Back to Journals » Blood and Lymphatic Cancer: Targets and Therapy » Volume 12
Neuropsychiatric Manifestations of Lymphoma-Associated Cerebral Glucose Hypometabolism Can Be Reversed by Intensive Glucose Supplementation
Authors Kase AM , Bullock C, Parrondo R, Alhaj Moustafa M , Iqbal M, Li KD, Parent EE, Tun H
Received 17 December 2021
Accepted for publication 7 March 2022
Published 23 March 2022 Volume 2022:12 Pages 17—21
DOI https://doi.org/10.2147/BLCTT.S353430
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Wilson Gonsalves
Adam M Kase,1 Catherine Bullock,2 Ricardo Parrondo,1 Muhamad Alhaj Moustafa,1 Madiha Iqbal,1 K David Li,3 Ephraim E Parent,4 Han Tun1
1Division of Hematology and Medical Oncology, Mayo Clinic Hospital, Jacksonville, FL, 32224, USA; 2Department of Internal Medicine, Mayo Clinic Hospital, Jacksonville, FL, 32224, USA; 3Department of Pathology, Mayo Clinic Hospital, Jacksonville, FL, 32224, USA; 4Nuclear Medicine Division of Radiology Department, Mayo Clinic, Jacksonville, FL, 32224, USA
Correspondence: Adam M Kase, Division of Hematology and Medical Oncology, Mayo Clinic, 4500 San Pablo Road South, Jacksonville, FL, USA, Tel +1 904-953-2000, Fax +1 904-953-2315, Email [email protected]
Abstract: Cerebral glucose hypometabolism (CGHM) is characterized by diffuse or focal reduction in uptake of glucose by the brain as determined on a FDG PET-CT. We report a case of lymphoma-associated cerebral glucose hypometabolism (LA-CGHM) in a patient with hepatosplenic T-cell lymphoma (HSTCL) whose neuropsychiatric symptoms were resolved with glucose supplementation. PET-CT scan showed diffuse cerebral hypometabolism in addition to focal hypermetabolism in the liver related to lymphomatous involvement. He responded rapidly to infusion of 10% dextrose with complete resolution of neurological symptoms on two separate occasions and was later maintained on oral glucose without relapse. While his neuropsychiatric symptoms improved, his aggressive lymphoma and chemo-refractory disease ultimately led to his demise. We suggest that LA-CGHM can cause neuropsychiatric manifestations which can be reversed by intensive glucose supplementation.
Keywords: cerebral hypometabolism, hepatosplenic T-cell lymphoma, non-Hodgkin’s lymphoma, glucose hypometabolism FDG PET-CT
Introduction
18F-Fluorodeoxyglucose (FDG) positron emission tomography (PET)-computed tomography (CT) is an established nuclear imaging modality for lymphoma staging and assessing treatment response.1 Since this imaging modality relies on glucose metabolism, the readout reflects hypo- or hypermetabolic areas based on glucose uptake. PET scans typically show high physiologic metabolic activity in the brain as glucose is the main metabolic substrate of the brain accounting for 20% of the total-body glucose metabolism.2 However, cerebral glucose hypometabolism (CGHM) has been described in multiple conditions including Alzheimer disease, epilepsy, and malignancies.3 In this case report, we present a patient with lymphoma associated-cerebral glucose hypometabolism (LA-CGHM) who achieved reversal of neuropsychiatric symptoms with the administration of glucose.
Case
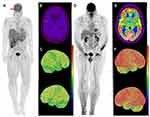
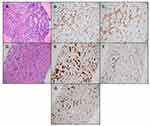
The patient is a 53-year-old male who was transferred to our institution for further diagnostic workup and management. Upon presentation, he reported persistent high fevers, chills, night sweats, and loss of 40 lbs. Imaging revealed splenomegaly and hepatomegaly. Bone marrow biopsy findings suggested dysplasia. The most striking clinical findings at our institution included chronically ill appearance with emaciation, flat affect, psychomotor slowing with poor interaction, and mental apathy. Cranial nerves were intact, normal deep tendon reflexes and no motor or sensory deficits. His wife reported he had been experiencing headache, confusion, personality changes with very slow responsiveness to questions, limited speech with few words, and visual hallucinations for about two months. Two months before presenting to our hospital, he had an acute abdomen with imaging scans showing rupture of enlarged spleen requiring splenectomy and evacuation of a hematoma. The outside pathology report did not indicate any significant findings in the spleen. In the setting of a fasting glucose of 96 mg/dL, 18F-fluorodeoxyglucose (18F-FDG) PET-CT was performed and revealed diffuse cerebral FDG hypometabolism without asymmetry or focality (Figure 1A) in addition to focal hypermetabolism in hepatic segment IVb and inferior right hepatic lobe (Figure 1A) with SUV-max of 5.7. Reference SUV-max values of the frontal lobe, parieto-occipital region, and cerebellum of the patient were 3.7, 4.0, and 3.4, respectively, compared to age-appropriate normal literature values of 9.72±1.97, 10.7±2.28, 6.80±1.21.4 Magnetic resonance imaging scan (MRI) of the brain was unremarkable excluding local lymphomatous involvement as the source of global hypometabolism. Bone marrow aspiration and biopsy and transjugular liver biopsy was performed with both specimens showing findings consistent with hepatosplenic T-cell lymphoma (Figure 2A–G). He was initiated on treatment with hyper-CVAD (cyclophosphamide, vincristine, doxorubicin, and dexamethasone). One day after initiation of chemotherapy, he was started on continuous infusion of NaCl (NS) 0.9% with 10% dextrose (D10) at 75 mL/hr with sliding scale insulin. Patient was normoglycemic at the start of dextrose infusion. His neuropsychiatric status improved dramatically the next day, and he became completely oriented, fully alert, headache-free, verbally responsive, and interactive. The D10 NS infusion was continued for a total of three days. After completing cycle 1 of hyper-CVAD, fevers and profuse sweating subsided, and he was discharged from the hospital. However, two weeks after discharge, his symptoms returned with low energy levels, altered mental status and hallucinations, despite normal fasting glucose of 95 mg/dL. The patient was readmitted and given NS 0.9% with D10 infusion again and was transitioned to maintenance with 4 grams glucose tablets every 6 hours. His neuropsychiatric symptoms responded again rapidly and did not relapse while on oral glucose maintenance. For further lymphoma treatment, he was treated with etoposide and high-dose Ara-C. Unfortunately, his lymphoma progressed again soon after the treatment. The patient decided to enroll into hospice where he passed away.
Discussion
CGHM is characterized by diffuse or focal reduction in uptake of glucose by the brain as determined on FDG PET-CT. FDG PET-CT scans are widely used for the evaluation of malignancies and its mechanism is based on the Warburg effect in which cancer cells use aerobic glycolysis as the predominant source of energy, rather than the more efficient oxidative phosphorylation.5 Human brain cells are obligate glucose metabolizers with the majority of cerebral glucose utilization occurring via oxidative metabolism. FDG becomes phosphorylated by hexokinase as the first step in the glycolytic pathway but then becomes trapped with no further metabolism possible. GLUT3, which is active in neurons, is independent of insulin activation and is a high-velocity hexose transporter, resulting in high FDG uptake in normal brain.6 PET scans typically show high physiologic metabolic activity in the brain as glucose is the main metabolic substrate of the brain accounting for 20% of the total-body glucose metabolism.2 CGHM has been reported and investigated in patients with lymphomas. In patients with non-Hodgkin’s lymphoma, it has been reported the global FDG uptake of the brain decreases as the total glycolytic volume (TGV) of the body increases resulting in a negative correlation between brain standardized uptake value (SUV) and TGV.7 In a retrospective study evaluating regional glucose metabolism of 30 lymphoma patients, pre-treatment lymphoma patients were found to have hypometabolism on 18F-FDG PET in the parieto-occipital region of the brain compared to normal controls. After chemotherapy, the cerebral glucose metabolism improved in bilateral frontal and parietal cortices in patients who achieved good therapeutic response.8 In another retrospective study by Adams et al, authors reported R-CHOP-treated DLBCL patients with low pretreatment glucose metabolism, measured by FDG-PET, had worse progression free survival and overall survival, however, after multivariate Cox regression, low pretreatment glucose metabolism was not an independent predictor of survival.9 This may have been attributed to small sample size. The pre- and post-R-CHOP therapy brain FDG-PET metabolism did not change with treatment which suggests R-CHOP does not impact brain glucose metabolism.
The symptoms of CGHM in patients with malignancy has not been well described in the literature and neuropsychiatric symptoms, as seen in our patient, may be inaccurately attributed to other etiologies such as a primary mood disorder or therapy side effects. Cerebral metabolism and symptomology have been investigated in neuropsychiatric disorders. In patients with Alzheimer’s disease, when compared to age-matched healthy controls, Alzheimer’s disease patients show regional metabolic reductions involving the parieto-temporal and posterior cingulate cortices.3 The FDG PET extent and topography correlates with symptom severity including cognition deficits and disturbance of consciousness.10 In patients with depression, FDG PET studies have shown decreased cerebral metabolism in the frontal lobe regimens. In our patient presented here, his diffuse cerebral hypometabolism caused multiple neuropsychiatric effects like patients with depression and Alzheimer’s disease. The clinician treating lymphomas should be aware of these neuropsychiatric symptoms and correlate to FDG PET as part of staging or treatment assessment. While the FDG PET imaging can be used to assess for the presence of CGHM, the management strategy of LA-CGHM has not been reported and remains unclear. Knowing the mechanism of CGHM in lymphoma patients could provide some insight into management strategies. The mechanism of LA-CGHM is not definitively known but has been hypothesized to be related to undiagnosed intravascular involvement of lymphoma causing occlusion of arterioles, capillaries and venules,8 paraneoplastic syndrome,11 or potentially shunting of glucose to rapidly dividing aggressive malignancies. Retrospective analyses have shown mixed conclusions with chemotherapy improving LA-CGHM from an imaging perspective, but these studies do not mention the presence or improvement of neuropsychiatric symptoms.8,9 In our patient, aggressive glucose administration improved his symptoms which suggests cerebral hypometabolism may be overcome with saturation. While we do not have a correlative FDG PET to show improved cerebral metabolism, his neuropsychiatric symptoms dramatically improved on two separate occasions and did not recur with continuous glucose supplementation.
In conclusion, CGHM in lymphoma patients can present with neuropsychiatric manifestations that may be inaccurately attributed to other etiologies such as primary mood disorder or therapy side effects. Aggressive glucose supplementation should be tried to reverse CGHM even if the underlying lymphoma is not responsive to treatment. More research is necessary to elucidate this cerebral metabolic complication of lymphoma.
Ethics Approval
Institutional approval was not required to publish the case details.
Consent
Patient consent was obtained for publication including images.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
There is no funding to report.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Cheson BD, Fisher RI, Barrington SF, et al. Recommendations for initial evaluation, staging, and response assessment of Hodgkin and non-Hodgkin lymphoma: the Lugano classification. J Clin Oncol. 2014;32:3059–3068.
2. Berti V, Mosconi L, Pupi A. Brain: normal variations and benign findings in fluorodeoxyglucose-PET/computed tomography imaging. PET Clin. 2014;9:129–140.
3. Mosconi L, Pupi A, De Leon MJ. Brain glucose hypometabolism and oxidative stress in preclinical Alzheimer’s disease. Ann N Y Acad Sci. 2008;1147:180–195.
4. Pourhassan Shamchi S, Khosravi M, Taghvaei R, et al. Normal patterns of regional brain (18) F-FDGuptake in normal aging. Hell J Nucl Med. 2018;21:175–180.
5. Liberti MV, Locasale JW. The Warburg Effect: how Does it Benefit Cancer Cells? Trends Biochem Sci. 2016;41:211–218.
6. Parent EE, Johnson DR, Gleason T, Villanueva-Meyer JE. Neuro-Oncology Practice Clinical Debate: FDG PET to differentiate glioblastoma recurrence from treatment-related changes. Neurooncol Pract. 2021;8:518–525.
7. Hanaoka K, Hosono M, Shimono T, et al. Decreased brain FDG uptake in patients with extensive non-Hodgkin’s lymphoma lesions. Ann Nucl Med. 2010;24:707–711.
8. Nonokuma M, Kuwabara Y, Takano K, Tamura K, Ishitsuka K, Yoshimitsu K. Evaluation of regional cerebral glucose metabolism in patients with malignant lymphoma of the body using statistical image analysis. Ann Nucl Med. 2014;28:950–960.
9. Adams HJA, de Klerk JMH, Fijnheer R, et al. Brain glucose metabolism in diffuse large B-cell lymphoma patients as assessed with FDG-PET: impact on outcome and chemotherapy effects. Acta Radiologica. 2015;57:733–741.
10. Mosconi L. Brain glucose metabolism in the early and specific diagnosis of Alzheimer’s disease. FDG-PET studies in MCI and AD. Eur J Nucl Med Mol Imaging. 2005;32:486–510.
11. Voltz R. Paraneoplastic neurological syndromes: an update on diagnosis, pathogenesis, and therapy. Lancet Neurol. 2002;1:294–305.
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.