Back to Journals » Infection and Drug Resistance » Volume 16
Neuropsychiatric Adverse Events Following Antiretroviral Therapy in People Living with HIV: A Real-World Study of Dynamic Trends and Risk Factors in Hangzhou, China
Authors Zhang W, Wang Y , Li E, Yan D, Yu J, Zhu M, Shi J , Zheng L
Received 31 May 2023
Accepted for publication 22 July 2023
Published 2 August 2023 Volume 2023:16 Pages 5007—5019
DOI https://doi.org/10.2147/IDR.S419308
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Prof. Dr. Héctor Mora-Montes
Wenhui Zhang,1,2,* Yi Wang,2,3,* Er Li,1 Dingyan Yan,1,2 Jianhua Yu,2 Mingli Zhu,4 Jinchuan Shi,2,* Liping Zheng1,*
1Department of Nursing, Affiliated Hangzhou Xixi Hospital, Zhejiang University School of Medicine, Hangzhou, People’s Republic of China; 2Department of Infection, Affiliated Hangzhou Xixi Hospital, Zhejiang University School of Medicine, Hangzhou, People’s Republic of China; 3Institute of Hepatology and Epidemiology, Affiliated Hangzhou Xixi Hospital, Zhejiang University School of Medicine, Hangzhou, People’s Republic of China; 4Medical Laboratory, Affiliated Hangzhou Xixi Hospital, Zhejiang University School of Medicine, Hangzhou, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Jinchuan Shi, Department of Infection, Affiliated Hangzhou Xixi Hospital, Zhejiang University School of Medicine, No. 2 Hengbu Street, Liuxia Town, Xihu District, Hangzhou, Zhejiang, People’s Republic of China, Email [email protected] Liping Zheng, Department of Nursing, Affiliated Hangzhou Xixi Hospital, Zhejiang University School of Medicine, No. 2 Hengbu Street, Liuxia Town, Xihu District, Hangzhou, Zhejiang, People’s Republic of China, Email [email protected]
Purpose: Neuropsychiatric adverse events (NPAEs) occur frequently in people living with human immunodeficiency virus (PLWH) receiving antiretroviral therapy (ART). This study aimed to assess the dynamic trends and risk factors of NPAEs among PLWH in Hangzhou taking efavirenz (EFV)- or dolutegravir (DTG)- or elvitegravir (EVG)-based regimens.
Patients and Methods: A total of 287 ART-naive PLWH were included in this study, EFV (400mg)- (n = 122), EFV (600mg)- (n = 37), DTG- (n = 73), EVG-based (n = 47) and other ART regimens (n = 8) as the initial ART regimen were administered for 12 months. All data were collected at five time points within a 12-month follow-up. The Pittsburgh Sleep Quality Index and Hospital Anxiety and Depression Scale were used to evaluate sleep disorders and anxiety and depression symptoms, respectively. The dynamic trends and potential risk factors of NPAEs were investigated using a generalized linear mixed model.
Results: Mean age was 29.4 (SD: 7.5) years with 97.2% males. After 12 months of ART, the prevalence of sleep disorders and anxiety decreased significantly, although only a slight improvement was observed for depression. In addition, there was a significant positive correlation between sleep disorders, anxiety, and depression. The risk factors for NPAEs differed slightly depending on the choice of ART regimen, but the seven factors most commonly associated with NPAEs were age, sex, marital status, education level, smoking status, body mass index, and WHO clinical stage. Treatment-induced changes in CD4-positive T-cell count and virological suppression did not depend on the particular choice of ART regimen.
Conclusion: The prevalence of sleep disorders and anxiety changed significantly over time on ART and the risks of these disorders were associated with seven common clinical and demographic factors.
Keywords: sleep disorders, anxiety, depression, antiretroviral therapy, people living with HIV
Introduction
Acquired immunodeficiency syndrome, the disease caused by human immunodeficiency virus (HIV), is a great threat to public health.1 People living with HIV (PLWH) face persistent worldwide discrimination that increases the risk for psychiatric disorders including sleep disorders, anxiety, depression, and others.2,3 Previous study reported that the neuropsychiatric problems such as sleep disorders, anxiety, and depression are prevalent among PLWH worldwide.4 Such as, a meta-analysis in China among PLWH estimated the prevalence of having depression was more than 50%,5 which was up to seven-fold higher than the general population.6 These neuropsychiatric problems are usually related to a wide range of negative outcomes such as decreased quality of life, poor psychosocial functioning, low patient adherence or even suicidal ideation.7
Currently, antiretroviral therapy (ART) is the most effective way to block viral replication and improve immune status, but this therapy does not relieve the burden of psychiatric disorders experienced by PLWH. Previous study revealed one of the main causes of neuropsychiatric problems in PLWH is side effects of ART.8 Neuropsychiatric adverse events (NPAEs) are the primary reason for treatment discontinuation, and the development of suitable personalized treatments for PLWH requires a deeper understanding of the symptoms and risk factors of NPAEs following ART.9
Antiviral drugs and treatment regimens are under constant and ongoing development that varies by country. Efavirenz (EFV)-based ART is a former worldwide first-line treatment that is still commonly used in some resource-limited settings.10 Although EFV is highly efficacious in reducing HIV viral loads, its clinical use is reported to be associated with NPAEs, including confusion, dizziness, nightmares, sleep disturbances, anxiety, and depression.3 So far, EFV-associated NPAEs have been reported in Africa,11–13 Europe,14 and other locations.15–18 The integrase strand-transfer inhibitors raltegravir, elvitegravir (EVG), dolutegravir (DTG), and bictegravir are approved by the US Food and Drug Administration for treatment-naive individuals initiating ART.19 Adoption of DTG-based ART is increasing in European countries and economically developed areas,20,21 and the risks of DTG-associated NPAEs including anxiety, depression and other symptoms have been studied.20–22 In China, ART commonly involves three-drug regimens with either EFV or DTG plus two nucleoside reverse transcriptase inhibitors (for example, tenofovir disoproxil fumarate and lamivudine),23 or EVG with tenofovir alafenamide and emtricitabine.24 Data on NPAEs has come primarily from studies with EFV-based regimens,18,25 and there is a lack of clinical studies on DTG or EVG-associated NPAEs in China.
In the study, we have addressed 2 vital issues. First, we report a real-world study of ART regimens-associated dynamic trends of NPAEs in PLWH in China based on 12-month follow-up. Secondly, the risk factors of three different ART (DTG or EVG or EFV)-associated NPAEs were identified. Our study contributes to proper treatment decision-making for PLWH by characterizing the efficacy of different ART regimens and further enriching symptom management knowledge for PLWH in China.
Materials and Methods
Study Populations
In this real-world, observational cohort study, we recruited 320 PLWH who initially received standardized ART treatment from 1 January 2021, to 31 December 2022, at Xixi Hospital in Hangzhou, the largest AIDS designated hospital in Zhejiang Province. Registered PLWH in our hospital mainly contain factory workers, university students and community population. The sample size was calculated by using the univariate repeat sampling method through PASS15 online software. The inclusion criteria were as follows: (1) a positive diagnosis of HIV confirmed by Western blot, (2) age ≥ 18 years, (3) no pregnancy in the previous 3 months, (4) willingness to complete the Pittsburgh Sleep Quality Index (PSQI) and Hospital Anxiety and Depression Scale (HADS) scales with regular follow-ups, and (5) signed written informed consent. Exclusion criteria were as follows: (1) any condition that prohibits completion of the study questionnaires, (2) diagnosis with an ongoing psychiatric illness or have a history of psychiatric illness, and (3) pharmacological treatment for psychiatric problems. Collected baseline data included demographic characteristics (age, sex, marital status, education level, body mass index (BMI), HIV transmission route, smoking status, drinking status, and mental health status before ART) and disease characteristics (baseline HIV-RNA, baseline CD4-positive T-cell count (CD4-count), WHO clinical stage, duration between HIV diagnosis and initiation of ART, syphilis status, and hepatitis B status) by the Electronics Medical Records (EMR) management system.
Study Procedures
All participants were followed for 12 months after beginning treatment by online questionnaires. They were assessed at baseline (M0) and at 1 month (M1), 3 months (M3), 6 months (M6), and 12 months (M12) for NPAEs after initiating ART with various regimens. At each time point, sleep disorders were evaluated using the PSQI, whereas anxiety and depression symptoms were assessed using the HADS. The data obtained from the questionnaire were captured, summarized and analysed by two case managers. During the follow-up, 33 PLWH were excluded for incomplete follow-ups (n = 15) and data (n = 18), leaving 287 PLWH for inclusion in the study (Figure 1).
Operational Definitions
BMI: WHO defines overweight as a BMI of 25 or higher and obesity as a BMI of 30 or higher. Previous study have verified the BMI cutoff values of 24 for overweight and 28 for obesity among Chinese adults.26 If your BMI is less than 18.5, it falls within the underweight range. If your BMI is 18.5 to < 24, it falls within the healthy weight range. If your BMI is 24 to < 28, it falls within the overweight range. If your BMI is 28 or higher, it falls within the obesity range.
WHO clinical stage: It is one of the indicators of patient status and classified as I, II, III and IV depending on some clinical parameters. The World Health Organization (WHO) classifies HIV into four stages.27 In this study, we have classified as I or II or III or IV. Clinical stage I: 3 clinical manifestations (asymptomatic infection, persistent generalized lymphadenopathy and acute retroviral infection); Clinical stage II: 4 clinical manifestations (unintentional weight loss of more than 10% body weight, minor mucocutaneous manifestations, herpes zoster within previous 5 years and recurrent upper respiratory tract infections); Clinical stage III: 8 clinical manifestations (unintentional weight loss of more than 10% body weight, chronic diarrhea for longer than 1 month, prolonged fever for longer than 1 month (constant or intermittent), oral candidiasis, oral hairy leukoplakia, pulmonary tuberculosis within the previous year, severe bacterial infections, vulvovaginal candidiasis); Clinical stage IV: 17 clinical manifestations (HIV wasting syndrome, pneumocystis carinii (juvenii) pneumonia (PCP)), toxoplasmosis of the brain, crytosporidiosis with diarrhea for longer than 1 month, isosporiasis with diarrhea for longer than 1 month, cryptococcosis, extrapulmonary, cytomegalovirus disease of an organ other than liver or spleen or lymph node, herpes simplex virus infection, mucocutaneous, progressive multifocal leukoencephalopathy, any disseminated endemic mycosis (eg histoplasmosis), candidiasis of the esophagus, trachea, bronchi, or lung, atypical mycobacteriosis, disseminated, non-typhoid Salmonella septicemia, extrapulmonary tuberculosis, lymphoma, kaposi’s sarcoma, HIV encephalopathy).
Evaluation of Sleep Quality
Sleep quality was assessed using the PSQI; the assessment has 19 items across 7 component dimensions (subjective sleep quality, sleep latency, sleep duration, habitual sleep efficiency, sleep disturbances, use of sleeping medications, and daytime dysfunction).28 Each component score ranges from 0–3 and the global score is the total of the seven component scores (range: 0–21).29 The global score reflects the severity of sleep disorders: 0–5, none; 6–10, mild; 11–15, moderate; and 16–21, severe.30 The Cronbach’s alpha coefficient of this scale for PLWH in China was 0.78.31
Evaluation of Anxiety and Depression
Anxiety and depression were assessed using the HADS;32 HADS-A consists of seven items assessing anxiety symptoms and HADS-D consists of seven items evaluating depressive symptoms.33,34 Each item is scored on a 4-point Likert scale (0–3) and total scores range from 0–21.35 For HADS-A and HADS-D, the following score reflects the severity of anxiety or depression: 0–7, none; 8–10, mild; 11–14, moderate; and 15–21, severe.36 The Cronbach’s alpha coefficient for this scale among PLWH in China was 0.904, with 0.869 for the anxiety subscale and 0.807 for the depression subscale.37
Exposure and Outcome Variables
The exposure variables include age, sex, transmission route, marital status, education level, smoking, BMI, WHO clinical stage, baseline HIV-RNA (copies/mL). The outcome variables consist of PSQI, HADS-A and HADS-D scores. A PSQI score > 5 yielded a diagnosis in distinguishing good and poor sleep quality. The higher score indicates the worse sleep quality. The patients with HADS-A or HADS-D scores of > 7 points were regarded as being just suggestive of the presence of anxiety or depression, and higher scores indicate worse severity of anxiety or depression.
Statistical Analysis
All statistical analysis was performed using R statistical software (v4.1.3) and IBM SPSS Statistics (v25.0). Normally distributed continuous variables are presented as means with standard deviations. Non-normal variables are described as medians with interquartile ranges (1st quartile-3rd quartile). For categorical variables, we reported the numbers and percentages of patients in each category and proportions were compared using the Pearson’s chi-squared test. To correct for multiple comparisons, we used the Friedman test followed by a post hoc two-tailed Bonferroni test. We used a nonparametric Spearman rank correlation to test for associations between sleep disorders, anxiety, depression, and demographic characteristics. MedCalc software (v18.2.1) was used to determine the cut-off values, sensitivity, and specificity of patient age in predicting the status of sleep disorders, anxiety, and depression among PLWH. The prevalence of sleep disorders, anxiety and depression over time were evaluated in the longitudinal analysis using a generalized estimating equation (GEE). A generalized linear mixed model (GLMM) was used to investigate the potential factors associated with sleep disorders, anxiety, and depression scores among PLWH under different ART regimens. A P-value < 0.05 was considered to be statistically significant.
Results
Baseline Characteristics of the Study Population
A total of 287 PLWH were enrolled in this study to evaluate ART treatment-associated dynamic trends and risk factors for NPAEs (Figure 1). Among them, the mean age was more than 29 years (SD: 7.5), 97.2% were male, and the mean BMI was 22.1 (SD: 3.2). At baseline (M0), the median scores of sleep disorders, anxiety, and depression were 5 (IQR: 4–7), 6 (IQR: 4–9) and 5 (IQR: 2–8), respectively. The ART regimens consist of EFV 400 mg (n = 122), EFV 600 mg (n = 37), DTG (n = 73), EVG (n = 47) and others (n = 8). The other clinical characteristics, complications, and laboratory indices of PLWH are shown in Table 1.
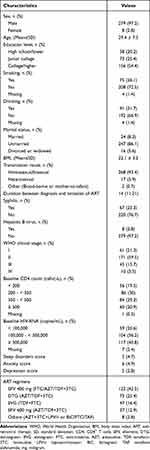 |
Table 1 Baseline Characteristics of All Participants (n = 287) |
Except for the evaluation the general dynamic trend of ART treatment-associated NPAEs and risk factors among 287 PLWH, we also compared the influence of three different ART regimens on the prevalence of NPAEs and their risk factors. Among 287 PLWH, except for 6 PLWH with ART discontinuation, 80 PLWH (including 32 EFV 400 mg, 23 EFV 600 mg and 25 DTG) experienced the adjustment of treatment due to medication convenience and financial problems or failure of antiviral therapy (Table S1). Considering the adjustment of treatment may affect the outcomes of NPAEs, 80 PLWH with adjustment of treatment were excluded in the comparison of different ART regimens induced-NPAEs and their risk factors. In addition, 19 PLWH continuously maintaining the original regimens (including 11 EFV 600 mg and 8 others), were also excluded in the subgroup analysis, due to small sample size lacked of statistical significance (Table S1). Thus, a total of 182 PLWH (EFV 400 mg- (EFV-) (n = 85), DTG- (n = 50) and EVG-based regimens (n = 47)) were included in the subgroup analysis about different ART regimens induced-NPAEs and their risk factors.
Dynamic Changes in the Prevalence and Severity of Sleep Disorders, Anxiety, and Depression Among 287 PLWH Following ART
Spearman correlation analysis revealed a weak correlation between PSQI (r = −0.086, P = 0.001) and HADS-A scores (r = −0.099, P = 0.0001) and time on ART, whereas the HADS-D score was not correlated (Table 2). The Friedman test revealed a decreasing trend over time (from M0 to M12) in scores on sleep disorders (P = 0.003, Figure 2 and Table 2) and anxiety (P = 0.0001, Figure 2 and Table 2). GEE analysis also showed that the prevalence of sleep disorder significantly decreased from M0 to M6 (P < 0.05) and M12 (P < 0.05) while the prevalence of anxiety had also decreased significantly by M1 and M3 (Figure 2 and Table 3). There was no obvious change in the prevalence of depression from M0 to M12 (Table 3).
 |
Table 2 The Median Scores of Sleep Disorders, Anxiety and Depression at M0, M1, M3, M6 and M12 After ART Among PLWH |
 |
Table 3 The Prevalence of Sleep Disorders, Anxiety and Depression Over Time After ART Among PLWH |
Correlational Between Sleep Quality, Anxiety, and Depression Among 287 PLWH Following ART
Spearman correlation analysis of all time points revealed a positive correlation between sleep disorders, anxiety, and depression among 287 PLWH (P < 0.01, Table 4). The correlation between anxiety and depression symptoms was greater than that between sleep disorders and anxiety and between sleep disorders and depressive symptoms. The correlation between sleep disorders and depression symptoms was weaker than the correlation between sleep disorders and anxiety symptoms (Table 4).
 |
Table 4 Correlational Relationship Between Sleep Quality, Anxiety and Depression Scores at M0-M12 After ART |
Influence of Different ART Regimens on the Prevalence and Severity of Sleep Disorders, Anxiety, and Depression
Then, we further compared the different ART regimens induced-NPAEs. The baseline characteristics of 182 PLWH EFV 400 mg- (EFV-) (n = 85), DTG- (n = 50) and EVG-based regimens (n = 47) are summarized in Table S2. There were no obvious differences in characteristics among EFV, DTG and EVG groups at baseline, except for age, marital status, WHO clinical stage and baseline CD4 count (Table S2).
After controlling for the demographic variables of time, age, marital status, and WHO clinical stage, we compared scores for sleep disorders, anxiety, and depression across different ART regimens among PLWH. Participants taking EFV- (P = 0.009 and 0.016) and DTG-based regimens (P = 0.038 and 0.004) scored significantly lower on PSQI (Figure 3a and Table S3) and HADS-A (Figure 3b and Table S3) than those taking EVG-based regimens, while there was no significant difference between the DTG and EFV groups. The HADS-D scores of the DTG group were significantly lower than those of the EVG group (P = 0.004), but the DTG group was comparable to those of the EFV group (P > 0.05, Figure 3c and Table S3).
Risk Factors for Developing Sleep Disorders After One Year on ART
Then, we focused on identification of factors associated with sleep disorders (PSQI > 5), anxiety (HADS-A > 7) and depression (HADS-D > 7) at M12, respectively. We calculated the cut-off value of age in predicting the outcome of sleep disorders, anxiety and depression scores. As shown in Table S4, the optimal cut-off values of age were all > 27 years.
A GLMM analysis of 287 PLWH revealed that the following eight variables were independent risk factors associated with sleep disorders (PSQI > 5) at the M12 time point: age, sex, transmission route, marital status, education level, smoking status, BMI, and WHO clinical stage (see Table 5).
 |
Table 5 Risk Factors for Developing Sleep Disorders, Anxiety, and Depression After One Year on ART (n = 287) |
Then, the risk factors for developing sleep disorders of PLWH with three different ART regimens were analyzed, respectively. Among participants taking EFV-based regimens (n = 85), the risk factors associated with sleep disorders were as follows: female sex (P < 0.001), divorced/widowed (P = 0.015) or unmarried status (P = 0.004), BMI of 18.5 - < 24 kg/m2 (P = 0.007) and 24 - < 28 kg/m2 (P < 0.001), WHO clinical stage II (P = 0.001) and III (P = 0.002), and baseline HIV-RNA level of 100,000 - < 500,000 copies/mL (P = 0.01) and ≥ 500,000 copies/mL (P = 0.018). Among participants taking DTG-based regimens (n = 50), the risk factors associated with sleep disorders were as follows: unmarried status (P = 0.004), a high school education or lower (P < 0.001), BMI of 24 - < 28 kg/m2 (P = 0.001) and ≥ 28 kg/m2 (P = 0.008), and WHO clinical stage IV (P < 0.001). Among participants taking EVG-based regimens (n = 47), the risk factors associated with sleep disorders were as follows: age > 27 years (P = 0.017), unmarried status (P = 0.027), and a baseline HIV-RNA level of 100,000 - < 500,000 copies/mL (P = 0.022) and ≥ 500,000 copies/mL (P = 0.026) (Table S5).
Risk Factors for Developing Anxiety After One Year on ART
A GLMM analysis was performed to assess the risk factors associated with anxiety (HADS-A > 7) among 287 PLWH at M12. The following risk factors for anxiety were slightly different from those for sleep disorders: age, sex, marital status, education level, smoking status, BMI, WHO clinical stage, and baseline HIV-RNA count were all associated with anxiety (Table 5).
Among participants taking EFV-based regimens (n = 85), the risk factors associated with anxiety were as follows: female sex (P = 0.013), heterosexual transmission route (P = 0.016), unmarried status (P < 0.001), and BMI of 18.5 - < 24 kg/m2 (P = 0.014) and 24 - < 28 kg/m2 (P = 0.001). Among participants taking DTG-based regimens (n = 50), the risk factors associated with anxiety were as follows: age > 27 years (P = 0.002), unmarried status (P = 0.002), a high school education or lower (P < 0.001), smoking status (P = 0.015), and WHO clinical stage II (P = 0.024) and III (P = 0.004). Among participants taking EVG-based regimens (n = 47), the risk factors associated with anxiety were as follows: age > 27 years (P = 0.002) and divorced/widowed (P = 0.042) or unmarried status (P < 0.001) (Table S6).
Risk Factors for Developing Depression After One Year on ART
In addition, age, sex, marital status, education level, smoking status, BMI, WHO clinical stage, and baseline HIV-RNA count were the risk factors associated with depression (HADS-D > 7) among 287 PLWH at M12 by a GLMM analysis (Table 5).
Among participants taking EFV-based regimens (n = 85), the risk factors associated with depression were as follows: heterosexual transmission route (P = 0.002) and baseline HIV-RNA level ≥ 500,000 copies/mL (P = 0.024). Among participants taking DTG-based regimens (n = 50), the risk factors associated with depression were as follows: age > 27 years (P < 0.001), unmarried status (P = 0.045), a high school education or lower (P = 0.002), smoking status (P = 0.009), and WHO clinical stage III (P = 0.006). Among participants taking EVG-based regimens (n = 47), the risk factors associated with depression were as follows: age > 27 years (P = 0.013), female sex (P = 0.014), heterosexual transmission route (P = 0.029), unmarried status (P < 0.001), a college education or higher (P = 0.01), smoking status (P = 0.005), and baseline HIV-RNA level from 100,000 - < 500,000 copies/mL (P = 0.03) and ≥ 500,000 copies/mL (P = 0.019) (Table S7).
Discussion
This real-world cohort study evaluated the neuropsychiatric impact of different ART regimens among PLWH in China; we observed dynamic changes in sleep disorders, anxiety, and depression scores 12 months following the start of ART. Our results are consistent with previous studies showing that symptoms of sleep disorders, anxiety, and depression are common among PLWH.16,17,25 Consistent with a previous study, our analysis revealed a dynamic correlation among sleep disorders, anxiety, and depression symptoms over the 12-month treatment period.4 Our data also demonstrate a greater correlation between sleep disorders and anxiety symptoms than between sleep disorders and depression symptoms in PLWH, and this is consistent with a previous study among healthy individuals.38
Considering the disparate trends between sleep disorders, anxiety, and depression, we further identified risk factors associated with each condition. Previous studies have only considered these risk factors regarding EFV-based ART regimens.18 Here we complement those studies by also evaluating DTG- and EVG-based regimens and find that the risk factors for sleep disorders, anxiety, and depression differ between EFV, DTG, and EVG groups. Some key risk factors (age, sex, marital status, education level, smoking status, BMI, and WHO clinical stage) were jointly associated with the prevalence of sleep disorders, anxiety, and depression. These factors could be useful for identifying high-risk patients requiring more in-depth evaluation and psychiatric health support along with HIV care.
In a previous study, EFV-based regimens led to improved neurological performance in PLWH,18 and our study replicates this finding. In addition, we found differences among the ART regimens regarding improvements in sleep disorders, anxiety, and depression after 12 months of treatment. DTG-based regimens produce greater neurological improvements than EVG-based regimens, although EFV-based regimens outperform EVG-based regimens regarding sleep disorders and anxiety but produce similar results for depression. Together, these data demonstrate a clinically meaningful improvement in neuropsychiatric symptoms (PSQI, HADS-A, and HADS-D scores) from a DTG-based 12-month ART regimen. Last but not least, similar to Hoffmann’ study,39 three was a higher rate of NAPEs among female PLWH initiating DTG-based ART regimen. Therefore, our results suggested that clinicians of contagion section should preferentially pay more attention to neuropsychiatric health among female PLWH.
This study comprehensively evaluated the dynamic trends and risk factors of NPAEs among PLWH taking EFV-, DTG- or EVG-based ART regimens in Hangzhou. Our findings suggest that PLWH should be regularly screened for sleep disorders, depression and anxiety, including assessments of the severity of these symptoms, and those with sleep disorders or depression or anxiety should adapt comprehensive clinical intervention measures to reduce those symptoms and effectively improve their quality of life.
There were several limitations to this study. The study included no healthy control subjects and thus we cannot separate the effects of HIV itself from the ART regimen. In addition, representative coverage of PLWH is relatively limited because study participants were recruited from a single center and included primarily men with a homosexual or bisexual transmission route. Lastly, we did not evaluate the relation between financial burden and psychological disorders for PLWH, since the financial/income status information of PLWH is absent. Future studies with enhanced coverage (including multiple centers and more female participants) and long-term follow-up would make analysis more accurate and generalizable.
Conclusions
In this study, we observed NPAEs (sleep disorders, anxiety, and depression) over time following the initiation of ART in PLWH. The prevalence of sleep disorders and anxiety improved significantly over time on ART. The data regarding DTG, the preferred drug under current clinical guidelines, are particularly notable. The risk factors of age, sex, marital status, education level, smoking, BMI, and WHO clinical stage were jointly associated with the prevalence of sleep disorders, anxiety, and depression among participants. In real-world applications of EFV-, DTG-, and EVG-based therapies, these findings can provide future guidance for mental health treatment for PLWH.
Data Sharing Statement
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Ethical Approval
This research was reviewed and approved by the Clinical Research Ethics Committee of the Hangzhou Xixi Hospital (No. 2022-Research ethics-60) in accordance with the tenets of the Declaration of Helsinki. This study was registered at www.medicalresearch.org.cn (MR-33-22-017948). All donors provided written informed consent. All samples were anonymised before use.
Acknowledgments
We would like to appreciate all patients who participated in this study.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
This research was supported by grants from the Infectious Diseases of Hangzhou Medical Key Subject, Hangzhou Biomedicine and Health Industry Development Project (2022WJCY174), Medical Science and Technology Project of Zhejiang Province (2023KY196 and 2023KY978), Guidance Programs of Hangzhou Bureau of Science and Technology (20211231Y050) and Medical Science and Technology Project of Hangzhou (A20210014).
Disclosure
Regarding the publication of this paper, the authors declare that they have no conflicts of interest.
References
1. Mallewa J, Szubert AJ, Mugyenyi P, et al. Effect of ready-to-use supplementary food on mortality in severely immunocompromised HIV-infected individuals in Africa initiating antiretroviral therapy (REALITY): an open-label, parallel-group, randomised controlled trial. Lancet HIV. 2018;5(5):e231–e240. doi:10.1016/S2352-3018(18)30038-9
2. Shadloo B, Amin-Esmaeili M, Motevalian A, et al. Psychiatric disorders among people living with HIV/AIDS in Iran: prevalence, severity, service utilization and unmet mental health needs. J Psychosom Res. 2018;110:24–31. doi:10.1016/j.jpsychores.2018.04.012
3. Dalwadi DA, Ozuna L, Harvey BH, et al. Adverse neuropsychiatric events and recreational use of efavirenz and other HIV-1 antiretroviral drugs. Pharmacol Rev. 2018;70(3):684–711. doi:10.1124/pr.117.013706
4. Wang N, Wang M, Xin X, et al. Exploring the relationship between anxiety, depression, and sleep disturbance among HIV patients in china from a network perspective. Front Psychiatry. 2021;12:764246. doi:10.3389/fpsyt.2021.764246
5. Wang T, Fu H, Kaminga AC, et al. Prevalence of depression or depressive symptoms among people living with HIV/AIDS in China: a systematic review and meta-analysis. BMC Psychiatry. 2018;18(1):160. doi:10.1186/s12888-018-1741-8
6. Yuan Q, Li F, Ruan Y, et al. Meta-analysis of the prevalence of depression among Chinese HIV/AIDS patients. Chin J AIDS STD. 2021;27(1):45–49.
7. Cai S, Liu L, Wu X, et al. Depression, anxiety, psychological symptoms and health-related quality of life in people living with HIV. Patient Prefer Adherence. 2020;14:1533–1540. doi:10.2147/PPA.S263007
8. Heaton RK, Franklin DR, Deutsch R, et al. Neurocognitive change in the era of HIV combination antiretroviral therapy: the longitudinal CHARTER study. Clin Infect Dis. 2015;60(3):473–480. doi:10.1093/cid/ciu862
9. Lapadula G, Bernasconi DP, Bai F, et al. Switching from efavirenz to rilpivirine improves sleep quality and self-perceived cognition but has no impact on neurocognitive performances. AIDS. 2020;34(1):53–61. doi:10.1097/QAD.0000000000002377
10. Phongsamart W, Jantarabenjakul W, Chantaratin S, et al. Switching efavirenz to rilpivirine in virologically suppressed adolescents with HIV: a multi-centre 48-week efficacy and safety study in Thailand. J Int AIDS Soc. 2022;25(1):e25862. doi:10.1002/jia2.25862
11. Mugusi S, Ngaimisi E, Janabi M, et al. Neuropsychiatric manifestations among HIV-1 infected African patients receiving efavirenz-based cART with or without tuberculosis treatment containing rifampicin. Eur J Clin Pharmacol. 2018;74(11):1405–1415. doi:10.1007/s00228-018-2499-0
12. Masimirembwa C, Dandara C, Leutscher PD. Rolling out efavirenz for HIV precision medicine in Africa: are we ready for pharmacovigilance and tackling neuropsychiatric adverse effects? OMICS. 2016;20(10):575–580. doi:10.1089/omi.2016.0120
13. Abah IO, Akanbi M, Abah ME, et al. Incidence and predictors of adverse drug events in an African cohort of HIV-infected adults treated with efavirenz. Germs. 2015;5(3):83–91. doi:10.11599/germs.2015.1075
14. Law JKC, Butler LT, Hamill MM. Predictors of discontinuation of efavirenz as treatment for HIV, due to neuropsychiatric side effects, in a multi-ethnic sample in the United Kingdom. AIDS Res Hum Retroviruses. 2020;36(6):459–466. doi:10.1089/aid.2019.0193
15. Xu L, Peng W, Song X, et al. Correction to: pharmacodynamics of efavirenz 400 mg in treatment-naïve Chinese HIV-infected patients in a prospective cohort study. BMC Infect Dis. 2021;21(1):185. doi:10.1186/s12879-021-05880-8
16. Fernández-Bargiela N, Rotea-Salvo S, Margusino-Framiñán L, et al. Discontinuation due to neuropsychiatric adverse events with efavirenz- and dolutegravir-based antiretroviral therapy: a comparative real-life study. Eur J Hosp Pharm. 2022;29(4):207–211. doi:10.1136/ejhpharm-2020-002374
17. Fahrni ML, Misran NFL, Abidin ZZ, et al. Clinical predictors of efavirenz-based regimen treatment durability: a two-year case-control study of antiretroviral-naïve patients. J Infect Public Health. 2023;16(1):96–103. doi:10.1016/j.jiph.2022.12.001
18. Hua W, Wang S, Wang X, et al. Neuropsychiatric adverse events during 12 months of treatment with efavirenz in treatment-naïve HIV-infected patients in china: a prospective cohort study. Front Psychiatry. 2021;12:579448. doi:10.3389/fpsyt.2021.579448
19. Scarsi KK, Havens JP, Podany AT, et al. HIV-1 integrase inhibitors: a comparative review of efficacy and safety. Drugs. 2020;80(16):1649–1676. doi:10.1007/s40265-020-01379-9
20. Chan P, Goh O, Kroon E, et al. Neuropsychiatric outcomes before and after switching to dolutegravir-based therapy in an acute HIV cohort. AIDS Res Ther. 2020;17(1):1. doi:10.1186/s12981-019-0257-8
21. Mwebaza J, Meya D, Musiime V, et al. Prevalence of neuropsychiatric adverse events and associated factors among adult patients on dolutegravir attending Mulago ISS clinic. HIV Med. 2023;24(4):491–501. doi:10.1111/hiv.13428
22. Yagura H, Watanabe D, Kushida H, et al. Impact of UGT1A1 gene polymorphisms on plasma dolutegravir trough concentrations and neuropsychiatric adverse events in Japanese individuals infected with HIV-1. BMC Infect Dis. 2017;17(1):622. doi:10.1186/s12879-017-2717-x
23. Zhong M, Li M, Qi M, et al. A retrospective clinical study of dolutegravir- versus efavirenz-based regimen in treatment-naïve patients with advanced HIV infection in Nanjing, China. Front Immunol. 2023;13:1033098. doi:10.3389/fimmu.2022.1033098
24. Chen GJ, Lee YL, Lee CH, et al. Impact of archived M184V/I mutation on the effectiveness of switch to co-formulated elvitegravir, cobicistat, emtricitabine and tenofovir alafenamide among virally suppressed people living with HIV. J Antimicrob Chemother. 2020;75(10):2986–2993. doi:10.1093/jac/dkaa287
25. Xiao J, Liu Y, Li B, et al. Anxiety, depression, and sleep disturbances among people on long-term efavirenz-based treatment for HIV: a cross-sectional study in Beijing, China. BMC Psychiatry. 2022;22(1):710. doi:10.1186/s12888-022-04366-4
26. Zhou BF; Cooperative Meta-Analysis Group of the Working Group on Obesity in China. Predictive values of body mass index and waist circumference for risk factors of certain related diseases in Chinese adults--study on optimal cut-off points of body mass index and waist circumference in Chinese adults. Biomed Environ Sci. 2002;15(1):83–96.
27. Weinberg JL, Kovarik CL. The WHO Clinical Staging System for HIV/AIDS. Virtual Mentor. 2010;12(3):202–206. doi:10.1001/virtualmentor.2010.12.3.cprl1-1003
28. Xi S, Gu Y, Guo H, et al. Sleep quality status, anxiety, and depression status of nurses in infectious disease department. Front Psychol. 2022;13:947948. doi:10.3389/fpsyg.2022.947948
29. Al Maqbali M. Sleep disturbance among frontline nurses during the COVID-19 pandemic. Sleep Biol Rhythms. 2021;19(4):467–473. doi:10.1007/s41105-021-00337-6
30. Fabbri M, Beracci A, Martoni M, et al. Measuring subjective sleep quality: a review. Int J Environ Res Public Health. 2021;18(3):1082. doi:10.3390/ijerph18031082
31. Redman KN, Karstaedt AS, Scheuermaier K. Increased CD4 counts, pain and depression are correlates of lower sleep quality in treated HIV positive patients with low baseline CD4 counts. Brain Behav Immun. 2018;69:548–555. doi:10.1016/j.bbi.2018.02.002
32. Zigmond AS, Snaith RP. The Hospital Anxiety and Depression Scale. Acta Psychiatr Scand. 1983;67(6):361–370. doi:10.1111/j.1600-0447.1983.tb09716.x
33. Liu X, Haagsma J, Sijbrands E, et al. Anxiety and depression in diabetes care: longitudinal associations with health-related quality of life. Sci Rep. 2020;10(1):8307. doi:10.1038/s41598-020-57647-x
34. Lkhagvasuren B, Hiramoto T, Tumurbaatar E, et al. The brain overwork scale: a population-based cross-sectional study on the psychometric properties of a new 10-item scale to assess mental distress in Mongolia. Healthcare. 2023;11(7):1003. doi:10.3390/healthcare11071003
35. Karakizlis H, Doerr JM, Becker A, et al. Neuropsychological Assessment of Cognitive Impairment in Kidney Transplantation (NAsKiT) and its related risk factors: a study protocol. J Nephrol. 2022;35(7):1933–1941. doi:10.1007/s40620-022-01376-z
36. Christodoulou C, Michopoulos J, Tournikioti K, et al. Hospital Anxiety and Depression Scale. A quantitative analysis in medical outpatients, psychiatric outpatients and normal subjects. Psychiatriki. 2010;21(4):279–286.
37. Xie N, Yan H, Ding J, et al. Reliability and validity analysis of the Hospital Anxiety and Depression Scale among HIV/AIDS patients. Chin J AIDS STD. 2020;26(12):1328–1331.
38. Zhang YT, Huang T, Zhou F, et al. Correlation between anxiety, depression, and sleep quality in college students. Biomed Environ Sci. 2022;35(7):648–651. doi:10.3967/bes2022.084
39. Hoffmann C, Welz T, Sabranski M, et al. Higher rates of neuropsychiatric adverse events leading to dolutegravir discontinuation in women and older patients. HIV Med. 2017;18(1):56–63. doi:10.1111/hiv.12468
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.