Back to Journals » Infection and Drug Resistance » Volume 16
New Attempts to Inhibit Methicillin-Resistant Staphylococcus aureus Biofilm? A Combination of Daptomycin and Azithromycin
Authors Zhou Y, Liu MJ, Liao XY, Chen YT, Liao QX, Lin JD, Lin HR, Huang YH
Received 1 August 2023
Accepted for publication 9 October 2023
Published 6 November 2023 Volume 2023:16 Pages 7029—7040
DOI https://doi.org/10.2147/IDR.S433439
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Sandip Patil
Ye Zhou,1,* Ming-Jun Liu,2,* Xiu-Yu Liao,1 Yu-Ting Chen,3 Qiu-Xia Liao,1 Jian-Dong Lin,1 Hai-Rong Lin,4 Ying-Hong Huang1
1Department of Intensive Care Unit, First Affiliated Hospital of Fujian Medical University, Fuzhou, 350000, People’s Republic of China; 2Department of Infection, People’s Hospital of YangJiang, YangJiang, 529500, People’s Republic of China; 3Department of Gastroenterology, Fuzhou NO. 1 Hospital, Fuzhou, 350000, People’s Republic of China; 4Department of Intensive Care Unit, National Regional Medical Center, Binhai Campus of the First Affiliated Hospital, Fujian Medical University, Fuzhou, 350212, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Xiu-Yu Liao; Ying-Hong Huang, Department of Intensive Care Unit, First Affiliated Hospital of Fujian Medical University, No. 20 of Chazhong Road, TaiJiang District, Fuzhou, 350000, People’s Republic of China, Tel +86 15959021313 ; +86 13306901596, Fax +86 591-87982028, Email [email protected]; [email protected]
Objective: To investigate the antibacterial impact of daptomycin and azithromycin in vitro on methicillin-resistant Staphylococcus aureus (MRSA) biofilm.
Methods: (1) Measure the strain growth curve and the biofilm formation curve. (2) Determine the minimum inhibitory concentrations (MICs) of daptomycin and azithromycin. (3) Investigate the antibacterial impact of the combination of daptomycin and azithromycin. (4) Perform the evaluation of the intervention impact of antimicrobial agents on MRSA biofilm. (5) Observe the biofilm after intervention with the antibacterial agent.
Results: (1) MRSA exhibited three phases: lag phase (0– 4 h), logarithmic growth (4– 8 h) and stationary phase after 18 h; its biofilm began to form at 6 h, semi-matured at 24 h, and reached maturity after 48 h. (2) The MICs of daptomycin and azithromycin were 8 μg/mL and greater than 256 μg/mL, respectively. (3) The combination of daptomycin and azithromycin has an additive effect on MRSA (Fractional Inhibitory Concentration Index [FICI] 0.625) (FICI = MIC of drug A in combination/MIC of drug A alone + MIC of drug B in combination/MIC of drug B alone). Evaluation criteria: Synergistic effect is considered when FICI ≤ 0.5; additive effect is considered when 0.5 < FICI ≤ 1; irrelevant effect is considered when 1 < FICI ≤ 2; antagonistic effect is considered when FICI > 2). (4) Daptomycin or azithromycin at MICs inhibited not only the growth of planktonic bacteria but also the formation of biofilm. (5) The combination of both, in which group the ratio of live/dead bacteria is low and the biofilm morphology was incomplete, was more productive than monotherapy in against biofilm.
Conclusion: Both daptomycin and azithromycin have anti-MRSA biofilm activity, and daptomycin is dominant. The fact that the combination of both can significantly inhibit the further maturation of MRSA biofilm and destroy already formed biofilm demonstrates the superiority of the combination over the monotherapy.
Keywords: azithromycin, bacterial resistance, biofilm, daptomycin, MRSA
Introduction
Bacterial infections are prevalent and their severity depends on the characteristics of the microorganisms involved and the high detection rate of nosocomial drug-resistant bacteria.1 Hospital-acquired infections are a major public health concern, with a high incidence, increasing the healthcare cost.2 Moreover, the data from the World Health Organization (WHO) revealed that an average of 8.7% of hospitalized patients suffered from hospital-acquired infections, and more than 1.4 million people suffered from complications of nosocomial infections, worldwide. Frequently, hospital infections may be endogenous (sources of infection came from patient’s own microbiota) or exogenous (pathogen came from other patients, healthcare workers, or the hospital environment such as water, air, and surfaces of objects).3 Numerous studies have identified the sterile hospital environment as a potential site for multi-drug resistant (MDR) bacteria, Staphylococcus aureus accounted for the third highest number of clinical isolates in antibiotic resistance surveillance in 2022.4 Intensive care units (ICUs) were one of the most common sources for methicillin-resistant S. aureus (MRSA) colonization and infection,5 with a 5- to 10-fold increased incidence of hospital-acquired infections than in general wards.6,7 Majority of ICU patients are critically ill and immunocompromised and are at high risk of MRSA infection; the longer the patient stays in the ICU, the higher the risk. Patients with MRSA infection have greater medical costs, morbidity, and mortality rates than patients with methicillin-susceptible S. aureus (MSSA). MRSA is presently resistant to numerous widely used antibiotics. Moreover, resistance to newer antimicrobial agents such as linezolid, vancomycin, teicoplanin, and daptomycin has been reported.8 Although the rate of MRSA infection has decreased in recent years, the infection risk remains high and poses a significant challenge to clinical anti-infective therapy due to the restricted number of antimicrobial drugs in development.
Infections caused by MRSA after biofilm formation are characterized by high environmental adaptability, susceptibility to drug resistance, and difficulty in being cleared by the host’s immune system, making it more challenging to find an appropriate treatment. Biofilm formation is seen in 78.2% of chronic wounds in patients in the ICU who have undergone surgical procedures; the persistence of biofilm causes an inflammatory response and the wound does not heal.9,10 In addition, with the aging of society, there are more and more joint replacement surgeries, and infection around prostheses is a catastrophic complication after joint replacement surgery. The reason for this is that bacterial biofilm has always been a key factor in the existence of chronic infections.11 Staphylococcus aureus is one of the most common pathogens in periprosthetic infections. Infections caused by the biofilm of Staphylococcus aureus are difficult to treat because bacterial biofilms have stronger resistance compared to planktonic forms of bacteria, which makes antibacterial drugs and disinfectants ineffective.12,13 Moreover, once medication is stopped, surviving bacteria can continue to reproduce rapidly and quickly restore bacterial biofilm to its original state.
Therefore, the role of biofilms is of great interest to scientists worldwide. Daptomycin exerts bactericidal activity by disrupting bacterial cell membranes; the percentage of drug resistance has not increased significantly in the past 10 years since the introduction of daptomycin to the market, demonstrating its unique bactericidal advantages.14,15 Macrolide drugs are weakly alkaline antibiotics produced by Streptomyces, which contain a lactone structure of 14 or 16 yuan ring, and have strong inhibitory effect on Streptococcus pneumoniae, so they are important drugs in the treatment of community-acquired pneumonia. Its main pharmacological mechanism is that it acts on bacterial ribosome 50S subunit, blocking bacterial protein synthesis and messenger RNA translocation, and has good antibacterial effect on atypical pathogens such as chlamydia, mycoplasma, Legionella and Helicobacter pylori. It is found that the application of macrolides alone can affect the adhesion of Pseudomonas aeruginosa and the permeability of biofilm.11,16 At the same time, macrolides not only inhibit the growth of bacteria but also have certain immunomodulatory and anti-inflammatory effects, thus assisting the body to resist infection. Azithromycin is a kind of macrolide antibacterial drug commonly used in clinic, which can affect the ribosome of bacteria and produce a series of biochemical reactions, and inhibit the further synthesis of protein needed for the growth and reproduction of bacteria, and finally inhibit the growth and reproduction of bacteria. Imperi et al17 found that azithromycin has a certain effect on Pseudomonas aeruginosa infection. Not only in bacteriostatic effect, macrolide antibacterial drugs such as azithromycin have strong penetration ability on bacterial biofilm, and this penetration ability is considered to be the main mechanism of their anti-bacterial biofilm. At the same time, with the deepening of research, it is found that there is a quorum sensing (QS) system in the group behavior between bacteria, and this system can affect the formation of biofilm. In this system, bacteria activate receptors through an information substance called autoinducer (AI) to express the corresponding genes. After gene expression, bacteria can perceive the existence of other bacteria and coordinate the relationship between individuals and groups. Azithromycin can just inhibit the production of AI and block QS. However, at present, there are few studies on the inhibition of MRSA biofilm by azithromycin. In this study, daptomycin and azithromycin were used in combination at different concentrations to observe their in vitro effects, so as to provide a basis for effectively treating MRSA infection caused by biofilm formation and reducing the premature drug resistance caused by daptomycin alone, and help reduce the family, medical care and social burden of patients.
Methods
Experimental Materials
Strain source: MRSA standard strain ATCC33591 was provided by Shanghai North Connaught Biotechnology Co., Ltd.
Biofilm carrier: 96 pore plate (U-shaped bottom plate) made of polyvinyl chloride (PVC), provided by Dalian Chenyu Biotechnology Co., Ltd.
Experimental instruments: Air-bath thermostatic shaker; electronic balance; electric thermostatic incubator; 6, 24, and 96-well cell culture plates; ultra-low temperature freezer; Thermo Scientific™ Multiskan™ FC Microplate Photometer (USA), confocal laser scanning microscopy (CLSM) (TCS-SP2).
Reagents and the preparation methods: Daptomycin was purchased from TargetMol, and azithromycin was purchased from MACKLIN. Daptomycin and azithromycin were prepared into 1 g/L mother liquor with sterile double-distilled water. Fluorescein isothiocyanate-labeled concanavalin A (FITC-ConA), propidium iodide (PI), and tryptic soy broth (TSB) medium were all provided by Dalian Chenyu Biotechnology Co., Ltd; 0.1% crystal violet and glutaraldehyde fixative were provided by Dalian Chenyu Biotechnology Co., Ltd
Experimental Methods
Biofilm Formation
The formation of MRSA ATCC33591 biofilm was observed using crystalline violet staining method and by referring to the study of Shi et al.18,19 Take MRSA single colonies from the plate and inoculate them into a test tube containing 10mL of TSB culture medium. Place the test tube on a 37 °C constant temperature shaker with a rotational speed of 120r/min for about 8 hours; dilute the concentration of the bacterial suspension to 0.5 micronaire turbidity standard. Dilute the bacterial suspension and TSB culture solution in a 1:100 ratio in a flask, place them on a 37 °C constant temperature shaking bed for constant temperature shaking cultivation, and take out 200μ L bacterial suspension samples every 2 hours, three wells were taken at a time, and the OD value was measured at 595nm on the enzyme-linked immunosorbent assay (ELISA) to obtain the MRSA growth curve. Construction of an MRSA biofilm model and determination of its formation curve: Before constructing an in vitro biofilm model, bacterial suspensions were diluted and inoculated on a PVC 96 well plate at 6h, 12h, 16h, and 24h under constant temperature oscillation. After standing in a 37 °C incubator for cultivation, their biofilm formation status was observed. It was found that the biofilm formation status was optimal when inoculating the bacterial suspension at 16h. Therefore, the bacterial suspension at this stage was selected for biofilm cultivation. Take a single MRSA colony and place it in a test tube containing 10mL of TSB culture medium. Place it in a constant temperature shaking table at 37 °C and shake for about 16 hours. Dilute the bacterial suspension to 0.5 McFarland standard with TSB culture medium Dilute the bacterial suspension to TSB culture medium in a ratio of 1:100, and pipette 200 with a Micropipette Add the bacterial suspension to a 96 well PVC plate, set anti-drying holes around the plate and seal them with a sealing film. Place the plate in a 37 °C incubator for constant temperature cultivation. Take out the plate at 6h, 12h, 24h, 48h, 72h, and 7d, and observe the formation of the biofilm. Observation of biofilm using crystal violet staining method: Discard the bacterial solution, use a pipette to absorb sterile physiological saline and lightly wash the pore plate three times to remove floating bacteria on the pore plate while avoiding mechanical damage to the biofilm. Let it stand at room temperature for 5–10 minutes and then stain with 0.1% crystal violet for 15 minutes. Discard the dye solution and then lightly wash it with physiological saline. After naturally drying the pore plate, observe the biofilm status; the quantitative results of biomembrane in different time periods were determined: the blank alcohol group was used as the negative control, 95% ethanol was used to dissolve the crystal violet dye solution, and the microplate reader was used to select 595nm to measure its OD value.
Micro Broth Dilution Method Was Used to Measure the Minimum Inhibitory Concentrations (MICs) of Daptomycin and Azithromycin
The antimicrobial impact of the combination of daptomycin and azithromycin was detected using the microdilution checkerboard technique and drug sensitivity test. Calculation of fractional inhibitory concentration index (FICI) and the evaluating criteria: FICI = MIC of drug A in combination/MIC of drug A alone + MIC of drug B in combination/MIC of drug B alone. Evaluation criteria: Synergistic effect is considered when FICI ≤ 0.5; additive effect is considered when 0.5 < FICI ≤ 1; irrelevant effect is considered when 1 < FICI ≤ 2; antagonistic effect is considered when FICI > 2.
In vitro Intervention of Antimicrobial Drugs on Semi Mature MRSA Biofilm (24 h) and Mature Biofilm (48 h)
Daptomycin and azithromycin were diluted in multiple ratios so that the concentration for standby was between 2 MIC and 1/4 MIC. The prepared bacterial suspension was inoculated with 100 μL in 96-well plate made of PVC, and various concentrations of antimicrobial agents were added to the corresponding wells at 24 h (vs 48 h), and equal volumes of TSB culture solution were added to the control group. The biofilm formation was observed using crystalline violet staining at 6 h, 12 h, 24 h, 48 h, 72 h, and 7 d, and finally, the quantitative results of biofilm were acquired by measuring the absorbance values with an enzyme marker.
Observation of the Biofilm After Antimicrobial Agent Intervention with CLSM
The coexistence of biofilm and planktonic bacteria was visualized. The bacteria were inoculated on a PVC sheet or sterile coverslip, placed in constant temperature incubation in a thermostat incubator, and after 24 h (without discarding the bacterial solution), intervention with separate or combined antimicrobial agents were performed for 24 h, respectively; the changes in MRSA biofilm morphology and the numbers of bacteria within the biofilm were observed with CLSM.
Statistical Methods
SPSS 19.0 statistical software was used to analyze the data. The measurement data are expressed as mean ± standard deviation ( ), and t-test was performed to compare the data between the two groups; P < 0.05 was considered statistically significant difference.
), and t-test was performed to compare the data between the two groups; P < 0.05 was considered statistically significant difference.
Results
The bacteria inoculated with TSB medium, grew slowly from 0 to 4 h, grew quickly after 4–8 h, then grew steadily from 16 to 18 h (Figure 1). Biofilm was generated by inoculated bacteria on the PVC 96-well plates at 6 h, semi-matured at 24 h, and reached maturation and stable existence at 48 h (Figure 2). The MRSA biofilm model may be constructed successfully using PVC material as the adhesion carrier. As shown in Figure 3, there was no colony growth on solid medium at daptomycin intervention concentrations of 8 mg/L (3a) and no colony growth on solid medium at azithromycin intervention concentration of 512 mg/L (3d); colonies started to appear when the concentration was reduced to 256 mg/L. The MICs of daptomycin and azithromycin measured by micro-broth dilution method were 512 mg/L for daptomycin and 8 mg/L for azithromycin >256 mg/L.
 |
Figure 1 The growth curve of ATCC 33591. |
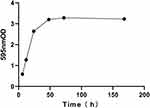 |
Figure 2 The biofilm formation curve of ATCC 33591. |
As shown in Figure 4, in case of daptomycin combined with azithromycin, the results of the combination using the tessellation dilution method and inhibition test revealed that compared with monotherapy, the MIC of daptomycin decreased to 1/8 MIC and the MIC of azithromycin decreased to 1/2 MIC when they were combined. The calculation formula gave the FICI of 0.625, indicating that the combination had an additive impact on MRSA2, thereby effectively reducing the clinical drug dosage and side effects. As shown in Figures 5–7, in addition to inhibiting the growth of planktonic bacteria, daptomycin and azithromycin at their MICs also inhibited and destroyed the biofilm. For mature biofilm, daptomycin at 2 MIC (16 mg/L) could almost prevent biofilm formation (95.3%), but azithromycin at MIC (512 mg/L) could inhibit nearly 80% of MRSA biofilm formation; considering azithromycin has a high value of MIC, azithromycin at 2 MIC was not set up for the next experiment. When the concentration of azithromycin was 1/2 MIC (256 mg/L), 43.2% of biofilms were eliminated by it.
Staining observation after 24 h of the combined antimicrobial agents on mature biofilm demonstrated that MRSA biofilm could be totally eliminated by the combination of daptomycin at concentration of 1 mg/L combined with azithromycin at 256 mg/L, demonstrating the additive effect of daptomycin and azithromycin when combined. As shown in Table 1, during the early phase of biofilm formation (24 h), there was a significant inhibitory effect on biofilm formation when daptomycin (16 to 8 mg/L) was combined with azithromycin (512 to 64 mg/L), respectively, and the OD (595 nm) values were significantly lower than in the control group (P < 0.001). Additionally, biofilm density increased gradually as daptomycin concentration decreased (P < 0.001); there was no significant change observed in biofilm density with decreasing azithromycin concentration (P = 0.5), indicating that both antibiotics had anti-biofilm effects, but daptomycin played a more significant role.
 |
Table 1 Semi-Quantitative Results of the Intervention of Daptomycin and Azithromycin on the MRSA Biofilm for 24 h ( |
As shown in Figure 8, microscopically, a large green fluorescence was observed in the control group, which consisted of living bacteria within the biofilm; following daptomycin (8 mg/L) or azithromycin (512 mg/L) MIC intervention, yellow-green fluorescence was detected microscopically, indicating that both of them have an inhibitory effect on MRSA with biofilm and can kill some bacteria within the biofilm; bright-yellow fluorescence was observed microscopically for the MIC combination of daptomycin (8 mg/L) and azithromycin (512 mg/L) intervention, implying that the combination further inhibited MRSA in biofilm and the number of colonies was significantly reduced; increasing daptomycin to 2 MIC (16 mg/L), the yellow fluorescence was more apparent microscopically, which demonstrated that the number of dead bacteria increased; when the combination was observed with CLSM, high bright yellow fluorescence was observed, indicating that the MRSA within the biofilm was further inhibited.
Discussion
MRSA can lead to high mortality and has high drug resistance, reducing the clinical prognosis of infected patients, particularly those with serious illnesses. It poses a formidable challenge for clinical treatment and prevention, making it a widespread global clinical problem. The constant search for novel antimicrobial drugs to strengthen the treatment of MRSA infections is imperative. It is essential to determine the MRSA growth curve and to understand its growth pattern.
Currently, glycopeptide antibacterial drugs such as vancomycin are widely used; however, the MIC of vancomycin against MRSA is rising annually,20 and vancomycin-mediated and vancomycin-resistant strains are developing clinically, leading to an increase in the rate of vancomycin failure for MRSA infection.21 In addition, studies have revealed that vancomycin is ineffective against MRSA that has formed biofilms.22 Daptomycin is a lipopeptide antibiotic used to treat Gram-positive bacterial infections. It was approved by the US FDA in 2003 to treat complex skin infections and structural skin infections caused by Gram-positive bacteria, and in 2006 to treat bacteremia caused by Staphylococcus aureus and right endocarditis.23. The minimum inhibitory concentration (MIC) of pathogenic bacteria against daptomycin has not changed significantly since its approval for over 10 years,24 and the drug resistance rate is very low, demonstrating the unique advantages of daptomycin. At present, the use of daptomycin in clinical practice is safe and has a low incidence of adverse reactions. Common adverse reactions include diarrhea, vaginitis, nausea, headache, dizziness, indigestion, rash, etc. A very small number of patients may experience symptoms of muscle pain or weakness, accompanied by an increase in phosphocreatine kinase.25 However, the mechanism of action on bacteria is not yet fully understood. Schriever et al26 believed that daptomycin can depolarize the cell membrane and cause bacterial cell death: first, daptomycin binds to Ca2+, and then its lipophilic tail is inserted into the bacterial plasma membrane; The second step is the oligomerization of daptomycin, which causes its lipophilic tail to act as an “ion channel” on the bacterial cell membrane, damaging the cell membrane and causing a large amount of intracellular ions to flow out; Step 3: The loss of K+ causes rapid depolarization of cells, disrupting ion gradients. Lack of appropriate ion gradients inhibits the synthesis of adenosine triphosphate in bacterial cells, resulting in the loss of the ability to synthesize DNA, RNA, and macromolecular proteins, leading to cell death. In addition, research by Boaretti et al27 shows that the target of daptomycin is not penicillin binding protein, but it binds to the bacterial cell membrane. By disrupting the transport of amino acids by the cell membrane, it blocks the biosynthesis of phosphorus wall (acid) lipids of bacterial cell wall peptidoglycan, interferes with cell replication, and leads to rapid bacterial death. This time, we have chosen Daptomycin, which has more advantages, but we have not conducted in-depth research on the specific antibacterial mechanism of Daptomycin.
The goal of this study was to determine the anti-MRSA effect of daptomycin in combination with azithromycin, as well as its inhibitory effect on MRSA biofilm formation and its damaging effect on the biofilm. The MICs of the two antibacterial drugs were determined as per the requirements of the Clinical & Laboratory Standards Institute (CLSI),28 and the drug sensitivity tests for the combination were then performed. The antibacterial activity of daptomycin combined with azithromycin was evaluated using the chessboard microdilution method. The results demonstrated a good additive effect of both.
The drug-resistance mechanism of MRSA is mainly comprised of resistance mediated by β-lactamases,29 by mec A gene,30 and by active efflux system,31 in addition to its ability to form biofilms.32 There are five phases to the production of biofilm: colonization, adhesion, aggregation, differentiation and maturation, and dispersal. MRSA can secrete intercellular polysaccharide adhesin, which allows it to adhere to foreign bodies and biological materials. It is one of the essential components for biofilm aggregation, increases the maturation and architecture of biofilms, and reduces the antimicrobial activity of antibacterial drugs. As the number of aggregated bacteria increases gradually a mature and stable biofilm is formed, which has the function of transporting nutrients, metabolites, and enzymes to the membrane; Eventually, a portion of the bacteria in the mature biofilm detach and disperse, thus initiating the formation of the next biofilm.33 In comparison to the exponential growth of planktonic bacteria, bacteria within mature biofilms in nutrient-deficient conditions survive via anaerobic or microaerobic growth. Compared to free bacteria, biofilm formation significantly increases the resistance of the strain of bacteria.34 Biofilm allows the bacteria to escape from the host immune system and phagocytosis, resulting in recurrent and prolonged infections, as well as helps bacteria against antibacterial drugs.35 Currently, the dominant theories on the resistance mechanism of biofilm to antimicrobial drugs are as follows: the biofilm restricts the penetration of antimicrobial drugs,36 nutrient limitation reduces bacterial metabolic rate,37 drug-resistant bacteria are present in biofilms,38 and there is enhanced expression of efflux pumps.39 Therefore, it is important to have a deeper understanding of the growth mechanism of biofilms so that the formation of bacterial biofilms can be inhibited at an early stage and the formed biofilms can be destroyed, thus reducing the antimicrobial agent dosage and adverse drug reactions. The construction of a successful biofilm model is the precondition for understanding biofilm properties.
In this study, we adopted widely used methods to observe the growth performance of the MRSA biofilm at different time points and to describe its growth pattern. We found that the biofilm continued to develop and exist stably on PVC plates. Despite the obvious nutrient-depleted state of the bacteria in the late biofilm phase, it is imperative to regularly and strictly disinfect medical equipment in the ICU,40 as well as require physicians to more strictly administer drugs in a timely manner. Subsequently, the anti-biofilm effect of daptomycin and azithromycin (MIC concentration) was then examined at six time intervals. The results demonstrated that both antibacterial drugs at MIC inhibited the growth of planktonic bacteria and had good anti-biofilm activity on either mature or semi-mature biofilms, and daptomycin demonstrated superior anti-biofilm activity, which is consistent with the findings of Sutrave et al15,41
Crystalline violet staining, semi-quantitative assay, and CLSM microscopy were used to investigate the anti-MRSA biofilm effect of daptomycin in combination with azithromycin. According to preliminary observations, the growth of MRSA biofilm was completely inhibited when 8 μg/mL of daptomycin was combined with 256 μg/mL of azithromycin and when 512 μg/mL of azithromycin was combined with 1 μg/mL of daptomycin. This demonstrated that the combination of daptomycin and azithromycin significantly decreased the concentration of antibacterial drugs required to inhibit biofilm formation, which is consistent with the results of previous studies related to the combination of drugs.
Subsequently, in order to simulate the state of coexistence of bacteria and biofilm in the clinic, the planktonic bacteria and biofilm were designed to coexist, and the drug intervention was then implemented: MRSA suspension in the late logarithmic growth phase was diluted with TSB and inoculated on a 96-well plate made of PVC, drainage holes were set up, which were then sealed, and the plates were incubated in a thermostat incubator for 24 h. Antibacterial agents were added directly without discarding the bacterial solution (when planktonic bacteria and biofilm coexisted). After 24 h (the aim was to simulate clinical infection after 24 h), the state of the biofilm was observed by removing the plate. The results showed that the combination had a good antibacterial and anti-biofilm effect (P < 0.001), and daptomycin played the dominant role. The change in the proportion of live/dead bacteria within the biofilm at this time was visually observed under CLSM microscopy, which again demonstrated that the combination of daptomycin and azithromycin can significantly inhibit the maturation of biofilm and can destroy already formed biofilm thereby killing bacteria within the biofilm. In the extended experiment, the drug was promptly administered within 3 h after bacterial inoculation. When the bacterial suspension in the wells was spread on the plate and cultured, no growing colonies were found, indicating that the administration at this time completely inhibited the growth of planktonic bacteria and the formation of biofilm. This suggests that the earlier the antibiotics are used, the better the control over the infection. This also demonstrates that it may be easier to achieve effective antibacterial effect by applying antibacterial agents prior to biofilm formation.
The results of this study are meant for reference for the in vitro use of daptomycin/azithromycin; however, for in vivo efficacy, relevant animal models need to be designed for validation. In addition, crystalline violet staining method was used to observe the biofilm, but this method is unable to clarify the structure, thickness, and surface area of the biofilm and the various components, and further investigation with electron microscopy is needed. Finally, the drug resistance mechanism of biofilm is complex and variable, and further studies related to the genetic-molecular mechanism are needed.
Conclusion
In conclusion, both daptomycin and azithromycin have anti-MRSA biofilm activity, among which daptomycin is more dominant, and the combination of both antimicrobial agents can significantly inhibit the further maturation of immature MRSA biofilms, while destroying already formed biofilm, which demonstrates the superiority of the combination of antimicrobial agents over monotherapy.
Human beings have battled against bacteria for thousands of years, and the current prevalence of MDR strains and the increase in bacterial resistance due to the biofilm make the situation much more dire, accelerating the search for new antibacterial drugs. The results of this study confirmed the anti-biofilm activity of daptomycin and azithromycin, the combination of the two had a significant in vitro antibacterial effect on MRSA with biofilm formation, and may provide a reference on how to clinically decrease drug dosage and adverse effects in the treatment of bacterial infection.
Funding
The Natural Science Foundation of Fujian Province (2016J01539).
Disclosure
The authors report no conflicts of interest in this work.
References
1. Haque M, Sartelli M, McKimm J, et al. Health care-associated infections – an overview. Infect Drug Resist. 2018;11:2321–2333. doi:10.2147/IDR.S177247
2. Gómez-De Rueda F, Martinez-Nogueras R, Tena-Sempere ME, et al. Epidemiological aspects and prevalence study of nosocomial infections in a general hospital of specialties: retrospective analysis 2012–2017. Eur J Hosp Pharm. 2019;26(6):339–342. doi:10.1136/ejhpharm-2018-001577
3. Sehulster L, Chinn RY; Centers for Disease Control and Prevention. Guidelines for environmental infection control in health-care facilities. Recommendations of CDC and the Healthcare Infection Control Practices Advisory Committee (HICPAC). MMWR Recomm Rep. 2003;52(RR–10):1–42.
4. Hu FP, Guo Y, Zhu DS, et al. CHINET surveillance of antimicrobial resistance among the bacterial isolates in 2021. Chin J Infect Chemother. 2022;22(05):521–530.
5. Bloemendaal AL, Fluit AC, Jansen WM, et al. Acquisition and cross-transmission of Staphylococcus aureus in European intensive care units. Infect Control Hosp Epidemiol. 2009;30(2):117–124. doi:10.1086/593126
6. Chen W, Chen XE, Jin HL, et al. Investigation of bacterial contamination on object surface in intensive care unit. Chin J Hosp Infect. 2016;26(24):5740–5741+5750.
7. Fu XL, Li LL, Hu XJ, Wang CQ, CAo Y, Zhang YX. Bacterial contamination of high-touch surfaces in neonatal intensive care unit. J Nurs Sci. 2016;31(19):8–10.
8. Kaur DC, Chate SS. Study of antibiotic resistance pattern in methicillin resistant Staphylococcus aureus with special reference to newer antibiotic. J Glob Infect Dis. 2015;7(2):78–84. doi:10.4103/0974-777X.157245
9. Sen CK, Gordillo GM, Roy S, et al. Human skin wounds: a major and snowballing threat to public health and the economy. Wound Repair Regen. 2009;17(6):763–771. doi:10.1111/j.1524-475X.2009.00543.x
10. Malone M, Swanson T. Biofilm-based wound care: the importance of debridement in biofilm treatment strategies. Br J Community Nurs. 2017;22(Sup6):S20–S25. doi:10.12968/bjcn.2017.22.Sup6.S20
11. Barshak MB, Durand ML. The role of infection and antibiotics in chronic rhinosinusitis. Laryngoscope Investig Otolaryngol. 2017;2(1):36–42. doi:10.1002/lio2.61
12. Pant N, Eisen DP. Non-antimicrobial adjuvant strategies to tackle biofilm-related Staphylococcus aureus prosthetic joint infections. Antibiotics. 2021;10(9):1060. doi:10.3390/antibiotics10091060
13. Sousa A, Carvalho A, Pereira C, et al. Economic impact of prosthetic joint infection - an evaluation within the Portuguese National Health System. J Bone Jt Infect. 2018;3(4):197–202. doi:10.7150/jbji.28508
14. Davis CA, Janssen EM. Environmental fate processes of antimicrobial peptides daptomycin, bacitracins, and polymyxins[J]. Environ Int. 2020;134(6):105271. doi:10.1016/j.envint.2019.105271
15. Sutrave S, Kikhney J, Schmidt J. Effect of daptomycin and vancomycin on staphylococcus epidermidis biofilms: an in vitro assessment using fluorescence in situ hybridization[J]. PLoS One. 2019;14(8):e0221786. doi:10.1371/journal.pone.0221786
16. Attaran B, Falsafi T, Ghorbanmehr N. Effect of biofilm formation by clinical isolates of Helicobacter pylori on the efflux-mediated resistance to commonly used antibiotics. World J Gastroenterol. 2017;23(7):1163–1170. doi:10.3748/wjg.v23.i7.1163
17. Imperi F, Leoni L, Visca P. Antivirulence activity of azithromycin in Pseudomonas aeruginosa. Front Microbiol. 2014;5:178. doi:10.3389/fmicb.2014.00178
18. Shi HY, Liu QD, Wan XY. Effect of quorum sensing system on the infection of MRSA and the intervention of erythromycin on it. Chin Med J. 2016;96(37):3014–3019. doi:10.3760/cma.j.issn.0376-2491.2016.37.013
19. Shi HY. Study on the Effect of Density-Sensing Signaling System on MRSA Infection and Erythromycin Intervention. Liaoning: Dalian Medical University; 2016:6.
20. Zhu X, Liu C, Gao S, et al. Vancomycin intermediate-resistant Staphylococcus aureus (VISA) isolated from a patient who never received vancomycin treatment. Int J Infect Dis. 2015;33:185–190. doi:10.1016/j.ijid.2014.12.038
21. Elstrøm P, Astrup E, Hegstad K, et al. The fight to keep resistance at bay, epidemiology of carbapenemase producing organisms (CPOs), vancomycin resistant enterococci (VRE) and methicillin resistant Staphylococcus aureus (MRSA) in Norway, 2006–2017. PLoS One. 2019;14(2):e0211741. doi:10.1371/journal.pone.0211741
22. Alonso B, Cruces R, Perez A, et al. Activity of maltodextrin and vancomycin against staphylococcus aureus biofilm. Front Biosci. 2018;10(2):300–308. doi:10.2741/s517
23. Lee SY, Fan HW, Kuti JL, et al. Update on daptomycin: the first approved lipopeptide antibiotic[J]. Expert Opin Pharmacother. 2006;7(10):1381–1397. doi:10.1517/14656566.7.10.1381
24. Humphries RM, Pollett S, Sakoulas G. A current perspective on daptomycin for the clinical microbiologist[J]. Clin Microbiol Rev. 2013;26(4):759–780. doi:10.1128/CMR.00030-13
25. Kim PW, Sorbello AF, Wassel RT, et al. Eosinophilic pneumonia in patients treated with daptomycin: review of the literature and US FDA adverse event reporting system reports[J]. Drug Saf. 2012;35(6):447–457. doi:10.2165/11597460-000000000-00000
26. Schriever CA, Fernández C, Rodvold KA, et al. Daptomycin: a novel cyclic lipopeptide antimicrobial. Am J Health Syst Pharm. 2005;62(11):1145–1158. doi:10.1093/ajhp/62.11.1145
27. Boaretti M, Canepari P. Purification of daptomycin binding proteins (DBPs) from the membrane of Enterococcus hirae[J]. New Microbiol. 2000;23(3):305–317.
28. Clinical and Laboratory Standards Institute. Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria.That Grow Aerobically.
29. Hashizume H, Takahashi Y, Masuda T, et al. In vivo efficacy of β-lactam/tripropeptin C in a mouse septicemia model and the mechanism of reverse β-lactam resistance in methicillin-resistant Staphylococcus aureus mediated by tripropeptin C. J Antibiot. 2017. doi:10.1038/ja.2017.88
30. Aguayo-Reyes A, Quezada-Aguiluz M, Mella S, et al. Bases moleculares de la resistencia a meticilina en Staphylococcus aureus [Molecular basis of methicillin-resistance in Staphylococcus aureus]. Rev Chilena Infectol. 2018;35(1):7–14. Spanish. doi:10.4067/s0716-10182018000100007
31. Mo XN, Li JG, Tang YC, et al. The action of active efflux system on multi-drug resistance in methicillin resistant Staphylococcus aureus. Chin J Tuberc Respir. 2007;30(01):40–43.
32. Johnson NB, Hayes LD, Brown K, et al. CDC National Health Report: leading causes of morbidity and mortality and associated behavioral risk and protective factors--United States, 2005–2013. MMWR Suppl. 2014;63(4):3–27.
33. Stephens C. Microbiology: breaking down biofilms. Curr Biol. 2002;12(4):R132–R134. doi:10.1016/s0960-9822(02)00706-6
34. Sabirova JS, Hernalsteens JP, De Backer S, et al. Fatty acid kinase A is an important determinant of biofilm formation in Staphylococcus aureus USA300. BMC Genomics. 2015;16:861. doi:10.1186/s12864-015-1956-8
35. Nadell CD, Drescher K, Foster KR. Spatial structure, cooperation and competition in biofilms. Nat Rev Microbiol. 2016;14(9):589–600. doi:10.1038/nrmicro.2016.84
36. Pechère JC. Comment les bactéries résistent aux antibiotiques: une première forme d’intelligence collective ? [How bacteria resist antibiotics: a primary form of collective intelligence?]. Bull Acad Natl Med. 2004;188(8):1249–1256. French.
37. Lewis K. Persister cells. Annu Rev Microbiol. 2010;64(1):357–372. doi:10.1146/annurev.micro.112408.134306
38. Al-Dhaheri RS, Douglas LJ. Absence of amphotericin B-tolerant persister cells in biofilms of some Candida species. Antimicrob Agents Chemother. 2008;52(5):1884–1887. doi:10.1128/AAC.01473-07
39. Li X, Wang M. PP-001 Study on relationship between active efflux system in methicillin-resistant Staphylococci aureus and multi-antibiotic resistance. Int J Infect Dis. 2009;13(S1):S51. doi:10.1016/S1201-9712(09)60395-7
40. Bahamondez-Canas TF, Heersema LA, Smyth H. Current status of in vitro models and assays for susceptibility testing for wound biofilm infections. Biomedicines. 2019;7(2):34. doi:10.3390/biomedicines7020034
41. El-Khawaga AM, Elsayed MA, Fahim YA, et al. Promising photocatalytic and antimicrobial activity of novel capsaicin coated cobalt ferrite nanocatalyst. Sci Rep. 2023;13(1):5353. doi:10.1038/s41598-023-32323-y
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.







