Back to Journals » OncoTargets and Therapy » Volume 12
Neutrophil-To-Lymphocyte Ratio Predicted Long-Term Chemotherapy Benefits In Stage IIIB-IV Non-Squamous Non-Small Cell Lung Cancer Patients Without Sensitive Mutations
Authors Li X , Zeng WH, Zhou YQ, Ji YY, Li WZ, Zhang LY, Guo YF, Feng DY , Zhang TT
Received 31 July 2019
Accepted for publication 23 September 2019
Published 23 October 2019 Volume 2019:12 Pages 8779—8787
DOI https://doi.org/10.2147/OTT.S225544
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Leo Jen-Liang Su
Xing Li,1,* Wei-Hua Zeng,2,* Yu-Qi Zhou,3,* Yan-Ying Ji,4 Wei-Zhan Li,2 Li-Yi Zhang,3 Yue-Fei Guo,5 Ding-Yun Feng,3 Tian-Tuo Zhang3
1Department of Oncology and Guangdong Key Laboratory of Liver Disease, The Third Affiliated Hospital of Sun Yat-sen University, Guangzhou 510630, People’s Republic of China; 2Department of Oncology, Panyu Central Hospital, Guangzhou 511400, People’s Republic of China; 3Department of Pulmonary and Critical Care Medicine, The Third Affiliated Hospital of Sun Yat-sen University, Institute of Respiratory Diseases of Sun Yat-sen University, Guangzhou 510630, People’s Republic of China; 4Department of Pathology, The Third Affiliated Hospital of Sun Yat-sen University, Guangzhou 510630, People’s Republic of China; 5Department of Radiology, The Third Affiliated Hospital of Sun Yat-sen University, Guangzhou 510630, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Tian-Tuo Zhang; Ding-Yun Feng
Department of Pulmonary and Critical Care Medicine, Third Affiliated Hospital of Sun Yat-sen University, Institute of Respiratory Diseases of Sun Yat-sen University, 600 Tianhe Road, Guangzhou 510630, People’s Republic of China
Tel +86-20-85252241
Email [email protected]; [email protected]
Purpose: To investigate the predictive capability of clinical parameters for long-term chemotherapy benefits among stage IIIB-IV non-squamous non-small cell lung cancer (NSCLC) patients without sensitive mutations.
Patients and methods: We investigated the clinical features of 206 stage IIIB-IV non-squamous NSCLC patients without sensitive mutations and assessed their predictive value for disease control rate (DCR) at 6 and 12 months post-treatment.
Results: Seventy-two patients received docetaxel and platinum-based chemotherapy while 134 received pemetrexed and platinum-based chemotherapy. The 6-month and 12-month DCR were 33 (45.8%) and 6 (8.3%) in the docetaxel group and 69 (51.5%) and 19 (14.2%) in the pemetrexed group, respectively. Univariate Cox regression revealed that age, sex, smoking history, adrenal gland metastasis, stage IV disease, neutrophil-to-lymphocyte ratio (NLR), and serum albumin were associated with unfavorable progression-free survival (PFS). Age, stage IV disease, and NLR were identified as independent predictors of PFS using multivariate analysis. NLR was the only parameter that could predict 3-month and 6-month DCRs. NLR and age were able to predict 12-month DCR, with NLR presenting a larger area under the curve. Kaplan–Meier curves demonstrated that patients with NLR > 2.231 displayed significantly reduced long-term disease control. The group with higher NLR had more male patients, lower ALB levels, and serum sodium levels as well as higher platelet counts.
Conclusion: NLR was an independent predictor of long-term chemotherapy benefits among non-squamous NSCLC patients without sensitive mutations. Patients with lower NLR were optimal candidates for chemotherapy. Patients with high NLR may receive alternative treatments or be included in clinical trials.
Keywords: non-small cell lung cancer, chemotherapy, disease control rate, neutrophil-to-lymphocyte ratio
Introduction
In this era of emerging targeted therapies, the treatment of non-small cell lung cancer (NSCLC) has become increasingly precise with biomarkers directly reflecting the efficacy of targeted agents.1 However, chemotherapy is still the fundamental treatment option for patients without sensitive mutation or when targeted agents fail.2,3 In recent years, the use of immunotherapy and chemo-immunotherapy combinations have increased in clinical practice for the treatment of advanced/metastatic NSCLC,4,5 re-launching the role of chemotherapy in NSCLC. Moreover, neoadjuvant and adjuvant chemotherapy are still major strategies for NSCLC amenable to surgery, especially for N2 patients.6 However, the progression-free survival (PFS) of chemotherapy was reported to be less than 6 months,7 which was inferior to that of tyrosine kinase inhibitors (TKIs) among patients with sensitive mutations.8 Thus, identification of long-term chemotherapy benefits is imperative to improve the efficacy of chemotherapy.
Evaluation of the efficacy of chemotherapy is mainly based on overall survival (OS), PFS and objective response rate (ORR).9 These measures are suitable for clinical trials but are not adequate for the identification of NSCLC patients with persistent response to chemotherapy since more than 50% of patients displayed disease progression during 6–8 cycles of chemotherapy. According to the World Health Organization (WHO) criteria for chemotherapy efficacy,10 the treatment response should be sustained for at least 6–8 weeks after initial assessment of treatment response. Thus, we speculated a PFS longer than 12 months is a satisfying benefit for NSCLC patients. Those expected to present disease progression within 6 months after chemotherapy may receive alternative treatments11 or be included in clinical trials.12 Thus, selection of patients whose disease control is longer than 12 months may be an optimal strategy in identifying chemotherapy benefits.
Neutrophil-to-lymphocyte ratio (NLR) was indicated as a significant prognostic predictor in various tumor types,13 including lung cancer.14–17 However, inflammatory indicators in differential blood cell counts, such as red blood cells, platelets and NLR, remain uninvestigated in predicting long-term chemotherapy benefits among NSCLC patients without sensitive mutations. The present study investigated the predictive capability of clinical parameters for long-term chemotherapy benefits among stage IIIB-IV non-squamous NSCLC patients without sensitive mutations.
Materials And Methods
Patients
We retrospectively enrolled 206 patients diagnosed with non-squamous NSCLC who regularly received docetaxel or pemetrexed combined with platinum chemotherapy at the Third Affiliated Hospital of Sun Yat-sen University or Panyu Central Hospital from January 2016 to December 2018. The inclusion criteria were as follows: non-squamous NSCLC was pathologically diagnosed; stage IIIb or IV according to the American Joint Committee on Cancer (AJCC) 8th edition; received docetaxel or pemetrexed combined with platinum drugs chemotherapy for more than two cycles without combination of target therapies or radiation therapy; no additional cancer history; no sensitive mutations; and laboratory test results were obtained before treatment. The exclusion criteria were as follows: patients with missing or incomplete PFS data; who underwent surgery, radiotherapy, or other anti-cancer treatments after chemotherapy; and who presented with acute infections, such as fever and pneumonia (Figure 1). Clinical data were obtained from the patients’ medical records. Written informed consent was obtained from each patient or his/her immediate family members. The study was approved by the ethics committee of the Third Affiliated Hospital of Sun Yat-sen University and Panyu Central Hospital.
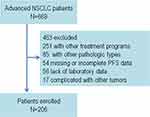 |
Figure 1 The study flowchart. |
Data Collection
The clinical data were collected as follows: age, sex, Eastern Cooperative Oncology Group Performance Status (ECOG PS) scores, blood routine parameters and biochemical profiles 1 week before chemotherapy, disease control rate (DCR), and PFS. Data of relevant laboratory indicators were collected before chemotherapy.
Statistical Analysis
The 12-month DCR was the main endpoint of this study, with 3-month and 6-month DCR the secondary endpoints. PFS was defined as the time interval between the first chemotherapy and death or the last follow-up. Multivariate analysis using a stepwise Cox proportional hazards model was used to test for the independent significance of baseline characteristics and explanatory variables. The performance of relevant parameters was assessed using the Kaplan–Meier method, and differences in survival between groups were compared using the log-rank test. In addition, the receiver operating characteristic (ROC) curves of relevant parameters for predicting 3-month, 6-month, and 12-month DCRs for NSCLC patients were plotted. Sensitivity of a score less than the cutoff point indicated DCR for a certain time, while specificity of that greater than the cutoff point indicated PFS beyond certain time, both of which could be evaluated for each possible cutoff point. The cutoff point representing the highest Youden index (specificity+specificity−1) was selected as the optimal threshold value. Hazard ratios (HRs) and 95% confidence intervals (CIs) were pooled to measure the effects of relevant parameters on prognosis. An HR greater than 1 indicated a worse prognosis in patients with a relevant parameter, while an HR less than 1 indicated a better prognosis. The criterion for statistical significance was set at an α of 0.05, and all p values were based on two-sided tests. Statistical analysis was performed using IBM SPSS Statistics, version 20 (IBM Corp, Armonk, NY, USA).
Results
Patient Characteristics
The study included 206 advanced NSCLC patients in total, of which 126 (61.2%) patients were male, 66 (32.0%) patients had a smoking history, and 180 (87.4%) patients had stage IV disease. Docetaxel and platinum-based chemotherapy were administered to 72 patients while 134 patients received pemetrexed and platinum-based chemotherapy. The baseline characteristics of the two groups were similar. The docetaxel group had more males, less brain metastasis, less stage IV disease, and higher mean corpuscular hemoglobin concentration (MCHC). First-line chemotherapy was administered to 59 (81.9%) patients in the docetaxel group and 114 (85.1%) patients in the pemetrexed group. After 2 cycles of chemotherapy PR and SD were achieved by 22 (30.6%) and 32 (44.4%) patients in the docetaxel group and 54 (40.3%) and 52 (38.8%) in the pemetrexed group, respectively. The 6-month and 12-month DCRs were 33 (45.8%) and 6 (8.3%) in the docetaxel group and 69 (51.5%) and 19 (14.2%) in the pemetrexed group, respectively (Table 1).
 |
Table 1 Clinical Characteristics Differences Between Docetaxel And Pemetrexed Groups |
Identification Of The Predictors For PFS
Univariate analysis by Cox regression revealed that age, sex, smoking history, adrenal gland metastasis, stage IV disease, NLR, platelet count, and serum albumin were associated with unfavorable PFS. Importantly, patients with higher NLR presented a significant unfavorable prognosis. The independence of the above parameters as predictors for PFS was determined using the enter model of multivariate Cox regression. As a result, NLR, age, platelet count, and stage IV disease were confirmed as independent predictors for PFS among stage IIIB-IV non-squamous NSCLC patients without sensitive mutations receiving chemotherapy. The other parameters were not significant. However, platelet count presented the lowest hazard ratio (HR) of 1.003, while disease stage presented the highest HR of 1.823 (Table 2).
 |
Table 2 Significant Univariate And Multivariate Cox Regression Analyses Of Factors Associated With 12-Month Progression-Free Survival |
Predictive Value Of NLR For Long-Term Benefit Of Chemotherapy
The ultimate aim of investigating prognostic factors is the identification of long-term chemotherapy benefits. Thus, ROC analysis was conducted to analyze the predictive value of these prognostic parameters for DCR, 3-month DCR, 6-month DCR, and 12-month DCR. All three prognostic factors were not able to predict ORR after 2 cycles of chemotherapy. However, NLR was the only parameter that could predict 3-month DCR and 6-month DCR. NLR and age were able to predict 12-month DCR with NLR presenting a larger AUC. The cutoff point for 12-month DCR was 2.231 with the highest predictive performance (Figure 2). In order to illustrate survival differences among different levels of NLR and age, these two parameters were dichotomized according to the cutoff point generated by ROC analysis for 12-month DCR. Kaplan–Meier curves demonstrated that patients with different levels of NLR and age displayed significantly different long-term disease control (Figure 3).
Comparison Of Patients With High And Low Level Of NLR
The characteristics of patients with high and low NLR levels were compared. The group with higher NLR had more male patients, lower lymphocytes, albumin and serum sodium, as well as higher platelets, neutrophils and white blood cells (WBCs). Patients were similar in age, smoking history, ECOG scores, distant metastasis, and other baseline characteristics (Table 3).
 |
Table 3 Clinical Characteristics Differences Between High And Low NLR Groups |
Discussion
The efficacy of chemotherapy should not only be observed as an objective response after the first two cycles of chemotherapy but should persist beyond initial treatment to achieve long-term tumor remission. However, the PFS of chemotherapy among advanced NSCLC patients was reported to be only less than 6 months,1,7 which is unsatisfactory since multiple alternative treatments are available.11,12 In the present study, the 6-month DCR was 49%, which was an unsatisfactory outcome for a toxic treatment. Thus, identification of long-term chemotherapy benefits is imperative in this era of precision medicine. In the present study, we found that NLR reliably predicted long-term treatment response, including 3-month, 6-month and 12-month DCR. These results indicate that patients with lower NLR may be the appropriate chemotherapy benefits.
Prognostic factors similar to NLR were also found to display prognostic value for NSCLC. Russo A, et al, found that derived neutrophil-to-lymphocyte ratio (dNLR, absolute neutrophil count/[white blood cell concentration – absolute neutrophil count]) predicted response to anti-PD1/PD-L1 agents instead of chemotherapy.18–20 Furthermore, some authors have combined dNLR values with LDH levels to create a score (LIPI), which was shown to be of prognostic importance to immunotherapy and chemotherapy in advanced NSCLC.19,21 These studies demonstrate the clinical potential of neutrophil-related prognostic factors, including NLR. The prognostic value of NLR has been investigated in NSCLC patients and many other types of cancer in several clinical studies.14–17,22 Previous studies revealed a potential prognostic role of the NLR in NSCLC patients receiving PD-1 axis inhibitors.14,16,23 However, high NLR levels were found to predict a lower risk of immune-related adverse events (irAEs).24 Yao Y et al, found that NLR predicted PFS and OS among NSCLC patients under chemotherapy.25 However, they did not exclude patients with sensitive mutations and chemotherapy strategy was not based on histology. Another study found NLR predicted OS among metastatic NSCLC patients with chemotherapy as an initial therapy, however, did not relate to PFS.15 Another study found that NLR predicted OS among stage I-IV NSCLC patients after chemotherapy instead of before chemotherapy.23 Thus, some studies did not confirm the prognostic value of NLR for PFS.23,26 Hence, the prognostic value of NLR on PFS is still controversial. However, these studies did not focus on patients without sensitive mutations. NLR was found to play a certain prognostic role in NSCLC patients receiving other anti-cancer therapies. NLR predicted OS for NSCLC patients receiving chemoradiation.27 A study found NLR predicted PFS and OS for TKIs treatment of patients with sensitive EGFR mutations.28 However, NLR failed to predict prognosis of patients who underwent radical surgery.26 Unfortunately, the long-term benefit of chemotherapy for NSCLC patients without sensitive mutations has not been investigated. In this study we found age, stage and NLR predicted PFS, but age and stage were not related to the persistence of the response to chemotherapy indicated by 3-month, 6-month, 12-month DCR. Thus, the present study indicates that NLR can be used to identify long-term chemotherapy benefits among stage IIIB-IV NSCLC patients without sensitive mutations.
NLR expresses the balance between the inflammatory and immune systems, making it a useful index that reflects systemic inflammatory responses.13 Our previous study indicated that elevation of neutrophils was associated with myeloid-derived suppressor cell (MDSC),29 which is a critical inducer of immune suppression in cancer. Other studies found that the increased neutrophil levels are related to a systemic release of chemokines and ILs, including vascular endothelial growth factor30 and angiopoietin-1.31 These mechanisms may potentially explain the prognostic value of NLR. In the present study, low levels of NLR were associated with females which was a prognostic factor. NLR related to serum albumin and sodium with minimal difference between patients with different levels of NLR. Previous studies indicated that albumin-related indexes, such as C-reactive protein-albumin ratio and albumin-to-alkaline phosphatase ratio, were prognostic factors for NSCLC, which partly explains the prognostic value of NLR.32–34 These results indicate that NLR is an independent prognostic factor in NSCLC. The mechanisms of the prognostic value of NLR are not yet understood. Further studies on NSCLC are needed to investigate the latent mechanism of NLR.
The present study did not investigate the OS due to a significant loss in the follow-up of patients after the cessation of chemotherapy. However, the patients in our study might have received later lines of chemotherapy, bevacizumab, ninetedanib, anlotinib, anti-PD-1 treatment and clinical trials after chemotherapy, which also influenced OS.1,11,12 The financial situation of patients influences the utilization of subsequent anti-cancer therapies which influences their OS and might affect the predictive value of NLR. However, the present study aimed to identify long-term chemotherapy benefits rather than predicting OS. Bevacizumab was not routinely used in our institutions, and thus, the predictive value of NLR on bevacizumab treatment was not investigated. Additionally, the present study did not include patients who underwent unsuccessful TKIs treatment or were diagnosed with squamous cell carcinoma. Squamous cell carcinoma patients benefit more from gemcitabine treatment7 unlike non-squamous cell carcinoma patients. Thus, they should be investigated separately.
Conclusion
In summary, the present study identified NLR as an independent predictor for long-term chemotherapy benefit among non-squamous NSCLC patients without sensitive mutations. Patients with lower NLR presented preferable 12-month DCR and were optimal candidates for chemotherapy. Patients with high NLR may receive combined treatment or be included in clinical trials.
Acknowledgments
This study is supported by Guangzhou Science and Technology Project (201904010461), special Funds for Fundamental Research Fund of Sun Yat-sen University(19ykpy17) and National Natural Science Foundation of China (81572368, 81871999).
Disclosure
The authors report no conflicts of interest in this work.
References
1. PDQ Adult Treatment Editorial Board.Non-Small Cell Lung Cancer Treatment (PDQ(R)): Health Professional Version. 2002.
2. Huang CY, Ju DT, Chang CF, Muralidhar Reddy P, Velmurugan BK. A review on the effects of current chemotherapy drugs and natural agents in treating non-small cell lung cancer. Biomedicine (Taipei). 2017;7:23. doi:10.1051/bmdcn/2017070423
3. Oronsky B, Ma P, Reid TR, et al. Navigating the “No Man’s Land” of TKI-failed EGFR-mutated Non-Small Cell Lung Cancer (NSCLC): a review. Neoplasia. 2018;20:92–98. doi:10.1016/j.neo.2017.11.001
4. Russo A, Franchina T, Ricciardi GRR, et al. The changing scenario of 1(st) line therapy in non-oncogene addicted NSCLCs in the era of immunotherapy. Crit Rev Oncol Hematol. 2018;130:1–12. doi:10.1016/j.critrevonc.2018.06.007
5. Herbst RS, Morgensztern D, Boshoff C. The biology and management of non-small cell lung cancer. Nature. 2018;553:446–454. doi:10.1038/nature25183
6. Kris MG, Gaspar LE, Chaft JE, et al. Adjuvant systemic therapy and adjuvant radiation therapy for stage I to IIIA completely resected non-small-cell lung cancers: american society of clinical oncology/cancer care ontario clinical practice guideline update. J Clin Oncol. 2017;35:2960–2974. doi:10.1200/JCO.2017.72.4401
7. Scagliotti G, Brodowicz T, Shepherd FA, et al. Treatment-by-histology interaction analyses in three phase III trials show superiority of pemetrexed in nonsquamous non-small cell lung cancer. J Thorac Oncol. 2011;6:64–70. doi:10.1097/JTO.0b013e3181f7c6d4
8. Vestergaard HH, Christensen MR, Lassen UN. A systematic review of targeted agents for non-small cell lung cancer. Acta Oncol. 2018;57:176–186. doi:10.1080/0284186X.2017.1404634
9. Schwartz LH, Litiere S, de Vries E, et al. RECIST 1.1-update and clarification: from the RECIST committee. Eur J Cancer. 2016;62:132–137. doi:10.1016/j.ejca.2016.03.081
10. Sylvester R.World Health Organization (WHO) Handbook for Reporting Results of Cancer Treatment: Offset Publication N48.48. Geneva (Switzeland); 1979.
11. Han B, Li K, Wang Q, et al. Effect of anlotinib as a third-line or further treatment on overall survival of patients with advanced non-small cell lung cancer: the ALTER 0303 phase 3 randomized clinical trial. JAMA Oncol. 2018;4:1569–1575. doi:10.1001/jamaoncol.2018.3039
12. Raphael J, Chan K, Karim S, et al. Antiangiogenic therapy in advanced non-small-cell lung cancer: a meta-analysis of phase III randomized trials. Clin Lung Cancer. 2017;18:345–353 e345. doi:10.1016/j.cllc.2017.01.004
13. Li X, Chen ZH, Ma XK, et al. Neutrophil-to-lymphocyte ratio acts as a prognostic factor for patients with advanced hepatocellular carcinoma. Tumour Biol. 2014;35:11057–11063. doi:10.1007/s13277-014-2360-8
14. Zer A, Sung MR, Walia P, et al. Correlation of neutrophil to lymphocyte ratio and absolute neutrophil count with outcomes with PD-1 axis inhibitors in patients with advanced non-small-cell lung cancer. Clin Lung Cancer. 2018;19:426–434 e421. doi:10.1016/j.cllc.2018.04.008
15. Liu ZL, Zeng TT, Zhou XJ, et al. Neutrophil-lymphocyte ratio as a prognostic marker for chemotherapy in advanced lung cancer. Int J Biol Markers. 2016;31:e395–e401. doi:10.5301/jbm.5000222
16. Takeda T, Takeuchi M, Saitoh M, Takeda S. Neutrophil-to-lymphocyte ratio after four weeks of nivolumab administration as a predictive marker in patients with pretreated non-small-cell lung cancer. Thorac Cancer. 2018;9:1291–1299. doi:10.1111/1759-7714.12838
17. Liu D, Jin J, Zhang L, Li L, Song J, Li W. The neutrophil to lymphocyte ratio may predict benefit from chemotherapy in lung cancer. Cell Physiol Biochem. 2018;46:1595–1605. doi:10.1159/000489207
18. Russo A, Franchina T, Ricciardi GRR, et al. Baseline neutrophilia, derived neutrophil-to-lymphocyte ratio (dNLR), platelet-to-lymphocyte ratio (PLR), and outcome in non small cell lung cancer (NSCLC) treated with nivolumab or docetaxel. J Cell Physiol. 2018;233:6337–6343. doi:10.1002/jcp.26609
19. Mezquita L, Auclin E, Ferrara R, et al. Association of the lung immune prognostic index with immune checkpoint inhibitor outcomes in patients with advanced non-small cell lung cancer. JAMA Oncol. 2018;4:351–357. doi:10.1001/jamaoncol.2017.4771
20. Kazandjian D, Gong Y, Keegan P, Pazdur R, Blumenthal GM. Prognostic value of the lung immune prognostic index for patients treated for metastatic non-small cell lung cancer. JAMA Oncol. 2019. doi:10.1001/jamaoncol.2019.1747
21. Sorich MJ, Rowland A, Karapetis CS, Hopkins AM. Evaluation of the lung immune prognostic index for prediction of survival and response in patients treated with atezolizumab for NSCLC: pooled analysis of clinical trials. J Thorac Oncol. 2019;14:1440–1446. doi:10.1016/j.jtho.2019.04.006
22. Sahin F, Aslan AF. Relationship between inflammatory and biological markers and lung cancer. J Clin Med. 2018;7:160.
23. Kiriu T, Yamamoto M, Nagano T, et al. The time-series behavior of neutrophil-to-lymphocyte ratio is useful as a predictive marker in non-small cell lung cancer. PLoS One. 2018;13:e0193018. doi:10.1371/journal.pone.0193018
24. Pavan A, Calvetti L, Dal Maso A, et al. Peripheral blood markers identify risk of immune-related toxicity in advanced non-small cell lung cancer treated with immune-checkpoint inhibitors. Oncologist. 2019;24:1128–1136. doi:10.1634/theoncologist.2018-0563
25. Yao Y, Yuan D, Liu H, Gu X, Song Y. Pretreatment neutrophil to lymphocyte ratio is associated with response to therapy and prognosis of advanced non-small cell lung cancer patients treated with first-line platinum-based chemotherapy. Cancer Immunol Immunother. 2013;62:471–479. doi:10.1007/s00262-012-1347-9
26. Zhu J, Lian L, Qin H, et al. Prognostic evaluation of patients with resectable lung cancer using systemic inflammatory response parameters. Oncol Lett. 2019;17:2244–2256. doi:10.3892/ol.2018.9858
27. Scilla KA, Bentzen SM, Lam VK, et al. Neutrophil-lymphocyte ratio is a prognostic marker in patients with locally advanced (Stage IIIA and IIIB) non-small cell lung cancer treated with combined modality therapy. Oncologist. 2017;22:737–742. doi:10.1634/theoncologist.2016-0443
28. Zhang Y, Feng YC, Zhu HG, et al. The peripheral blood neutrophil-to-lymphocyte ratio is a prognostic predictor for survival of EGFR-mutant nonsmall cell lung cancer patients treated with EGFR-TKIs. Medicine (Baltimore). 2018;97:e11648. doi:10.1097/MD.0000000000011648
29. Li X, Xing YF, Lei AH, et al. Neutrophil count is associated with myeloid derived suppressor cell level and presents prognostic value of for hepatocellular carcinoma patients. Oncotarget. 2017;8:24380–24388. doi:10.18632/oncotarget.15456
30. Gong Y, Koh DR. Neutrophils promote inflammatory angiogenesis via release of preformed VEGF in an in vivo corneal model. Cell Tissue Res. 2010;339:437–448. doi:10.1007/s00441-009-0908-5
31. Neagoe PE, Brkovic A, Hajjar F, Sirois MG. Expression and release of angiopoietin-1 from human neutrophils: intracellular mechanisms. Growth Factors. 2009;27:335–344. doi:10.3109/08977190903155043
32. Yamauchi Y, Safi S, Muley T, et al. C-reactive protein-albumin ratio is an independent prognostic predictor of tumor recurrence in stage IIIA-N2 lung adenocarcinoma patients. Lung Cancer. 2017;114:62–67. doi:10.1016/j.lungcan.2017.11.002
33. Li D, Yu H, Li W. Albumin-to-alkaline phosphatase ratio at diagnosis predicts survival in patients with metastatic non-small-cell lung cancer. Onco Targets Ther. 2019;12:5241–5249. doi:10.2147/OTT.S203321
34. Li SJ, Lv WY, Du H, et al. Albumin-to-alkaline phosphatase ratio as a novel prognostic indicator for patients undergoing minimally invasive lung cancer surgery: propensity score matching analysis using a prospective database. Int J Surg. 2019;69:32–42. doi:10.1016/j.ijsu.2019.07.008
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.


