Back to Journals » Cancer Management and Research » Volume 13
Neutrophil–Lymphocyte Ratio as a Predictive Factor of Growing Malignant Pulmonary Ground-glass Opacity
Authors Xue W, Zhang X, Niu Z, Xin Z, Zhang H, Zhao Q , He J, Hu Z, Duan G
Received 14 May 2021
Accepted for publication 22 June 2021
Published 13 July 2021 Volume 2021:13 Pages 5651—5655
DOI https://doi.org/10.2147/CMAR.S319190
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Bilikere Dwarakanath
Wenfei Xue1 1, Xiaopeng Zhang2 2, Zhancong Niu3 3, Zhifei Xin2 2, Hua Zhang2 2, Qingtao Zhao1 12 2, Jie He2 2, Zhonghui Hu2 2, Guochen Duan1 1
1Study Office of Thoracic Surgery, Hebei Medical University, Shijiazhuang, 050017, People’s Republic of China; 2Department of Thoracic Surgery, Hebei General Hospital, Shijiazhuang, 050000, People’s Republic of China; 3Department of Infectious Diseases, Hebei General Hospital, Shijiazhuang, 050000, People’s Republic of China
Correspondence: Guochen Duan
Study Office of Thoracic Surgery, Hebei Medical University, No. 361, Zhongshan East Road, Changan District, Shijiazhuang, 050017, People’s Republic of China
Tel +86 13513380703
Email [email protected]
Background: The object of the study was to elucidate the relationship between the neutrophil–lymphocyte ratio (NLR) and the growth of pulmonary ground-glass opacity (GGO) in stage IA lung adenocarcinoma.
Methods: All patients with GGO following surgical procedures were enrolled, with the time of follow-up and the variation tendency of GGO recorded. Meanwhile, laboratory parameters, age, gender, smoking history, histology, tumor size, and stage were recorded. Logistic regression was used to evaluate the value of NLR and the cutoff value was calculated by SPSS 22.0.
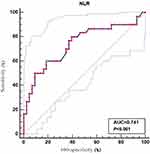
Results: In the whole cohort, 30 cases of growing GGO and 43 cases of stable GGO undergoing surgical procedures were diagnosed as lung adenocarcinoma. There was significant statistical difference between the two groups. Multivariable analysis showed that NLR could predict the GGOs with growth (odds ratio 5.198, 95% confidence interval (95%CI: 1.583– 14.581, P=0.002). Receiver operating characteristics analysis for NLR showed the optimal cutoff value of 2.38, with a sensitivity of 60.0% and specificity of 81.4%.
Conclusion: Our study demonstrated that the NLR appeared to have value as a promising clinical predictor of GGOs with growth. Further studies are needed to confirm this conclusion.
Keywords: neutrophil–lymphocyte ratio, ground-glass opacity, early lung adenocarcinoma
Introduction
With low-dose computed tomography widely used in lung cancer screening, pulmonary ground-glass opacity (GGO) was increasingly detected. According to the NCCN guideline,1 patients with GGOs are recommended to receive different follow-up strategy or surgical procedures. Many studies have suggested that lung cancer with a ground-glass component may have a better prognosis.2–4 Preinvasive lesions shown as GGO in the CT, including atypical adenomatous hyperplasia (AAH) or adenocarcinoma in situ (AIS), may be stable or slowly growing. AAH, AIS, and lepidic predominant lung adenocarcinomas grow along preexisting alveolar structures,5 which maintains the air space. During the follow-up duration, some small GGOs can gradually grow into solid nodules, while some large GGOs can remain stable. The critical risk factor was the growth of GGO, which determined how to choose the next follow-up strategy or surgical treatment.
Much evidence has shown that inflammation plays an important role in cancer development and progression.6 As biomarkers of systemic inflammation, neutrophil is related to tumor growth stimulation,7 while lymphocyte protected the organism from tumor cells through blocking their proliferation and migration.8 Therefore, several researchers demonstrated the role of inflammation in the prognostic of lung cancer.9,10 However, to the best of our knowledge, the relationship between NLR and malignant GGO with growth has not been researched in previous studies. If we find the risk factors associated with malignant growing GGOs, the clinical value will determine the procedure of follow-up or surgical method.
Materials and Methods
In this study, we retrospectively collected the data of patients with GGOs that were finally diagnosed as lung cancer at Hebei General Hospital in the CT follow-up from January 2015 to November 2020. The eligible criteria data included the following conditions: (1) the pathology of GGOs was confirmed as adenocarcinoma including AIS, minimal invasive adenocarcinoma (MIA), and invasive adenocarcinoma. (2) Patients with GGOs must be followed-up for at least three months according to the NCCN guideline, which were divided into the growing group and the stable group by two chief physicians. The definition of growing GGO was a increasing diameter by more than 10% according to the 2011 National Lung Screening Trial.11 (3) The types of GGO included pure GGO and subsolid GGO. (4) The CT scan must be taken in the same hospital during the follow-up and the data of NLR can be sought out at the time of the initial CT. The exclusion criteria included patients with inflammatory disease, missing preoperative complete blood cell count when finding the GGO, chemotherapy or radiotherapy before follow-up. The major GGO was selected in patients with multiple GGOs.
Clinical information of patients including sex, age, smoking history, lesion type, histology type, and tumor size were obtained from medical records. The TNM stage was assessed according to 2011 International Association for the Study of Lung Cancer, the American Thoracic Society, and the European Respiratory Society (IASLC/ATS/ERS).5 NLR was defined as the absolute neutrophil count divided by the absolute lymphocyte count.
Statistical Analyses
Statistical Package for Social Sciences version 22.0 software (SPSS Inc., IBM Corporation, Armonk, NY, USA) was used for the data analysis. The two-group difference was tested by the Student’s t-test for continuous variables and Pearson’s chi-squares test and Fisher’s Exact test for categorical variables. The relationship between various factors and growing GGO were assessed by univariable and multivariable analyses by the logistic regression test, while odds ratios (OR) with 95% confidence interval (95%CI) were also calculated. P<0.05 was considered with statistical significance. Receiver operating curve (ROC) analysis was plotted to investigate the optimal cutoff values that maximized sensitivity and specificity using the software MedCalc 19.0.
Results
The total number of patients with GGOs in the CT follow-up duration was 2584, but only 73 cases of these patients met the inclusion criteria. After several months of follow-up, patients with GGOs were divided into two groups: growing GGO group and stable GGO group. The longest follow-up period was more than 60 months and the shortest was three months. The distribution of surgical stage was 27 in stage 0, 24 in stage IA1, and 22 in stage IA2. The pathology of these cases included 51 preinvasive and 22 invasive adenocarcinomas. The overall clinical and pathological characteristics of all patients were shown in Table 1. There was no significant difference in the follow-up time between the two groups (P=0.208). In the pathological stage distribution between the two groups, there was no statistics difference (P=0.064) as shown in Table 1. As for pathological features, the preinvasive adenocarcinomas included AIS and MIA, and the number of invasive adenocarcinomas in the growing group was similar to the stable group (P=0.125).
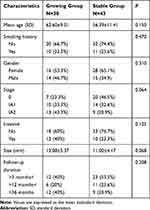 |
Table 1 Patient Characteristics and Pathological Findings in Two Groups |
As shown in Table 2, the mean counts of white cells (P=0.031), neutrophil (P=0.002), and NLR (P<0.001) in the growing GGO group were different from the stable GGO group. However, there was no significant difference in the mean values of lymphocytes, platelets, LMR, CEA, MONO, and PLR (P>0.05). Through the analysis of logistic regression between the two groups, we found that age (P=0.020) and NLR (P<0.001) were significantly associated with the growing GGOs in univariable analysis (Table 3), while multivariable analysis demonstrated that NLR was an independent risk factor for patients with growing GGOs (P=0.002). Through the receiver-operating curves, the cutoff value for NLR predicting the growth of GGO was shown in Figure 1. An optimal cutoff value was used to identify the maximized sum of sensitivity and specificity. Based on ROC, the best cutoff value for NLR was 2.38, which was 60.0% sensitive and 81.4% specific for the growing GGOs. NLR was independent predictor of growth in patients with GGO when using 2.38 as threshold value (OR: 5.198, 95%CI 1.583–14.581, P=0.002).
 |
Table 2 Laboratory Parameters |
 |
Table 3 Results of Univariable and Multivariable Analysis |
Discussion
In 1998, the first study published by Bellocq et al12 demonstrated the association between neutrophils and a poor outcome in the alveolar lumen of bronchioloalveolar carcinoma. Tumor-infiltrating lymphocytes (mostly CD8+) are correlated with better OS in 1290 patients with NSCLC.13 During these years, more and more studies have shown that the neutrophil–lymphocyte ratio (NLR) could be a representative prognostic marker in various malignancies.14,15 The most rational reason of why NLR was associated with the prognostic in human cancer may be the imbalance of immune response.16
The tumor microenvironment consists of immune and inflammatory cells. Therefore, immune and inflammatory response was important in tumor progression and aggressiveness. The circulating cytokines together with the NLR, which was associated with an increase in interleukin (IL), were shown by many studies.17,18 The granulocyte colony-stimulating factor, IL-1, IL-6 and tumor necrosis factor-alpha (TNF-α) may influence tumor-related leukocytosis and neutrophilia.19 A suitable environment provided by neutrophilia promotes the development of cancer.15,20 Through the suppression of recruiting T cells and activating chemokines, cancer-related inflammation leads to the improvement of tumors.21
Recently, research demonstrated the activation of immune responses and immune escape through immune checkpoints and suppressive interleukins that occurred at preinvasive stages of cancer development.22 In 2015, the World Health Organization (WHO) defined two new subtypes of lung cancer: AIS and MIA,23 while early lung cancer including AIS and MIA mainly occurs in patients with GGOs.24 During the period of follow-up, the natural course for these patients with GGO was growth or persistence. And the critical strategy was determined by the growth of GGO according to the 2020 NCCN guideline.1 Therefore, we assessed the relationship between the growth of GGO and clinicopathological characteristics. We found that age (P=0.020) and NLR (P=0.001) were significantly associated with the growing GGOs in the univariable analysis. In multivariable analysis, it is demonstrated that NLR was an independent risk factor for patients with growing GGOs (P=0.002).
To the best of our knowledge, this is the first study evaluating the association between NLR and early lung cancer with growing GGOs. We retrospectively collected the data from patients who underwent surgical procedures, and recorded the changes of CT in follow-up duration. To identify the influence factors which result in the growth of GGO, we analyzed the clinical and pathological characteristics including age, sex, smoking history, histological type, T stage, invasion, tumor marker, CEA, and TNM stage.
To avoid the influence of follow-up period, we made the analyses between the two groups, no significant statistical difference (P=0.208). Many studies concluded that PLR was an independent predictor of lung cancer prognosis, while we found the NLR was a high risk predictor in growing GGO. As various studies have assessed the pathological range of different GGOs,24 we found that growing large GGOs may still be preinvasive adenocarcinoma, while the stable GGOs had turned to invasive lung cancer. From preinvasive to invasive, there were many genetic changes in patients with lung cancer.22 This investigation concluded that NLR was significantly associated with the growth of GGO (OR: 5.198, 95%CI: 1.583–14.581, P=0.002). Adversely, this study may provide evidence of the microenvironment changes in early stage lung adenocarcinoma.
Just like all retrospective studies, this research has many limitations. The data of follow-up duration missed CT imaging and blood counts at the initial CT examination that made it difficult to collect more samples. In addition, selection bias also exists in our study. Further larger prospective studies will be needed to confirm the conclusion.
In conclusion, this research firstly provides the evidence that NLR was associated with GGOs growth. Therefore NLR has clinical value as an independently predictive factor upon growing GGO. We hope that it could serve as a biomarker in lung cancer screening, which will help the surgeon make more suitable strategies for patients with GGO.
Data Sharing Statement
The data used to support the findings of this study are available from the corresponding author upon request.
Acknowledgments
This work is not supported by any sponsors.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Network NCCN. Lung Cancer Screening Version1. 2020. NCCN org; 2020.
2. Matsuguma H, Yokoi K, Anraku M, et al. Proportion of ground-glass opacity on high-resolution computed tomography in clinical T1N0M0 adenocarcinoma of the lung: a predictor of lymph node metastasis. J Thorac Cardiovasc Surg. 2002;124:278–284. doi:10.1067/mtc.2002.122298
3. Seidelman JL, Myers JL, Quint LE. Incidental, subsolid pulmonary nodules at CT: etiology and management. Cancer Imaging. 2013;13:365–373. doi:10.1102/1470-7330.2013.9025
4. Hattori A, Matsunaga T, Takamochi K, et al. Importance of ground glass opacity component in clinical stage IA radiologic invasive lung cancer. Ann Thorac Surg. 2017;104:313–320. doi:10.1016/j.athoracsur.2017.01.076
5. Travis WD, Brambilla E, Noguchi M, et al. International association for the study of lung cancer/American thoracic society/european respiratory society international multidisciplinary classification of lung adenocarcinoma. J Thorac Oncol. 2011;6:244–285.
6. Mantovani A, Allavena P, Sica A, et al. Cancer-related inflammation. Nature. 2008;454(7203):436–444. doi:10.1038/nature07205
7. Souto JC, Vila L, Bru A. Polymorphonuclear neutrophils and cancer. Intense and sustained neutrophilia as a treatment against solidtumors. Med Res Rev. 2011;31:311–316.
8. Torre LA, Bray F, Siegel RL, et al. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65(2):87–108. doi:10.3322/caac.21262
9. Adamowicz K, Romanowska A, Zaucha RE. Prognostic value of the neutrophil-to-lymphocyte ratio (NLR) in advanced non-small cell lung cancer. Ann Oncol. 2018;29(8):viii538–viii539. doi:10.1093/annonc/mdy292.055
10. Amaral SR, Casal Moura M, Carvalho J, et al. Prognostic significance of neutrophil-to-lymphocyte ratio (NLR) and platelet-to-lymphocyte ratio (PLR) in non-small cell lung cancer (NSCLC) treated with immune checkpoint inhibitors. Ann Oncol. 2019;30(1):i3. doi:10.1093/annonc/mdz027.004
11. National Lung Screening Trial Research Team, Aberle DR, Berg CD, et al. The National Lung Screening Trial: overview and study design. Radiology. 2011;258(1):243–253.
12. Bellocq A, Antoine M, Flahault A, et al. Neutrophil alveolitis in bronchioloalveolar carcinoma: induction by tumor-derived interleukin-8 and relation to clinical outcome. Am J Pathol. 1998;152:83–92.
13. Ruffini E, Asioli S, Filosso PL, et al. Clinical significance of tumor-infiltrating lymphocytes in lung neoplasms. Ann Thorac Surg. 2009;87:365–371. doi:10.1016/j.athoracsur.2008.10.067
14. Sharaiha RZ, Halazun KJ, Mirza F, et al. Elevated preoperative neutrophil: lymphocyteratio as a predictor of postoperative disease recurrence in esophageal cancer. Ann Surg Oncol. 2011;18:3362–3369. doi:10.1245/s10434-011-1754-8
15. Mano Y, Shirabe K, Yamashita Y, et al. Preoperative neutrophil-to-lymphocyte ratio is a predictor of survival after hepatectomy for hepatocellular carcinoma: a retrospective analysis. Ann Surg. 2013;258:301–305. doi:10.1097/SLA.0b013e318297ad6b
16. Kusumanto YH, Dam WA, Hospers GA, et al. Platelets and granulocytes, in particular neutrophils, form important compartments for circulating vascular endothelial growth factor. Angiogenesis. 2003;6:283–287. doi:10.1023/B:AGEN.0000029415.62384.ba
17. Motomura T, Shirabe K, Mano Y, et al. Neutrophil-lymphocyte ratio reflects hepatocellular carcinoma recurrence after liver transplantation via inflammatory microenvironment. J Hepatol. 2013;58(1):58–64. doi:10.1016/j.jhep.2012.08.017
18. Kantola T, Klintrup K, Vayrynen JP, et al. Stage-dependent alterations of the serum cytokine pattern in colorectal carcinoma. Br J Cancer. 2012;107(10):1729–1736. doi:10.1038/bjc.2012.456
19. Kwon HC, Kim SH, Oh SY, et al. Clinical significance of preoperative neutrophil-lymphocyte versus platelet-lymphocyte ratio in patients with operable colorectal cancer. Biomarkers. 2012;17(3):216–222. doi:10.3109/1354750X.2012.656705
20. Fridman WH, Galon J, Dieu-Nosjean MC, et al. Immune infiltration in human cancer: prognostic significance and disease control. Curr Top Microbiol Immunol. 2011;344:1–24. doi:10.1007/82_2010_46
21. Bhatti I, Peacock O, Lloyd G, et al. Preoperative hematologic markers as independent predictors of prognosis in resected pancreatic ductal adenocarcinoma: neutrophil-lymphocyte versus platelet-lymphocyte ratio. Am J Surg. 2010;200(2):197–203. doi:10.1016/j.amjsurg.2009.08.041
22. Mascaux C, Angelova M, Vasaturo A, et al. Immune evasion before tumour invasion in earlylung squamous carcinogenesis. Nature. 2019;571(7766):570–575. doi:10.1038/s41586-019-1330-0
23. Travis WD, Brambilla E, Burke AP, et al. The 2015 World Health Organization classification of lung tumors: impact of genetic, clinical and radiologic advances since the 2004 classification. J Thorac Oncol. 2015;10(9):1243–1260. doi:10.1097/JTO.0000000000000630
24. Kim TJ, Goo JM, Lee KW, et al. Clinical, pathological and thin-section CT features of persistent multiple ground-glass opacity nodules: comparison with solitary ground-glass opacity nodule. Lung Cancer. 2009;64(2):171–178. doi:10.1016/j.lungcan.2008.08.002
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.