administration of antigenic inactivated vaccine12. Am J Epidemiol. 1969;89:422–434. doi:10.1093/oxfordjournals.aje.a120955
41. Polack FP, Alvarez-Paggi D, Libster R, et al. Fatal enhanced respiratory syncytial virus disease in toddlers. Sci Transl Med. 2021;13(616). doi:10.1126/scitranslmed.abj7843
42. Rey-Jurado E, Kalergis A. Immunological features of respiratory syncytial virus-caused pneumonia—implications for vaccine design. Int J Mol Sci. 2017;18:556. doi:10.3390/ijms18030556
43. Retamal-Díaz A, Covián C, Pacheco GA, et al. Contribution of resident memory CD8+ T cells to protective immunity against respiratory syncytial virus and their impact on vaccine design. Pathogens. 2019;8(3):147. doi:10.3390/pathogens8030147
44. Becker Y. Respiratory syncytial virus (RSV) evades the human adaptive immune system by skewing the Th1/Th2 cytokine balance toward increased levels of Th2 cytokines and IgE, markers of allergy—a review. Virus Genes. 2006;33:235–252. doi:10.1007/s11262-006-0064-x
45. Khan IU, Ahmad F, Zhang S, et al. Respiratory syncytial virus F and G protein core fragments fused to HBsAg-binding protein (SBP) induce a Th1-dominant immune response without vaccine-enhanced disease. Int Immunol. 2019;31:199–209. doi:10.1093/intimm/dxy078
46. van der Fits L, Bolder R, Heemskerk-van der Meer M, et al. Adenovector 26 encoded prefusion conformation stabilized RSV-F protein induces long-lasting th1-biased immunity in neonatal mice. NPJ Vaccines. 2020;5:49. doi:10.1038/s41541-020-0200-y
47. van Haren SD, Pedersen GK, Kumar A, et al. CAF08 adjuvant enables single dose protection against respiratory syncytial virus infection in murine newborns. Nat Commun. 2022;13:4234. doi:10.1038/s41467-022-31709-2
48. Zheng Y, Bian L, Zhao H, et al. Respiratory syncytial virus F subunit vaccine with AS02 adjuvant elicits balanced, robust humoral and cellular immunity in BALB/c mice. Front Immunol. 2020;11. doi:10.3389/fimmu.2020.526965
49. Riffault S, Meyer G, Deplanche M, et al. A new subunit vaccine based on nucleoprotein nanoparticles confers partial clinical and virological protection in calves against bovine respiratory syncytial virus. Vaccine. 2010;28:3722–3734. doi:10.1016/j.vaccine.2010.03.008
50. King AM, Stott EJ, Langer SJ, Young KK, Ball LA, Wertz GW. Recombinant vaccinia viruses carrying the N gene of human respiratory syncytial virus: studies of gene expression in cell culture and immune response in mice. J Virol. 1987;61:2885–2890. doi:10.1128/jvi.61.9.2885-2890.1987
51. Thomas LH, Wyld SG, Wertz GW, et al. Recombinant vaccinia viruses expressing the F, G or N, but not the M2, protein of bovine respiratory syncytial virus (BRSV) induce resistance to BRSV challenge in the calf and protect against the development of pneumonic lesions. J General Virol. 1997;78:3195–3206. doi:10.1099/0022-1317-78-12-3195
52. Bangham CR, Openshaw PJ, Ball LA, King AM, Wertz GW, Askonas BA. Human and murine cytotoxic t cells specific to respiratory syncytial virus recognize the viral nucleoprotein (N), but not the major glycoprotein (G), expressed by vaccinia virus recombinants. J Immunol. 1986;137:3973–3977. doi:10.4049/jimmunol.137.12.3973
53. Boxus M, Tignon M, Roels S, et al. DNA immunization with plasmids encoding fusion and nucleocapsid proteins of bovine respiratory syncytial virus induces a strong cell-mediated immunity and protects calves against challenge. J Virol. 2007;81:6879–6889. doi:10.1128/JVI.00502-07
54. Vaughan K, Rhodes GH, Gershwin LJ. DNA immunization against respiratory syncytial virus (RSV) in infant rhesus monkeys. Vaccine. 2005;23:2928–2942. doi:10.1016/j.vaccine.2004.10.046
55. Bueno SM, González PA, Cautivo KM, et al. Protective T cell immunity against respiratory syncytial virus is efficiently induced by recombinant BCG. Proc Natl Acad Sci. 2008;105:20822–20827. doi:10.1073/pnas.0806244105
56. Díaz FE, Guerra-Maupome M, McDonald PO, Rivera-Pérez D, Kalergis AM, McGill JL. A recombinant BCG vaccine is safe and immunogenic in neonatal calves and reduces the clinical disease caused by the respiratory syncytial virus. Front Immunol. 2021;12. doi:10.3389/fimmu.2021.664212
57. Abarca K, Rey-Jurado E, Muñoz-Durango N, et al. Safety and immunogenicity evaluation of recombinant BCG vaccine against respiratory syncytial virus in a randomized, double-blind, placebo-controlled phase I clinical trial. EClinicalMedicine. 2020;27:100517. doi:10.1016/j.eclinm.2020.100517
58. Céspedes PF, Rey-Jurado E, Espinoza JA, et al. A single, low dose of a CGMP recombinant BCG vaccine elicits protective T cell immunity against the human respiratory syncytial virus infection and prevents lung pathology in mice. Vaccine. 2017;35:757–766. doi:10.1016/j.vaccine.2016.12.048
59. Lindell DM, Morris SB, White MP, et al. A novel inactivated intranasal respiratory syncytial virus vaccine promotes viral clearance without Th2 associated vaccine-enhanced disease. PLoS One. 2011;6:e21823. doi:10.1371/journal.pone.0021823
60. Roux X, Dubuquoy C, Durand G, et al. Sub-nucleocapsid nanoparticles: a nasal vaccine against respiratory syncytial virus. PLoS One. 2008;3:e1766. doi:10.1371/journal.pone.0001766
61. Jordan E, Lawrence SJ, Meyer TPH, et al. Broad antibody and cellular immune response from a phase 2 clinical trial with a novel multivalent poxvirus-based respiratory syncytial virus vaccine. J Infect Dis. 2021;223:1062–1072. doi:10.1093/infdis/jiaa460
62. McFarland EJ, Karron RA, Muresan P, et al. Live respiratory syncytial virus attenuated by M2-2 deletion and stabilized temperature sensitivity mutation 1030s is a promising vaccine candidate in children. J Infect Dis. 2020;221:534–543. doi:10.1093/infdis/jiz603
63. Buchholz UJ, Cunningham CK, Muresan P, et al. Live respiratory syncytial virus (RSV) vaccine candidate containing stabilized temperature-sensitivity mutations is highly attenuated in RSV-seronegative infants and children. J Infect Dis. 2018;217:1338–1346. doi:10.1093/infdis/jiy066
64. McFarland EJ, Karron RA, Muresan P, et al. Live-attenuated respiratory syncytial virus vaccine candidate with deletion of RNA synthesis regulatory protein M2-2 is highly immunogenic in children. J Infect Dis. 2018;217:1347–1355. doi:10.1093/infdis/jiy040
65. Gomez RS, Guisle-Marsollier I, Bohmwald K, Bueno SM, Kalergis AM. Respiratory syncytial virus: pathology, therapeutic drugs and prophylaxis. Immunol Lett. 2014;162:237–247. doi:10.1016/j.imlet.2014.09.006
66. Kalergis AM, Soto JA, Gálvez NMS, et al. Pharmacological management of human respiratory syncytial virus infection. Expert Opin Pharmacother. 2020;21:2293–2303. doi:10.1080/14656566.2020.1806821
67. IMpact-RSV Study Group. Palivizumab, a humanized respiratory syncytial virus monoclonal antibody, reduces hospitalization from respiratory syncytial virus infection in high-risk infants. Pediatrics. 1998;102:531–537. doi:10.1542/peds.102.3.531
68. Ventre K, Randolph A. Ribavirin for respiratory syncytial virus infection of the lower respiratory tract in infants and young children. In: Ventre K, editor. Cochrane Database of Systematic Reviews. Chichester, UK: John Wiley & Sons, Ltd; 2007.
69. Conrad DA, Christenson JC, WANER JL, Marks MI. Aerosolized ribavirin treatment of respiratory syncytial virus infection in infants hospitalized during an epidemic. Pediatr Infect Dis J. 1987;6:152–158. doi:10.1097/00006454-198702000-00003
70. Johnson S, Oliver C, Prince GA, et al. Development of a humanized monoclonal antibody (MEDI‐493) with potent in vitro and in vivo activity against respiratory syncytial virus. J Infect Dis. 1997;176:1215–1224. doi:10.1086/514115
71. Morris JA, Blount RE, Savage RE. Recovery of cytopathogenic agent from chimpanzees with goryza. Exp Biol Med. 1956;92:544–549. doi:10.3181/00379727-92-22538
72. González PA, Bueno SM, Carreño LJ, Riedel CA, Kalergis AM. Respiratory syncytial virus infection and immunity. Rev Med Virol. 2012;22:230–244. doi:10.1002/rmv.1704
73. Wang E, Tang N. Immunoglobulin for preventing respiratory syncytial virus infection. In: Wang E, editor. The Cochrane Database of Systematic Reviews. Chichester, UK: John Wiley & Sons, Ltd; 1999.
74. Englund JA. Prevention strategies for respiratory syncytial virus: passive and active immunization. J Pediatr. 1999;135:38–44.
75. Wu H, Pfarr DS, Losonsky GA, Kiener PA. Immunoprophylaxis of RSV infection: advancing from RSV-IGIV to palivizumab and motavizumab. Human Antibody Therap Viral Dis. 2008;20:103–123.
76. Meissner HC, Rennels MB, Long SS, Pickering LK. Immunoprophylaxis with RespiGam. Pediatrics. 2004;113:629. doi:10.1542/peds.113.3.629-a
77. Mac S, Sumner A, Duchesne-Belanger S, Stirling R, Tunis M, Sander B. Cost-effectiveness of palivizumab for respiratory syncytial virus: a systematic review. Pediatrics. 2019;143. doi:10.1542/peds.2018-4064
78. Sanders SL, Agwan S, Hassan M, van Driel ML, Del Mar CB. Immunoglobulin treatment for hospitalised infants and young children with respiratory syncytial virus infection. Cochrane Database Syst Rev. 2019. doi:10.1002/14651858.CD009417.pub2
79. Homaira N, Rawlinson W, Snelling TL, Jaffe A. Effectiveness of palivizumab in preventing RSV hospitalization in high risk children: a real-world perspective. Int J Pediatr. 2014;2014:1–13. doi:10.1155/2014/571609
80. van Mechelen L, Luytjes W, de Haan CAM, Wicht O. RSV neutralization by palivizumab, but not by monoclonal antibodies targeting other epitopes, is augmented by Fc gamma receptors. Antiviral Res. 2016;132:1–5. doi:10.1016/j.antiviral.2016.05.003
81. Mochizuki H, Kusuda S, Okada K, et al. Palivizumab prophylaxis in preterm infants and subsequent recurrent wheezing. six-year follow-up study. Am J Respir Crit Care Med. 2017;196:29–38. doi:10.1164/rccm.201609-1812OC
82. Resch B. Respiratory syncytial virus infection in high-risk infants – an update on palivizumab prophylaxis. Open Microbiol J. 2014;8:71–77. doi:10.2174/1874285801408010071
83. Saez-Llorens X, Moreno MT, Ramilo O, Sanchez PJ, Top FH, Connor EM. Safety and pharmacokinetics of palivizumab therapy in children hospitalized with respiratory syncytial virus infection. Pediatr Infect Dis J. 2004;23:707–712. doi:10.1097/01.inf.0000133165.85909.08
84. Soto JA, Gálvez NMS, Pacheco GA, Bueno SM, Kalergis AM. Antibody development for preventing the human respiratory syncytial virus pathology. Mol Med. 2020;26:35. doi:10.1186/s10020-020-00162-6
85. Wu H, Pfarr DS, Johnson S, et al. Development of motavizumab, an ultra-potent antibody for the prevention of respiratory syncytial virus infection in the upper and lower respiratory tract. J Mol Biol. 2007;368:652–665. doi:10.1016/j.jmb.2007.02.024
86. Griffin MP, Khan AA, Esser MT, et al. Safety, tolerability, and pharmacokinetics of MEDI8897, the respiratory syncytial virus prefusion f-targeting monoclonal antibody with an extended half-life, in healthy adults. Antimicrob Agents Chemother. 2017;61. doi:10.1128/AAC.01714-16
87. Aliprantis AO, Wolford D, Caro L, et al. A phase 1 randomized, double‐blind, placebo‐controlled trial to assess the safety, tolerability, and pharmacokinetics of a respiratory syncytial virus neutralizing monoclonal antibody MK‐1654 in healthy adults. Clin Pharmacol Drug Dev. 2021;10:556–566. doi:10.1002/cpdd.883
88. Mejías A, Chávez-Bueno S, Raynor MB, et al. Motavizumab, a neutralizing anti-respiratory syncytial virus (RSV) monoclonal antibody significantly modifies the local and systemic cytokine responses induced by RSV in the mouse model. Virol J. 2007;4:109. doi:10.1186/1743-422X-4-109
89. Abarca K, Jung E, Fernández P, et al. Safety, tolerability, pharmacokinetics, and immunogenicity of motavizumab, a humanized, enhanced-potency monoclonal antibody for the prevention of respiratory syncytial virus infection in at-risk children. Pediatr Infect Dis J. 2009;28:267–272. doi:10.1097/INF.0b013e31818ffd03
90. Gómez RS, Mora JE, Cortés CM, et al. Respiratory syncytial virus detection in cells and clinical samples by using three new monoclonal antibodies. J Med Virol. 2014;86:1256–1266. doi:10.1002/jmv.23807
91. Robbie GJ, Criste R, Dall’Acqua WF, et al. A novel investigational Fc-modified humanized monoclonal antibody, motavizumab-YTE, has an extended half-life in healthy adults. Antimicrob Agents Chemother. 2013;57:6147–6153. doi:10.1128/AAC.01285-13
92. Cingoz O. Motavizumab. MAbs. 2009;1:439–442. doi:10.4161/mabs.1.5.9496
93. Carbonell-Estrany X, Simões EAF, Dagan R, et al. Motavizumab for prophylaxis of respiratory syncytial virus in high-risk children: a noninferiority trial. Pediatrics. 2010;125:e35–e51. doi:10.1542/peds.2008-1036
94. Griffin MP, Yuan Y, Takas T, et al. Single-dose nirsevimab for prevention of RSV in preterm infants. N Engl J Med. 2020;383:415–425. doi:10.1056/NEJMoa1913556
95. Keam SJ. Nirsevimab: first approval. Drugs. 2023;83:181–187. doi:10.1007/s40265-022-01829-6
96. Anderson L, Jadhao S, Paden C, Tong S. Functional features of the respiratory syncytial virus G protein. Viruses. 2021;13:1214. doi:10.3390/v13071214
97. McLellan JS, Chen M, Leung S, et al. Structure of RSV fusion glycoprotein trimer bound to a prefusion-specific neutralizing antibody. Science. 2013;340:1113–1117. doi:10.1126/science.1234914
98. Collarini EJ, Lee FE-H, Foord O, et al. Potent high-affinity antibodies for treatment and prophylaxis of respiratory syncytial virus derived from b cells of infected patients. J Immunol. 2009;183:6338–6345. doi:10.4049/jimmunol.0901373
99. Tripp RA, Power UF, Openshaw PJM, Kauvar LM. Respiratory syncytial virus: targeting the G protein provides a new approach for an old problem. J Virol. 2018;92. doi:10.1128/JVI.01302-17
100. Haynes LM, Caidi H, Radu GU, et al. Therapeutic monoclonal antibody treatment targeting respiratory syncytial virus (RSV) G protein mediates viral clearance and reduces the pathogenesis of RSV infection in BALB/c mice. J Infect Dis. 2009;200:439–447. doi:10.1086/600108
101. Oliveira AP, Simabuco FM, Tamura RE, et al. Human respiratory syncytial virus N, P and M protein interactions in HEK-293T cells. Virus Res. 2013;177:108–112. doi:10.1016/j.virusres.2013.07.010
102. Simabuco FM, Carromeu C, Farinha-Arcieri LE, Tamura RE, Ventura AM. Production of polyclonal antibodies against the human respiratory syncytial virus nucleoprotein and phosphoprotein expressed in Escherichia coli. Protein Expr Purif. 2007;53:209–215. doi:10.1016/j.pep.2006.12.016
103. Weiner GJ. Building better monoclonal antibody-based therapeutics. Nat Rev Cancer. 2015;15:361–370. doi:10.1038/nrc3930
104. Lu R-M, Hwang Y-C, Liu I-J, et al. Development of therapeutic antibodies for the treatment of diseases. J Biomed Sci. 2020;27:1. doi:10.1186/s12929-019-0592-z
105. Barr R, Green CA, Sande CJ, Drysdale SB. Respiratory syncytial virus: diagnosis, prevention and management. Ther Adv Infect Dis. 2019;6:204993611986579. doi:10.1177/2049936119865798
106. Feltes TF, Sondheimer HM, Tulloh RMR, et al. A randomized controlled trial of motavizumab versus palivizumab for the prophylaxis of serious respiratory syncytial virus disease in children with hemodynamically significant congenital heart disease. Pediatr Res. 2011;70:186–191. doi:10.1203/PDR.0b013e318220a553
107. Gonzales T, Bergamasco A, Cristarella T, et al. Effectiveness and safety of palivizumab for the prevention of serious lower respiratory tract infection caused by respiratory syncytial virus: a systematic review. Am J Perinatol. 2022. doi:10.1055/a-1990-2633
108. Checchia PA, Nalysnyk L, Fernandes AW, et al. Mortality and morbidity among infants at high risk for severe respiratory syncytial virus infection receiving prophylaxis with palivizumab: a systematic literature review and meta-analysis. Pediatr Crit Care Med. 2011;12:580–588. doi:10.1097/PCC.0b013e3182070990
109. Bates JT, Keefer CJ, Slaughter JC, Kulp DW, Schief WR, Crowe JE. Escape from neutralization by the respiratory syncytial virus-specific neutralizing monoclonal antibody palivizumab is driven by changes in on-rate of binding to the fusion protein. Virology. 2014;454–455:139–144. doi:10.1016/j.virol.2014.02.010
110. Nguyen TN, Power UF, Robert A, et al. The respiratory syncytial virus G protein conserved domain induces a persistent and protective antibody response in rodents. PLoS One. 2012;7:e34331. doi:10.1371/journal.pone.0034331
111. Bergeron HC, Murray J, Nuñez Castrejon AM, DuBois RM, Tripp RA. Respiratory syncytial virus (RSV) G protein vaccines with central conserved domain mutations induce CX3C-CX3CR1 blocking antibodies. Viruses. 2021;13:352. doi:10.3390/v13020352
112. Amitai N, Stafler P, Blau H, et al. Palivizumab following extremely premature birth does not affect pulmonary outcomes in adolescence. Chest. 2020;158:660–669. doi:10.1016/j.chest.2020.02.075
113. Prais D, Kaplan E, Klinger G, et al. Short- and long-term pulmonary outcome of palivizumab in children born extremely prematurely. Chest. 2016;149:801–808. doi:10.1378/chest.15-0328
114. Goulet DR, Atkins WM. Considerations for the design of antibody-based therapeutics. J Pharm Sci. 2020;109:74–103. doi:10.1016/j.xphs.2019.05.031
115. Hiatt A, Bohorova N, Bohorov O, et al. Glycan variants of a respiratory syncytial virus antibody with enhanced effector function and in vivo efficacy. Proc Natl Acad Sci. 2014;111:5992–5997. doi:10.1073/pnas.1402458111
116. Rajapaksa AE, Do LAH, Suryawijaya Ong D, et al. Pulmonary deposition of radionucleotide-labeled palivizumab: proof-of-concept study. Front Pharmacol. 2020;11. doi:10.3389/fphar.2020.01291
117. Céspedes PF, Bueno SM, Ramírez BA, et al. Surface expression of the HRSV nucleoprotein impairs immunological synapse formation with T cells. Proc Natl Acad Sci. 2014;111. doi:10.1073/pnas.1400760111
118. Wang S-Y, Weiner G. Complement and cellular cytotoxicity in antibody therapy of cancer. Expert Opin Biol Ther. 2008;8:759–768. doi:10.1517/14712598.8.6.759
119. Corbeil S, Seguin C, Trudel M. Involvement of the complement system in the protection of mice from challenge with respiratory syncytial virus long strain following passive immunization with monoclonal antibody 18A2B2. Vaccine. 1996;14:521–525. doi:10.1016/0264-410X(95)00222-M
120. Bonsignori M, Pollara J, Moody MA, et al. Antibody-dependent cellular cytotoxicity-mediating antibodies from an HIV-1 vaccine efficacy trial target multiple epitopes and preferentially use the VH1 gene family. J Virol. 2012;86:11521–11532. doi:10.1128/JVI.01023-12
121. Gupta N, LeGoff J, Chamat S, et al. Affinity-purified respiratory syncytial virus antibodies from intravenous immunoglobulin exert potent antibody-dependent cellular cytotoxicity. PLoS One. 2013;8:e69390. doi:10.1371/journal.pone.0069390
122. Chung S, Lin YL, Reed C, et al. Characterization of in vitro antibody-dependent cell-mediated cytotoxicity activity of therapeutic antibodies — impact of effector cells. J Immunol Methods. 2014;407:63–75. doi:10.1016/j.jim.2014.03.021
123. Tsao L-C, Crosby EJ, Trotter TN, et al. Trastuzumab/pertuzumab combination therapy stimulates antitumor responses through complement-dependent cytotoxicity and phagocytosis. JCI Insight. 2022;7. doi:10.1172/jci.insight.155636
124. Clynes RA, Towers TL, Presta LG, Ravetch JV. Inhibitory Fc receptors modulate in vivo cytoxicity against tumor targets. Nat Med. 2000;6:443–446. doi:10.1038/74704
125. Clynes R, Takechi Y, Moroi Y, Houghton A, Ravetch JV. Fc receptors are required in passive and active immunity to melanoma. Proc Natl Acad Sci. 1998;95:652–656. doi:10.1073/pnas.95.2.652
126. Chan KR, Ong EZ, Mok DZ, Ooi EE. Fc receptors and their influence on efficacy of therapeutic antibodies for treatment of viral diseases. Expert Rev Anti Infect Ther. 2015;13:1351–1360. doi:10.1586/14787210.2015.1079127
127. Madhi SA, Polack FP, Piedra PA, et al. Respiratory syncytial virus vaccination during pregnancy and effects in infants. N Engl J Med. 2020;383:426–439. doi:10.1056/NEJMoa1908380
128. Simões EAF, Center KJ, Tita ATN, et al. Prefusion F protein–based respiratory syncytial virus immunization in pregnancy. N Engl J Med. 2022;386:1615–1626. doi:10.1056/NEJMoa2106062
129. Hammitt LL, Dagan R, Yuan Y, et al. Nirsevimab for prevention of RSV in healthy late-preterm and term infants. N Engl J Med. 2022;386:837–846. doi:10.1056/NEJMoa2110275
130. Simões EAF, Forleo-Neto E, Geba GP, et al. Suptavumab for the prevention of medically attended respiratory syncytial virus infection in preterm infants. Clin Infect Dis. 2021;73:e4400–e4408. doi:10.1093/cid/ciaa951
131. Regeneron Pharmaceuticals Inc. Regeneron to discontinue development of suptavumab for respiratory syncytial virus. 2017.
132. Weltzin R, Hsu SA, Mittler ES, Georgakopoulos K, Monath TP. Intranasal monoclonal immunoglobulin a against respiratory syncytial virus protects against upper and lower respiratory tract infections in mice. Antimicrob Agents Chemother. 1994;38:2785–2791. doi:10.1128/AAC.38.12.2785
133. Weltzin R, Traina-Dorge V, Soike K, et al. Intranasal monoclonal IgA antibody to respiratory syncytial virus protects rhesus monkeys against upper and lower respiratory tract infection. J Infect Dis. 1996;174:256–261. doi:10.1093/infdis/174.2.256
134. Cunningham S, Piedra PA, Martinon-Torres F, et al. Nebulised ALX-0171 for respiratory syncytial virus lower respiratory tract infection in hospitalised children: a double-blind, randomised, placebo-controlled, Phase 2b trial. Lancet Respir Med. 2021;9:21–32. doi:10.1016/S2213-2600(20)30320-9
135. Broadbent L, Parke HG, Ferguson LJ, et al. Comparative therapeutic potential of ALX-0171 and palivizumab against respiratory syncytial virus clinical isolate infection of well-differentiated primary pediatric bronchial epithelial cell cultures. Antimicrob Agents Chemother. 2020;64. doi:10.1128/AAC.02034-19
136. Larios Mora A, Detalle L, Gallup JM, et al. Delivery of ALX-0171 by inhalation greatly reduces respiratory syncytial virus disease in newborn lambs. MAbs. 2018;10:778–795. doi:10.1080/19420862.2018.1470727
137. Palomo C, Mas V, Detalle L, et al. Trivalency of a nanobody specific for the human respiratory syncytial virus fusion glycoprotein drastically enhances virus neutralization and impacts escape mutant selection. Antimicrob Agents Chemother. 2016;60:6498–6509. doi:10.1128/AAC.00842-16
138. Scott R, de Landazuri MO, Gardner PS, Owen JJ. Human antibody-dependent cell-mediated cytotoxicity against target cells infected with respiratory syncytial virus. Clin Exp Immunol. 1977;28:19–26.
139. Tawar RG, Duquerroy S, Vonrhein C, et al. Crystal structure of a nucleocapsid-like nucleoprotein-RNA complex of respiratory syncytial virus. Science. 2009;326:1279–1283. doi:10.1126/science.1177634
140. MacLellan K, Loney C, Yeo RP, Bhella D. The 24-angstrom structure of respiratory syncytial virus nucleocapsid protein-RNA decameric rings. J Virol. 2007;81:9519–9524. doi:10.1128/JVI.00526-07
141. El Omari K, Dhaliwal B, Ren J, et al. Structures of respiratory syncytial virus nucleocapsid protein from two crystal forms: details of potential packing interactions in the native helical form. Acta Crystallogr Sect F Struct Biol Cryst Commun. 2011;67:1179–1183. doi:10.1107/S1744309111029228
142. Johnson SM, McNally BA, Ioannidis I, et al. Respiratory syncytial virus uses CX3CR1 as a receptor on primary human airway epithelial cultures. PLoS Pathog. 2015;11:e1005318. doi:10.1371/journal.ppat.1005318
143. Jeong K-I, Piepenhagen PA, Kishko M, et al. CX3CR1 is expressed in differentiated human ciliated airway cells and co-localizes with respiratory syncytial virus on Cilia in a G protein-dependent manner. PLoS One. 2015;10:e0130517. doi:10.1371/journal.pone.0130517
144. Anderson CS, Chu C-Y, Wang Q, et al. CX3CR1 as a respiratory syncytial virus receptor in pediatric human lung. Pediatr Res. 2020;87:862–867. doi:10.1038/s41390-019-0677-0
145. Anderson CS, Chirkova T, Slaunwhite CG, et al. CX3CR1 engagement by respiratory syncytial virus leads to induction of nucleolin and dysregulation of cilium-related genes. J Virol. 2021;95. doi:10.1128/JVI.00095-21
146. Caidi H, Miao C, Thornburg NJ, Tripp RA, Anderson LJ, Haynes LM. Anti-respiratory syncytial virus (RSV) G monoclonal antibodies reduce lung inflammation and viral lung titers when delivered therapeutically in a BALB/c mouse model. Antiviral Res. 2018;154:149–157. doi:10.1016/j.antiviral.2018.04.014
147. Hayes C, Wilkerson S, Bibb K, Schuschke L, Guest A. Effectiveness of RSV-IVIG in premature infants: success in the home. Pediatrics. 1999;103(3):698. doi:10.1542/peds.103.3.698
148. Soto JA, Galvez NMS, Rivera DB, et al. From animal studies into clinical trials: the relevance of animal models to develop vaccines and therapies to reduce disease severity and prevent HRSV infection. Expert Opin Drug Discov. 2022;17:1237–1259. doi:10.1080/17460441.2022.2123468
149. MedImmune SYNAGIS® (PALIVIZUMAB) for Intramuscular Administration. Gaithersburg; 1999.
150. Fedechkin SO, George NL, Wolff JT, Kauvar LM, DuBois RM. Structures of respiratory syncytial virus G antigen bound to broadly neutralizing antibodies. Sci Immunol. 2018;3. doi:10.1126/sciimmunol.aar3534
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
