Back to Journals » International Journal of Chronic Obstructive Pulmonary Disease » Volume 19
Neutrophil-to-Lymphocyte Ratio (NLR), Platelet-to-Lymphocyte Ratio (PLR) and Monocyte-to-Lymphocyte Ratio (MLR) as Biomarkers in Diagnosis Evaluation of Acute Exacerbation of Chronic Obstructive Pulmonary Disease: A Retrospective, Observational Study
Authors Cai C, Zeng W, Wang H, Ren S
Received 27 December 2023
Accepted for publication 6 April 2024
Published 15 April 2024 Volume 2024:19 Pages 933—943
DOI https://doi.org/10.2147/COPD.S452444
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Jill Ohar
Chuang Cai,1,* Wentan Zeng,2,* Hongwei Wang,3 Shuqi Ren2
1Cancer Research Institute of Zhongshan City, Zhongshan City People’s Hospital, Zhongshan City, Guangdong Province, People’s Republic of China; 2Department of Laboratory Medicine, Tanzhou People’s Hospital of Zhongshan, Zhongshan City Hospital of Integration of TCM & Western Medicine, Zhongshan City, Guangdong Province, People’s Republic of China; 3Department of Pediatrics, Tanzhou People’s Hospital of Zhongshan, Zhongshan City hospital of integration of TCM & western medicine, Zhongshan City, Guangdong Province, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Shuqi Ren, Department of Laboratory Medicine, Tanzhou People’s Hospital of Zhongshan, Zhongshan City hospital of integration of TCM & western medicine, No. 10 Dexiu road, Zhongshan City, Guangdong Province, People’s Republic of China, Tel +8615521067602, Fax +860760-23639928, Email [email protected] Chuang Cai, Cancer Research Institute of Zhongshan City, Zhongshan City People’s Hospital, No. 2 Sunwen East Road, Zhongshan City, Guangdong Province, 528404, People’s Republic of China, Tel +86 18826431030, Fax +86 0760-88823566, Email [email protected]
Purpose: Hierarchical management is advocated in China to effectively manage chronic obstructive pulmonary disease (COPD) patients and reduce the incidence and mortality of acute exacerbation of COPD (AE-COPD). However, primary and community hospitals often have limited access to advanced equipment and technology. Complete blood count (CBC), which is commonly used in these hospitals, offers the advantages of being cost-effective and easily accessible. This study aims to evaluate the significance of routine blood indicators in aiding of diagnosing AE-COPD.
Patients and Methods: In this research, we enrolled a total of 112 patients diagnosed with AE-COPD, 92 patients with stable COPD, and a control group comprising 60 healthy individuals. Clinical characteristics, CBC parameters, and serum CRP levels were collected within two hours. To assess the associations between NLR/PLR/MLR and CRP by Spearman correlation test. The diagnostic accuracy of NLR, PLR and MLR in AE-COPD was assessed using Receiver Operating Characteristic Curve (ROC) and the area under the curve (AUC). Binary Logistic Regression analysis was conducted for the indicators of NLR, PLR and MLR.
Results: We found that patients with AE-COPD had significantly higher levels of NLR, PLR and MLR in contrast to patients with stable COPD. Additionally, the study revealed a noteworthy correlation between CRP and NLR (rs=0.5319, P< 0.001), PLR (rs=0.4424, P< 0.001), and MLR (rs=0.4628, P< 0.001). By utilizing specific cut-off values, the amalgamation of NLR, PLR and MLR augmented diagnostic sensitivity. Binary logistic regression analysis demonstrated that heightened NLR and MLR act as risk factors for the progression of AE-COPD.
Conclusion: The increasing levels of NLR, PLR and MLR could function as biomarkers, akin to CRP, for diagnosis and assessment of acute exacerbations among COPD patients. Further research is required to validate this concept.
Keywords: acute exacerbation of chronic obstructive pulmonary disease, AE-COPD, neutrophil-lymphocyte ratio, NLR, platelet-lymphocyte ratio, PLR, monocyte-lymphocyte ratio, MLR
Graphical Abstract:
Introduction
Chronic obstructive pulmonary disease (COPD) is an extensively occurring condition defined by continuous respiratory symptoms and airflow limitation that cannot be easily reversed, resulting in increased prevalence and mortality rates globally, which lead to an enormous burden on healthcare systems and society.1,2 In China, there is a concerning prevalence of COPD patients, with a staggering count of 105 million reported patients in 2020. This number is expected to continue increasing and is projected to reach 109 million by 2025.3,4 The overall prevalence of COPD in individuals aged 40 and above is 13.7%, mainly attributed to sustained exposure to cigarette smoke, environmental pollutants and the aging population.5 Given the increasing trend of an ageing population, China advocates the implementation of a hierarchical management in COPD patients. This approach not only helps to rationally allocate medical resources, but also better meets the individual needs of patients, thus improving treatment outcomes and quality of life.
Acute exacerbation of chronic obstructive pulmonary disease (AE-COPD) refers to a sudden worsening of respiratory symptoms in patients with COPD, such as cough, sputum and dyspnea, accompanied by the airway limitation and systemic inflammation.2 Clinically, the diagnosis of AE-COPD relies heavily on the patient’s medical history and clinical manifestations. However, the similarities between the symptoms of AE-COPD and a variety of other diseases, such as pneumonia and pulmonary embolism, have led to challenges in the diagnostic process. Furthermore, in the early stage of AE-COPD, as the symptoms are relatively mild, judgement by clinical manifestations alone may not be accurate enough, and may easily lead to missed or misdiagnosis. Therefore, at this stage, timely therapeutic interventions through auxiliary examination can rapidly control the condition, alleviate the symptoms, and prevent further deterioration of the disease, thus greatly improving the therapeutic effect of AE-COPD. Laboratory examinations play a crucial role in identifying and diagnosing acute exacerbations of COPD. However, the ability to detect these methods in primary and community hospitals’ laboratories is significantly restricted due to equipment limitations. Therefore, it is essential to identify easily accessible, affordable and effective method to aid in the diagnosis of AE-COPD.
More than half of the AE-COPD cases are triggered by bacterial and viral infections.6–9 Studies have shown that the inflammatory profile of COPD is associated with different types of inflammatory cells, including lymphocytes and neutrophils, which can lead to respiratory tissue damage.10,11 Hence, the exploration of inflammation-associated biomarkers could be a valuable approach for predicting and aiding the diagnosis of AE-COPD.
C-reactive protein (CRP) is usually used as a common indicator of inflammation in clinical practice, but not all primary and community hospitals carry out CRP testing for various reasons in China. In addition, the cost of CRP testing is indeed higher compared to Complete Blood Count (CBC). CBC is a fundamental clinical test that serves as a rapid laboratory aid, which is also carried out in primary and community hospitals’ laboratory.12 In the field of inflammation, lymphocytes and neutrophils are significant indicators observed in CBC. The CBC is able to reflect the systemic inflammatory response in different diseases.13 The presence of neutrophil-lymphocyte ratio (NLR) is recognized as a marker for systemic inflammation.14 Additionally, the platelet-lymphocyte ratio (PLR) has been evaluated as a predictor for various systemic inflammatory disorders.15 Moreover, the monocyte-lymphocyte ratio (MLR) is acknowledged as a new marker in patients with different types of malignant diseases.16 Recent studies have reported that three calculated indices derived from CBC tests in clinical practice are independent risk factors and helpful in diagnosing infection and the development of inflammation.17–19
Although previous studies have examined the diagnostic value of individual CBC parameters for AE-COPD, research on the relationship between CBC indicators and clinical diagnosis evaluation of AE-COPD patients is still limited.20 This study aims to comprehensively investigate all indicators of CBC, including NLR, PLR, and MLR, and explore their application value in the diagnosis and evaluation of AE-COPD.
Materials and Methods
Examples
In this retrospective study, 264 participates were enrolled and divided into three groups: 112 patients with AE-COPD, 92 patients with stable COPD and a control group of 60 healthy individuals. The control group is healthy elderly people without underlying diseases. The stable COPD group were patients with COPD who were regularly followed up. The AE-COPD group were patients who were admitted to the hospital to receive treatment. All participants’ blood test results were obtained from Tanzhou People’s Hospital of Zhongshan (Guangdong Province, China), from January 1, 2021 to December 31, 2022.
Participants were included in this study if they met the following criteria: (1) The control group was healthy elderly without underlying disease (eg, diabetes, cardiovascular disease, immune system disorders), and age and smoking rates were not statistically different from those of the COPD group. (2) All the patients with COPD were diagnosed by a pulmonologist based on past smoking history, clinical symptoms and spirometry measurement. According to the GOLD (2021 report) criteria, COPD patients had forced expiratory volume in 1 second [FEV1]/forced vital capacity [FVC]<70%. (3) Furthermore, individuals afflicted with AE-COPD experience a sudden exacerbation of respiratory manifestations including coughing, sputum production and dyspnea, accompanied by both airway restriction and systemic inflammation. The severity of these symptoms necessitates hospitalization. In China, steroid drugs have become a regular part of many patients’ home medical kits due to the chronic and recurrent nature of COPD. However, in actual medical practice, not all patients with AE-COPD receive hormone therapy before hospital admission due to the lack of knowledge about medication and insufficient medical resources. It is worth noting that patients with AE-COPD may have altered blood results after the use of steroid drugs, as these medications can impact the body’s hematopoietic and immune systems, leading to alterations in CBC values. The exclusion criteria were as follows: (1) Steroid drugs had been taken before the blood test. (2) Anti-infective medication has been administered prior to the blood test. (3) Patients with hematological disorders. (4) Patients with other respiratory disorders. (5) Patients with mental disorders or psychological disorders.
Data regarding demographics and clinical characteristics were gathered from every participant, encompassing information such as age, gender, and smoking background. A skilled and authorized nurse collected venous blood from the participant. The participants’ venous blood samples were gathered using K2 EDTA tubes and underwent analysis within a timeframe of 2 hours. NLR (Neutrophil-Lymphocyte ratio), PLR (Platelet–Lymphocyte ratio) and MLR (Monocyte–Lymphocyte ratio) was calculated derived from CBC per individual. Complete blood count was determined by Sysmex XN2800 (Kobe, Japan). The venous blood samples of participants were taken into sterile drying tubes, and the serum taken after centrifugation were detected the level of C-reactive protein (CRP) within 2 hours. CRP detection was determined by Genrui PA300 (Shenzhen, China). CBC and CRP results for all participants were obtained without receiving any therapeutic measures prior to blood collection. All blood samples were taken within 2 hours. All tests were carried out in strict accordance with the regulations and tested by professional laboratory technician. The instruments are in control during the testing period.
Statistical Analysis
All statistical analyses were performed using SPSS 25.0 for Windows (IBM, Chicago, USA). Data are represented as mean ± standard deviation. Control, stable-COPD and AE-COPD were compared by one-way analysis of variance parametric tests for the comparison of categorical variables and continuous variables. The correlation between NLR, PLR, and MLR and CRP was assessed using Spearman correlation test. To evaluate the sensitivity and specificity of NLR, PLR, and MLR, Receiver Operating Characteristic Curve (ROC) analysis was employed. In addition, we analyzed the sensitivity and specificity of two or three-markers combinations. To evaluate the diagnostic accuracy, we employed the area under the curve (AUC), which measures the ability to discriminate, with greater values denoting increased discriminatory capability. Binary logistic regression analyses incorporated individual risk factors. Statistical significance was established for group differences at P<0.05.
Results
Characteristics of Included Subjects
From January 2021 to December 2022, a total of 264 participants were included in this retrospective study. There were 112 patients with AE-COPD (71.43% male; mean age 76.866 ± 7.039 years), 92 patients with stable-COPD (78.26% male; mean age 76.870 ± 6.385 years) and 60 healthy individuals (78.33% male; mean age 75.433 ± 3.846 years). There was no statistically significant difference between the ages, gender and smoking history of the study participants. Table 1 displays the demographic traits of the subjects included in the study groups and the control group.
 |
Table 1 The Demographic Characteristics of Study Groups and the Control Group |
As shown in Table 2, elevated levels of leukocytes, erythrocytes, neutrophils, lymphocytes, monocytes, eosinophils, hemoglobin, mean corpuscular volume (MCV), hematocrit (HCT), red cell distribution width (RDW), plateletcrit (P-CT), mean platelet volume (MPV), platelet distribution width (PDW), Platelet-large cell rate (P-LCR), NLR, PLR, MLR and CRP were observed in both stable and exacerbation periods of COPD groups when compared to the control group (P < 0.05). Conducting the comparison in the COPD observation group, we found only the levels of lymphocytes, NLR, PLR, MLR, and CRP in AE-COPD group were significantly higher than stable COPD patients (P < 0.05). Table 2 displays the laboratory characteristics of study groups and the control group.
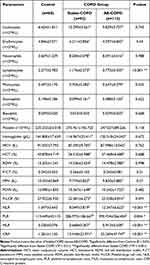 |
Table 2 Clinical Complete Blood Count Parameters of the Study Patients |
The Level of CRP, NLR, PLR and MLR
CRP is a sensitive acute phase reactant that increase in serum levels during infection and chronic inflammatory diseases.21 According to existing literature, CRP has emerged as a conventional diagnostic indicator to differentiate infection-induced AE-COPD progression.22 In this study, the average CRP levels among individuals with stable and exacerbated COPD, as well as controls, yielding values of 13.418±23.971, 35.269±47.747 and 1.281±1.130, respectively (P < 0.001).
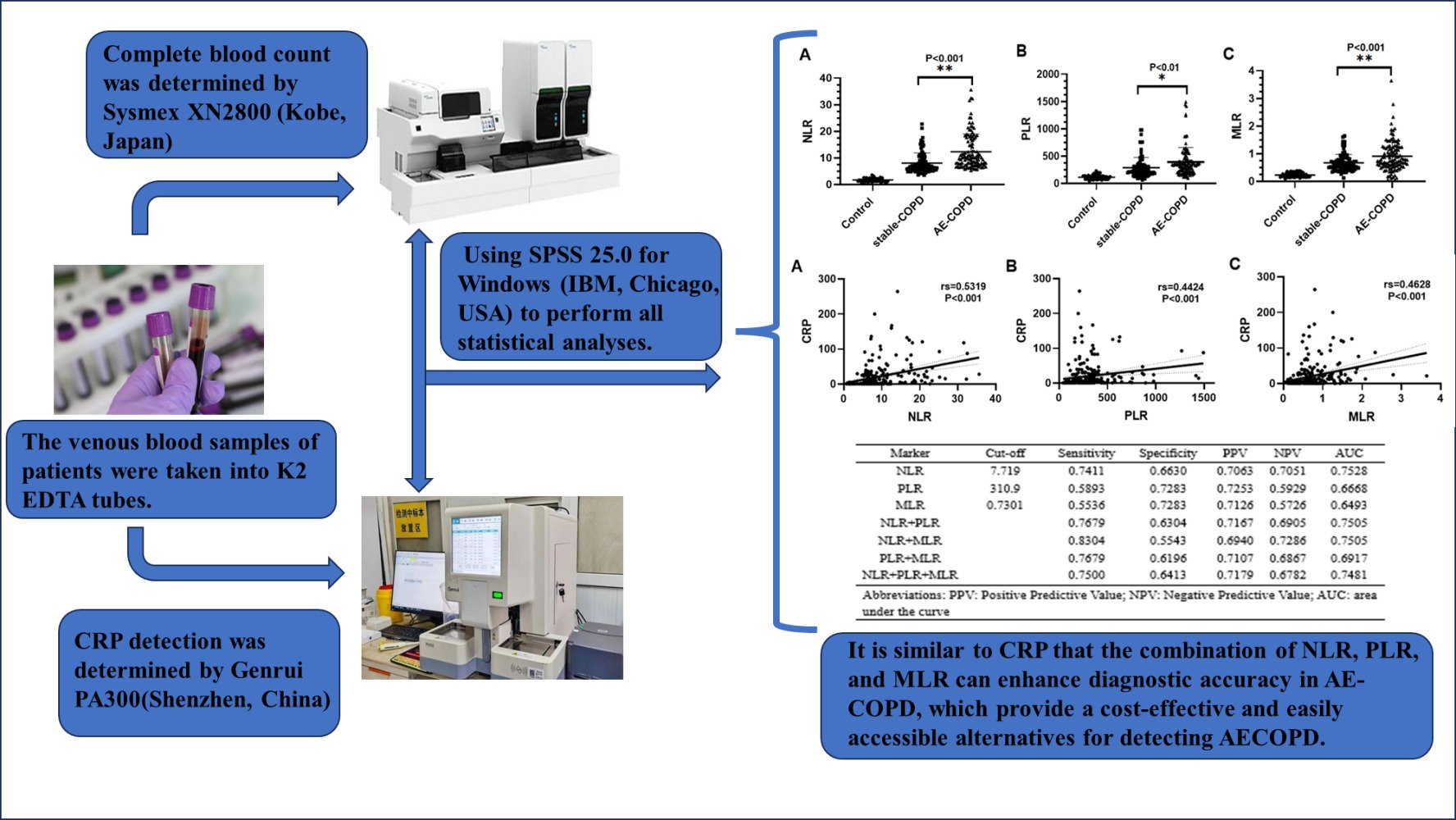
The mean values of NLR for control, Stable-COPD and AE-COPD were 1.697±0.643, 8.049±3.819 and 12.347±6.623, respectively. The mean values of PLR for control, Stable-COPD and AE-COPD were 113.449 ± 42.115, 286.973 ± 186.667 and 390.924 ± 266.454, respectively. The mean values of MLR for control, Stable-COPD and AE-COPD were 0.230±0.076, 0.668±0.307 and 0.912±0.558, respectively (Table 2). As show in Figure 1, the AE-COPD group exhibited notably increased levels of NLR, PLR, and MLR when compared to both the stable COPD and control groups (P<0.05).
The Correlation of CRP with NLR, PLR and MLR
Spearman correlation analysis indicated a significant correlation of CRP with NLR (rs=0.5319, P < 0.001), PLR (rs=0.4424, P < 0.001) and MLR (rs=0.4628, P < 0.001) (Figure 2). All the participants were taken into the Spearman correlation analysis (Table 3).
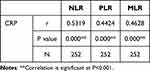 |
Table 3 Correlations Between All Inflammatory Markers |
Diagnostic Accuracy of NLR, PLR and MLR
Figure 3 displays the ROC curve analysis results for NLR, PLR, and MLR. The evaluation of AE-COPD using NLR resulted in a sensitivity of 74.11% and a specificity of 66.30% at a cut-off value of 7.719. The AUC for NLR was calculated to be 0.7528. When PLR was used to assess AE-COPD, the optimal cut-off PLR was determined to be 310.9, with an AUC of 0.6668. The sensitivity and specificity for PLR were 58.93% and 72.83%, respectively. Similarly, MLR exhibited a sensitivity of 55.36% and a specificity of 72.83% at a cut-off value of 0.7301, resulting in an AUC of 0.6493. In this study, we found that NLR had the highest diagnostic accuracy in AE-COPD, whereas PLR and MLR show a better diagnostic specificity. We further examined the diagnostic accuracy of two or three-markers combinations and found that they can improve the diagnostic sensitivity, which showed best sensitivity of 83.04% achieved by combining NLR and MLR. The diagnostic accuracy of both single markers and marker combinations is listed in Table 4.
 |
Table 4 Diagnostic Accuracy of the NLR, PLR and MLR |
Risk Factors for the Development of AE-COPD
A binary logistic regression analysis was performed with COPD groups (both stable-COPD and AE-COPD) as dependent variable and NLR, PLR and MLR as covariate. The results demonstrated NLR (OR=1.167, P<0.001), PLR (OR=1.000, P=0.843) and MLR (OR=2.695, P=0.019), indicating that elevated NLR and MLR are risk factors for the patients with AE-COPD (Table 5).
 |
Table 5 Binary Logistic Regression Analysis of NLR, PLR and MLR |
Discussion
COPD is a disabling condition characterized by poorly reversible airflow limitation and inflammation. AE-COPD exhibits a rapid deterioration in respiratory symptoms and airway function. This manifestation is predominantly triggered by infection, leading to increased hospitalization rates and mortality among COPD patients. Additionally, the economic burden on these patients is substantial, encompassing high direct and indirect medical expenses. As a most commonly clinical test and a rapidly laboratory aid, CBC can detect relevant indicators of inflammation, reflecting initially the infection of organism, and providing valuable information for the diagnosis and evaluation of AE-COPD patients.
In the lungs of COPD patients, the structure and function of lung tissues are damaged due to long-term chronic inflammation and oxidative stress, making the airways more vulnerable to external pathogens. In this case, pathogen-associated molecular patterns (PAMPs) may be released into the lung tissue and recognized by pattern recognition receptors (PRRs) (eg, Toll-like receptors). Once PAMPs are recognized by PRRs, an immune response is initiated, leading to the recruitment and activation of inflammatory cells, further exacerbating the damage and inflammatory response in lung tissues.23–27 In this study, we found significantly higher levels of leukocytes, neutrophils, and monocytes in the COPD and AE-COPD groups compared to the control group. However, patients with COPD have a suppressed immune system as a result of repeated use of steroid medications, such as budesonide, over a long period of time. In this state of immunosuppression, the increase in the number of leukocytes, neutrophils, and monocytes in AE-COPD patients may not be significant or sufficient to effectively clear pathogens. The One-way Anova test showed that there was no statistically significant difference in leukocytes, neutrophils and monocytes between stable-COPD and AE-COPD. In addition, lymphocyte counts may decrease due to immunosuppression, which further weakens the immunological function.28–32 The level of lymphocytes in AE-COPD group was significantly lower than stable-COPD group, with a statistically significant difference between the two groups. NLR, PLR, and MLR were also found to be significantly elevated in AE-COPD compared to stable-COPD.
CRP is a classical inflammatory maker that increases in response to tissue damage, inflammation, or infection. A systematic review33 discovered that individuals afflicted with COPD exhibited heightened CRP levels, with an average increase of 1.86 mg/l compared to the control group, which is considered clinically significant. Stolz et al34 demonstrated that CRP levels were significantly elevated in AE-COPD. This study found that the group of AE-COPD had the highest serum levels of CRP compared to the Control and Stable-COPD groups. Additionally, Spearman correlation analysis showed a positive correlation between CRP and NLR/PLR/MLR. It can be seen that NLR, PLR and MLR not only reflect CRP levels to a certain extent, but can also be used as auxiliary tools to help physician diagnose and monitor the development of AE-COPD.
ROC curve analysis was conducted to assess the diagnostic effectiveness of NLR, PLR, and MLR individually and in combination for AE-COPD. The results showed that NLR is a strongest parameter in diagnosing AE-COPD, with an optimal AUC of 0.7528, while PLR and MLR show a better ability in diagnostic specificity. Compared with NLR, it showed a better diagnostic sensitivity of two or three-marker combinations of NLR, PLR and MLR. A combination of NLR and MLR have showed the strongest diagnostic sensitivity of 0.8304 (AUC=0.7505). Therefore, it is recommended to interpret the results of NLR, PLR and MLR together for better diagnostic accuracy.
According to binary logistic regression analysis, elevated NLR and MLR are risk factors for the patients with AE-COPD. As a result, the accuracy of the PLR as a diagnostic tool needs to be reevaluated and discussed with caution.
Compared to other common clinical inflammatory indicators such as CRP and procalcitonin (PCT), CBC has the advantage of being convenient and cost-effective. It is the most common laboratory examination due to its rapid and easily accessible feature, especially for primary and community hospitals. Currently, the diagnosis of AE-COPD mainly depends on clinical symptoms and lacks specific serum biomarkers. Nonetheless, if routine checkup of patients with stable COPD reveals elevated levels of NLR, PLR and MLR, it becomes crucial to remain vigilant regarding any alterations in the patient’s health status.
In this study, we applied the data of COPD patients in our hospital for retrospective analysis, all of which were obtained before anti-infective treatment. However, there were still several limitations. Firstly, it is necessary to conduct retrospective cohort study across multiple hospitals and a large number of samples to confirm the diagnosis accuracy of NLR, PLR and MLR in patients with AE-COPD. Secondly, it is meaningful to explore the correlation between the severity of AE-COPD and the level of NLR, PLR and MLR, that physicians can classify and manage patients with AE-COPD based on the level of NLR, PLR and MLR. This approach is helpful for the clinical management and optimal allocation of limited medical resource. Thirdly, future study should take a CBC test regularly throughout treatment to investigate the relationship between the level of NLR/PLR/MLR and prognosis of AE-COPD. Fourthly, follow-up visits with CBC examination should also be conducted after discharge to determine the relevance of NLR/PLR/MLR and patient survival rates. Fifthly, future work should examine the ability of NLR, PLR and MLR to discriminating bacterial or viral infections in AECOPD, which plays a guiding role in the treatment of antibiotics.
Conclusion
In patients with AE-COPD, markers for increased inflammation, such as NLR, PLR and MLR, can be utilized akin to CRP. This similarity indicates that elevated NLR, PLR, and MLR may serve as indicators of heightened inflammation. Moreover, they provide a cost-effective and easily accessible alternatives for detecting AECOPD, which is helpful for primary hospitals and community hospitals. This study demonstrated that utilizing the combination of NLR, PLR, and MLR can enhance diagnostic accuracy in AE-COPD, making them a valuable tool in diagnosing and evaluating acute exacerbation.
Ethics Statement
The study protocol was reviewed and approved by the Zhongshan Tanzhou People’s Hospital Clinical Research and Animal Experimentation Ethic Committee (Approval No. 2020001) and was conducted in accordance with the 1964 Declaration of Helsinki and its subsequent amendments or similar ethical standards. This study was a retrospective study that did not involve the disclosure of patients’ private information, and informed consent was waived by our institutional review board. I solemnly promise to strictly comply with relevant laws and regulations to ensure the security and confidentiality of patients’ information and to respect their right to privacy. I am well aware that any information that identifies a patient is sensitive information, and therefore I promise not to disclose any patient identifying information in public information.
Acknowledgments
The authors would like to acknowledge the personnel of the Department of Laboratory Medicine, Tanzhou People’s Hospital of Zhongshan for their assistance for this study. The authors would like to acknowledge Health Commission of Guangdong Province (A2020389) and Zhongshan Science and Technology Bureau (2022B1073) funding for this study.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Patel AR, Patel AR, Singh S, Singh S, Khawaja I. Global initiative for chronic obstructive lung disease: the changes made. Cureus. 2019;11(6):e4985. doi:10.7759/cureus.4985
2. Vestbo J, Hurd SS, Agustí AG, et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: GOLD executive summary. Am J Respir Crit Care Med. 2013;187(4):347–365. doi:10.1164/rccm.201204-0596PP
3. Adeloye D, Chua S, Lee C, et al. Global Health Epidemiology Reference Group (GHERG). Global and regional estimates of COPD prevalence: systematic review and meta-analysis. Glob J Health Sci. 2015;5(2):020415. doi:10.7189/jogh.05.020415
4. GBD 2013 Mortality and Causes of Death Collaborators. Global, regional, and national age-sex specific all-cause and cause-specific mortality for 240 causes of death, 1990–2013: a systematic analysis for the global burden of disease study 2013. Lancet. 2015;385(9963):117–171. doi:10.1016/S0140-6736(14)61682-2
5. Wang C, Xu J, Yang L, et al. China Pulmonary Health Study Group. Prevalence and risk factors of chronic obstructive pulmonary disease in China (the China Pulmonary Health [CPH] study): a national cross-sectional study. Lancet. 2018;391(10131):1706–1717. doi:10.1016/S0140-6736(18)30841-9
6. Welte T, Miravitlles M. Viral, bacterial or both? Regardless, we need to treat infection in COPD. Europ resp J. 2014;44(1):11–13. doi:10.1183/09031936.00041914
7. Nishimura K, Izumi T, Tsukino M, Oga T. Dyspnea is a better predictor of 5-year survival than airway obstruction in patients with COPD. Chest. 2002;121(5):1434–1440. doi:10.1378/chest.121.5.1434
8. Wang H, Anthony D, Selemidis S, Vlahos R, Bozinovski S. Resolving viral-induced secondary bacterial infection in COPD: a concise review. Front Immunol. 2018;9:2345. doi:10.3389/fimmu.2018.02345
9. Seemungal T, Harper-Owen R, Bhowmik A, et al. Respiratory viruses, symptoms, and inflammatory markers in acute exacerbations and stable chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2001;164(9):1618–1623. doi:10.1164/ajrccm.164.9.2105011
10. Isles HM, Herman KD, Robertson AL, et al. The CXCL12/CXCR4 signaling axis retains neutrophils at inflammatory sites in zebrafish. Front Immunol. 2019;10:1784. doi:10.3389/fimmu.2019.01784
11. Zhu J, Mallia P, Footitt J, et al. Bronchial mucosal inflammation and illness severity in response to experimental rhinovirus infection in COPD. J Allergy Clin Immunol. 2020;146(4):840–850.e7. doi:10.1016/j.jaci.2020.03.021
12. Li K, Peng YG, Yan RH, Song WQ, Peng XX, Ni X. Age-dependent changes of total and differential white blood cell counts in children. Chinese Med J. 2020;133(16):1900–1907. doi:10.1097/CM9.0000000000000854
13. Wood SK, Wood CS, Lombard CM, et al. Inflammatory factors mediate vulnerability to a social stress-induced depressive-like phenotype in passive coping rats. Biol Psychiatry. 2015;78(1):38–48. doi:10.1016/j.biopsych.2014.10.026
14. Faria SS, Fernandes PC, Silva MJ, et al. The neutrophil-to-lymphocyte ratio: a narrative review. Ecancermedicalscience. 2016;10:702. doi:10.3332/ecancer.2016.702
15. Yang W, Wang X, Zhang W, et al. Neutrophil-lymphocyte ratio and platelet-lymphocyte ratio are 2 new inflammatory markers associated with pulmonary involvement and disease activity in patients with dermatomyositis. Int j Clin Chem. 2017;465:11–16. doi:10.1016/j.cca.2016.12.007
16. Xiang J, Zhou L, Li X, et al. Preoperative monocyte-to-lymphocyte ratio in peripheral blood predicts stages, metastasis, and histological grades in patients with ovarian cancer. Transl Oncol. 2017;10(1):33–39. doi:10.1016/j.tranon.2016.10.006
17. Luo S, Yang WS, Shen YQ, et al. The clinical value of neutrophil-to-lymphocyte ratio, platelet-to-lymphocyte ratio, and D-dimer-to-fibrinogen ratio for predicting pneumonia and poor outcomes in patients with acute intracerebral hemorrhage. Front Immunol. 2022;13:1037255. doi:10.3389/fimmu.2022.1037255
18. Wang X, Ni Q, Wang J, Wu S, Chen P, Xing D. Systemic inflammation response index is a promising prognostic marker in elderly patients with heart failure: a retrospective cohort study. Front Cardiovasc Med. 2022;9:871031. doi:10.3389/fcvm.2022.871031
19. Martini DJ, Liu Y, Shabto JM, et al. Novel risk scoring system for patients with metastatic renal cell carcinoma treated with immune checkpoint inhibitors. oncologist. 2020;25(3):e484–e491. doi:10.1634/theoncologist.2019-0578
20. Paliogiannis P, Fois AG, Sotgia S, et al. Neutrophil to lymphocyte ratio and clinical outcomes in COPD: recent evidence and future perspectives. Eur Respir Rev. 2018;27(147):170113. doi:10.1183/16000617.0113-2017
21. Polzin A, Pletz M, Erbes R, et al. Procalcitonin as a diagnostic tool in lower respiratory tract infections and tuberculosis. Europ resp J. 2003;21(6):939–943. doi:10.1183/09031936.03.00055103
22. Mohamed KH, Abderabo MM, Ramadan ES, et al. Procalcitonin as a diagnostic marker in acute exacerbation of COPD. Egypt J Chest Dis Tuberculosis. 2012;61(4):301–305. doi:10.1016/j.ejcdt.2012.08.011
23. Greene CJ, Nguyen JA, Cheung SM, et al. Macrophages disseminate pathogen associated molecular patterns through the direct extracellular release of the soluble content of their phagolysosomes. Nat Commun. 2022;13(1):3072. doi:10.1038/s41467-022-30654-4
24. Chow KT, Gale M, Loo YM. RIG-I and Other RNA Sensors in Antiviral Immunity. Ann Rev Immunol. 2018;36(1):667–694. doi:10.1146/annurev-immunol-042617-053309
25. Sato K, Kawakami K. PAMPs and host immune response in cryptococcal infection. Med Mycol J. 2022;63(4):133–138. doi:10.3314/mmj.22.005
26. Ciaston I, Dobosz E, Potempa J, Koziel J. The subversion of toll-like receptor signaling by bacterial and viral proteases during the development of infectious diseases. Mol Aspect Med. 2022;88:101143. doi:10.1016/j.mam.2022.101143
27. Thoma-Uszynski S, Stenger S, Takeuchi O, et al. Induction of direct antimicrobial activity through mammalian toll-like receptors. Science. 2001;291(5508):1544–1547. doi:10.1126/science.291.5508.1544
28. Coxon A, Tang T, Mayadas TN. Cytokine-activated endothelial cells delay neutrophil apoptosis in vitro and in vivo. A role for granulocyte/macrophage colony-stimulating factor. J Exp Med. 1999;190(7):923–934. doi:10.1084/jem.190.7.923
29. Dale DC, Boxer L, Liles WC. The phagocytes: neutrophils and monocytes. Blood. 2008;112(4):935–945. doi:10.1182/blood-2007-12-077917
30. Ley K, Hoffman HM, Kubes P, et al. Neutrophils: new insights and open questions. Sci Immunol. 2018;3(30):eaat4579. doi:10.1126/sciimmunol.aat4579
31. Maeda K, Malykhin A, Teague-Weber BN, Sun XH, Farris AD, Coggeshall KM. Interleukin-6 aborts lymphopoiesis and elevates production of myeloid cells in systemic lupus erythematosus-prone B6. Sle1.Yaa animals. Blood. 2009;113(19):4534–4540. doi:10.1182/blood-2008-12-192559
32. Barnes PJ. Oxidative Stress in Chronic Obstructive Pulmonary Disease. Antioxidants. 2022;11(5):965. doi:10.3390/antiox11050965
33. Gan W, Man SF, Senthilselvan A, et al. Association between chronic obstructive pulmonary disease and systemic inflammation: a systematic review and meta-analysis. Thorax. 2004;559:74–580.
34. Stolz D, Christ-Crain M, Morgenthaler N, et al. Copeptin, C-reactive protein, and procalcitonin as prognostic biomarkers in acute exacerbation of COPD. Chest. 2007;131(4):1058–1067. doi:10.1378/chest.06-2336
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.



